Abstract
Fast excitatory postsynaptic potentials (fEPSPs) occur in bursts in the myenteric plexus during evoked motor reflexes in the guinea-pig ileum in vitro. This study used electrophysiological methods to study fEPSPs during stimulus trains to mimic bursts of synaptic activity in vitro. The amplitude of fEPSPs or fast excitatory postsynaptic currents (EPSCs) declined (rundown) during stimulus trains at frequencies of 0.5, 5, 10 and 20 Hz. At 0.5 Hz, fEPSP or fEPSC amplitude declined by 50% after the first stimulus but remained constant for the remainder of the train. At 5, 10 and 20 Hz, synaptic responses ran down completely with time constants of 0.35, 0.21 and 0.11 s, respectively. Recovery from rundown occurred with a time constant of 7 seconds. Mecamylamine, a nicotinic cholinergic receptor antagonist, or PPADS, a P2X receptor antagonist, reduced fEPSP amplitude, but they had no effect on rundown. Responses caused by trains of ionophoretically-applied ATP or ACh (to mimic fEPSPs or fEPSCs) did not rundown. Blockade of presynaptic inhibitory muscarinic, adenosine A1, opioid, α2-adrenergic and 5-HT1A receptors or pertussis toxin treatment did not alter rundown. Antidromic action potentials followed a 10 Hz stimulus train. Iberiotoxin (100 nM), which was used to block large conductance calcium activated K+ (BK) channels, did not alter rundown. Iberiotoxin increased fEPSP amplitude and action potential duration. These data suggest that synaptic rundown is not due to: a) action potential failure; b) nicotinic or P2X receptor desensitization; c) presynaptic inhibition mediated by pertussis-toxin sensitive G-proteins, or d) BK+ channel activation. Synaptic rundown is likely due to depletion of a readily releasable pool of neurotransmitter.
Keywords: Enteric nervous system, synaptic transmission, electrophysiology, P2X receptors, nicotinic acetylcholine receptors
1. Introduction
Regulation of gastrointestinal (GI) motility depends on the coordinated activity of enteric neurons. Electrophysiological studies have identified two types of myenteric neuron: S-type and AH type (Hirst et al., 1974; Bornstein et al. 1994). S-type neurons receive fast excitatory synaptic input as single electrical stimulation applied to interganglionic never fibers elicits a fast excitatory postsynaptic potential (fEPSP) (Hirst et al., 1974; Nishi and North, 1973). The action potential in S neurons is mediated by voltage-gated Na+ channels thus they are totally blocked by tetrodotoxin (TTX) (Hirst et al., 1974; Bornstein et al. 1994). S-neurons are interneurons and motorneurons (Bornstein et al., 1994; Costa et al., 1996; Furness, 2000). AH neurons produce an action potential that is followed by an afterhyperpolarization of 1−20 seconds duration (Hirst et al., 1974; Furness et al. 1998). The action potential in AH neurons is caused by currents carried by calcium and sodium ions and it is only partly blocked by TTX (Hirst et al., 1974, Furness et al., 1998). AH neurons may be intrinsic primary afferent neurons (IPANs) (Furness et al., 2004).
Fast excitatory synaptic transmission in the enteric nervous system (ENS) plays an important role in controlling GI motility (Kunze and Furness, 1999; Galligan, 2002). In the guinea-pig small intestine, nicotinic acetylcholine receptors (nAChRs) and P2X receptors are co-expressed by many myenteric S neurons and synaptically-released ACh or ATP acting at nAChRs and P2X receptors respectively mediate most fEPSPs (Galligan and Bertrand, 1994; Galligan, 2002). P2X receptors and nAChRs are ligand-gated ion channels that are permeable to sodium, potassium and calcium ions. Electrophysiological studies in myenteric neurons maintained in primary culture have shown that nAChRs desensitize much more quickly than P2X receptors expressed by the same neurons (Zhou and Galligan, 1998; Zhou et al., 2002; Brown and Galligan, 2003). Different receptor desensitization rates may have functional significance for the control of GI motility as it has been shown that mechanical stimulation of the mucosa in isolated segments of guinea-pig ileum elicits bursts of fEPSPs that occur at frequencies ranging between 15 − 40 Hz (Bornstein et al., 1991; Smith et al., 1992). Similar bursts of fEPSPs were also elicited when chemical stimulation was applied to mucosa (Bertrand et al., 1997). As P2X receptors desensitize more slowly than nAChRs, these receptor classes may make quantitatively different contributions to fEPSPs during prolonged bursts of synaptic activity. That is, nAChRs may make the largest contribution to the fEPSP early in a train of fEPSPs while P2X receptors may make a larger contribution later in the burst after nAChRs have begun to desensitize. This hypothesis was tested in the present study where conventional electrophysiological methods were used to investigate the dynamics of fEPSPs during short trains of electrical stimulation in vitro. Short trains of stimulation were used to mimic the bursts of fEPSPs that might occur during motor reflexes elicited by mucosal stimulation.
2. Methods and materials
2.1 Tissue preparation
Animal use procedures were approved by the All University Committee on Animal Use and Care at Michigan State University. Male guinea pigs weighing 300−400 g were anesthetized via halothane inhalation, stunned and exsanguinated by severing the major neck blood vessels. A segment of ileum was harvested 15−20 cm proximal to the ileocecal junction and placed in oxygenated (95% O2 and 5% CO2) Krebs’ solution of the following composition (millimolar): NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; glucose, 11. The Krebs’ solution contained nifedipine (1 μM) to block longitudinal muscle contractions and scopolamine (1 μM) to block muscarinic cholinergic receptors. A 1.5 cm segment of ileum was cut open along the mesenteric border and pinned out flat with the mucosal surface up in a petri dish lined with a silastic elastomer (DOW CORNING Co., Midland, MI). A longitudinal muscle-myenteric plexus preparation was made by peeling away the mucosal, submucosal, and circular muscle layers using fine forceps and scissors. A 5 mm2 piece of longitudinal muscle-myenteric plexus was transferred to a 2 ml volume recording chamber. The preparation was stretched lightly and pinned to the chamber bottom using stainless steel pins (50 μm diameter). The preparation was superfused with 36°C oxygenated Krebs’ solution at a flow rate of 4 ml/min.
2.2 Intracellular electrophysiological recording
Individual myenteric ganglia were visualized at 200× magnification using an inverted microscope (Olympus CK-2) with differential interface contrast optics (Hoffman Modulation). Intracellular recordings were obtained from single neurons using glass microelectrodes filled with 2 M KCl and with a tip resistance of 80−120 MΩ. An amplifier (Axoclamp 2A, Axon Instruments, Foster City, CA) was used to record membrane potentials in current clamp mode. In some experiments, the single electrode voltage clamp mode was used to record fast excitatory synaptic currents (fEPSCs) or currents caused by ionophoretic application of ATP or acetylcholine (ACh). In these voltage clamp experiments, the switching rate was adjusted to 3 kHz and the gain setting was 2−3. In most current clamp experiments, the membrane potential was adjusted to −70 mV using constant DC current to avoid action potentials when evoking fEPSPs. When recording fEPSCs, the membrane potential was held at −60 mV. Signals were filtered at 1 kHz using a four-pole, low-pass Bessel filter (Warner Instruments, Hamden, CT), and digitized at 5 kHz using Digitdata 1200 analog/digital converter (Axon Instruments). Data were acquired and analyzed using Axotape 2.02 or Axoscope 8.2 software (Axon Instruments) and a desktop computer. A digital average of 8 fEPSPs/fEPSCs or ionophoretic responses was used to measure the amplitude of responses under control conditions and after various treatments.
Synaptic responses were evoked using a Krebs’ solution-filled glass pipette (tip diameter 40−60 μm) as a focal stimulating electrode. The stimulating electrode was positioned over an interganglionic nerve strand supplying the ganglion containing the impaled neuron. Nerve fibers were stimulated using a train of 20 stimuli delivered at frequencies of 0.5, 5, 10 and 20 Hz. The interval between successive stimulus trains in an individual experiment from a single neuron was at least 2 minutes.
2.3 Drug application
Antagonists were applied at a known concentration by addition to the superfusing Krebs’ solution. Antagonists were applied for 5−10 minutes prior to measuring the amplitude of evoked responses. ACh or ATP was applied by ionophoresis from an electrode (10−20 MΩ tip resistance) placed directly above the impaled neuron. The concentration of ACh or ATP used for ionophoresis was 1 M. When ACh ionophoresis was applied, a retaining current of −6 nA was used and cathode current pulses of 1−5 ms duration and 100−199 nA intensity were used to eject the agonist. When ATP ionophoresis was applied, a retaining current of +8 nA was used and an anodal current pulses of 1−5 ms duration and 100−199 nA intensity were used to eject the agonist.
2.4 Drugs
All drugs and chemicals were purchased from Sigma Chemical (St. Louis, MO). All reagents and drugs were diluted in distilled, deionized water except for nifedipine, which was dissolved in 95% ethanol to make a 10 mM concentrated stock solution. The final working concentration of all drugs was made daily by diluting concentrated stock solutions in Krebs’ solution.
2.5 Statistics
All antagonist effects on fEPSP/fEPSC amplitude or the amplitude of ACh or ATP responses were expressed as percent change from control amplitude. Changes in fEPSP/fEPSC amplitude during trains of stimulation were expressed as a percentage of the first response in the stimulus train. All data are expressed as the mean ± SEM and “n’ values indicate the number of neurons from which the data were obtained. The time course for decay of fEPSP amplitude during trains of stimulation or recovery from rundown after a stimulus train was fitted using the following exponential function:
where τ is the time constant for decay or recovery from rundown.
Student's t-test for paired data or analysis of variance was used to establish significant differences between control and treatment groups. A P value < 0.05 was considered statistically significant.
3. Results
3.1 fEPSCs declined in amplitude during trains of stimulation
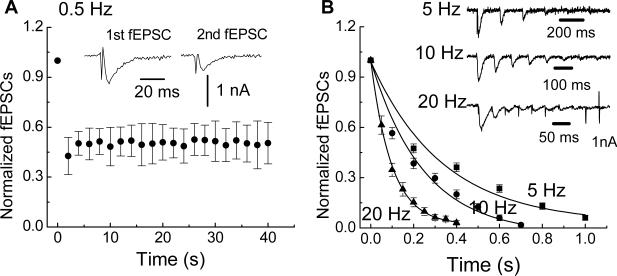
Trains of stimulation elicit slow excitatory post-synaptic potentials (sEPSPs) in S and AH type neurons in the enteric nervous system (Grafe et al., 1980; Morita and North, 1985; Wood and Kirchgessner, 2004). The depolarization occurring during sEPSPs would reduce the electrochemical driving force for sodium and calcium influx through nAChRs and P2X receptors thereby reducing fEPSP amplitude during a stimulus train. Therefore, we used single electrode voltage clamp methods to hold the membrane potential at −60 mV in order to record fEPSC amplitude at a constant membrane potential. Holding the membrane potential constant would eliminate changes in driving force as a cause for alterations in the amplitude of fast synaptic responses during trains of stimulation. It was found that successive fEPSCs declined in amplitude in a frequency-dependent manner (Fig. 1). At 0.5 Hz, the fEPSC declined in amplitude by approximately 50% after the first stimulus but was maintained throughout the remainder of the stimulus train (Fig. 1A). However, at stimulus frequencies of 5, 10 and 20 Hz, the amplitude of fEPSCs ran down completely and the rate of amplitude decline was frequency dependent and was fitted by a single exponential decay (Fig. 1B). The time constants (τ) for rundown of the fEPSCs at 5, 10 and 20 Hz were 0.35 ± 0.05, 0.21 ± 0.02 and 0.11 ± 0.02 s, respectively. These data indicate that fast excitatory synaptic responses rundown in amplitude during trains of stimulation and that synaptic rundown is not caused by changes in driving force that might occur during sEPSPs. Mosts of subsequent studies of synaptic rundown were done in current clamp mode to measure fEPSPs.
Fig. 1.
Trains of electrical stimulation cause fast synaptic rundown. (A) At 0.5 Hz, the fEPSC declined by 50% after the first stimulus but remained at half amplitude for the remainder of the stimulus train. Data are mean ± SEM (n = 17). Inset shows representative fEPSCs after the first and second stimuli. (B) Stimulus trains at 5, 10 and 20 Hz caused complete fEPSC rundown. Inset traces show representative recordings at each stimulation frequency. The rate of decline in fEPSC amplitude was frequency-dependent and was fitted by a monoexponential decay (solid lines). Symbols in the figure are the mean ± SEM of data obtained from 21 neurons at 5 Hz, 32 neurons at 10 Hz and 19 neurons at 20 Hz.
3.2 fEPSPs exhibit paired pulse depression
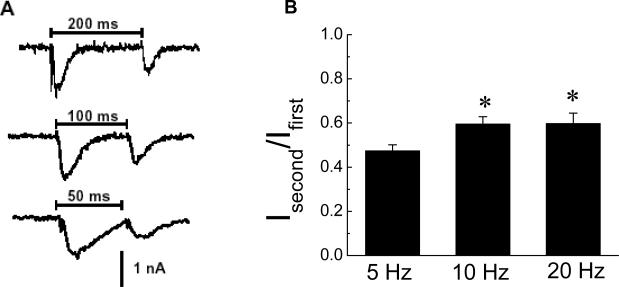
Paired pulse facilitation (PPF) is a property of many synapses in the peripheral and central nervous systems. We tested for the presence of PPF at synapses in the myenteric plexus by measuring the ratio of the amplitude of fEPSCs elicited by pairs of stimuli applied at intervals of 200, 100 and 50 ms (5, 10 and 20 Hz frequencies, respectively)(Fig. 2A). The second fEPSC in the pair was always smaller than the first indicating that synaptic connections in the myenteric plexus exhibit paired pulse depression rather than PPF (Fig. 2). It was also noted that the second fEPSC evoked at the 10 and 20 Hz frequencies exhibited less depression that that evoked at 5 Hz (Fig. 2B).
Fig. 2.
Paired stimuli caused synaptic depression. (A) The second fEPSC in a pair evoked by stimuli at 5 (200 ms interval), 10 (100 ms interval) and 20 (50 ms interval) Hz was smaller in amplitude than the first fEPSC. (B) Mean data from experiments similar to that shown in A. Data are the ratio of the second fEPSC vs. the first fEPSC (Isecond/Ifirst) in a stimulus pair.
*indicates that synaptic depression at 10 (n = 32) and 20 (n = 19) Hz is less than that occurring at 5 Hz (n = 21). Data are mean ± SEM.
3.3 Recovery from synaptic rundown
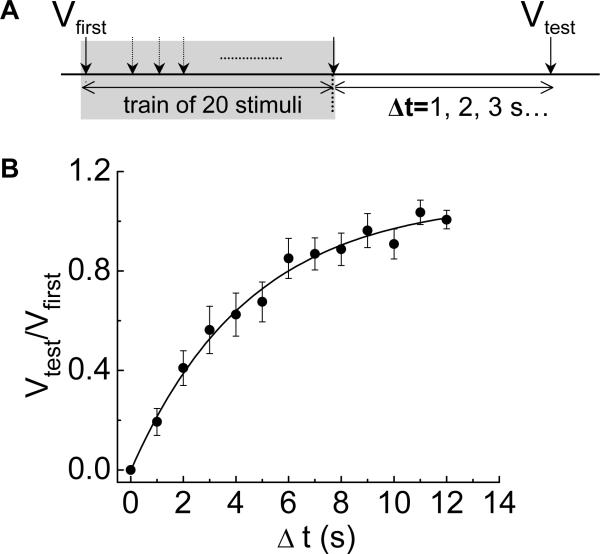
We next investigated the time course of recovery from synaptic rundown following a 10 Hz train of stimulation. In these experiments a train of 20 stimuli at 10 Hz caused fEPSPs to decline in amplitude to 0 mV. Recovery from rundown was tested by waiting for periods of 1 to 12 s before a single stimulus was used to elicit a fEPSP (Vtest); this amplitude was expressed as a fraction of the amplitude of the first EPSP in the preceding stimulus train (Vfirst)(Fig. 3A). Recovery from synaptic rundown occurred in less than 12 s and the recovery time course was fitted by a monoexponential function; the recovery time constant was 7 ± 1.6 s (Fig. 3B).
Fig. 3.
Time-dependent recovery from synaptic rundown. (A) Diagram of the protocol used to measure the time course of recovery from synaptic rundown. A conditioning train of 20 stimuli at 10 Hz was used to cause fEPSP rundown. A single test stimulus applied at various intervals after the conditioning train was used elicit a fEPSP to determine the extent of recovery. Δt is the time interval between the last stimulation in the conditioning and the test stimulus. Recovery is expressed as the ratio of the amplitude of the fEPSP caused by the test stimulus (Vtest) vs. the amplitude of the fEPSP caused by the first stimulus (Vfirst) in the conditioning train. (B) The time course of recovery was fit by a single exponential function with a τ = 7 ± 1.7 s. Data points are the mean ± SEM of observations made on 14 neurons.
3.4 Cholinergic and purinergic components of fEPSP rundown during stimulus trains
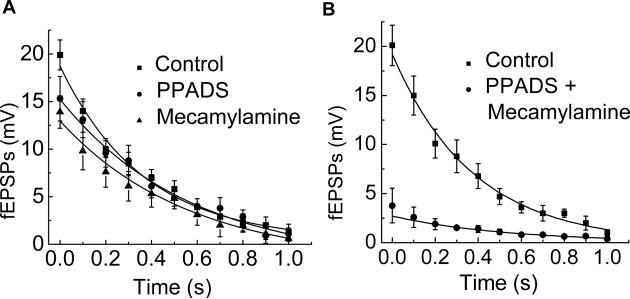
As ACh, acting at nAChRs, and ATP, acting at P2X receptors, contribute to most fEPSPs recorded from myenteric neurons in the guinea pig ileum we next examined if there was selective rundown of one of these fEPSP components during a 10 Hz train of stimulation. The P2X-mediated component of the fEPSP was isolated by using mecamylamine (10 μM), to block nAChRs. Under these conditions, it was found that the remaining fEPSP declined in amplitude with a time course (τ = 0.3 ± 0.07 s, n = 6) that was similar to that occurring in the absence of mecamylamine (τ = 0.3 ± 0.07 s, n = 6) (Fig. 4A). The cholinergic component of the fEPSP was isolated by using PPADS (10 μM) to block P2X receptors. The cholinergic fEPSP randown with a time course (τ = 0.4 ± 0.08 s, n = 8) similar to that measured under control conditions and in the presence of mecamylamine (Fig. 4A). More than 90% of the fEPSP was abolished by co-application of mecamylamine and PPADS (Fig. 4B) indicating that we were studying a mixed cholinergic-purinergic fEPSP.
Fig. 4.
Mecamylamine or PPADS did not alter fEPSP rundown. (A) The amplitude of fEPSPs was reduced by mecamylamine or PPADS (each at 10 μM) but the time course of rundown was not changed by the antagonists. The rate of synaptic rundown was fitted by a monoexponential function with τ values of = 0.3 ± 0.07, 0.3 ± 0.07 and 0.4 ± 0.08 s under control conditions and in the presence of mecamylamine and PPADS, respectively. Each point is the mean ± SEM (n = 6). (B) Co-application of mecamylamine or PPADS inhibited the fEPSP by more than 90%. Data are mean ± SEM (n = 6).
3.5 Rundown of fEPSPs was not due to nAChR or P2X receptor desensitization
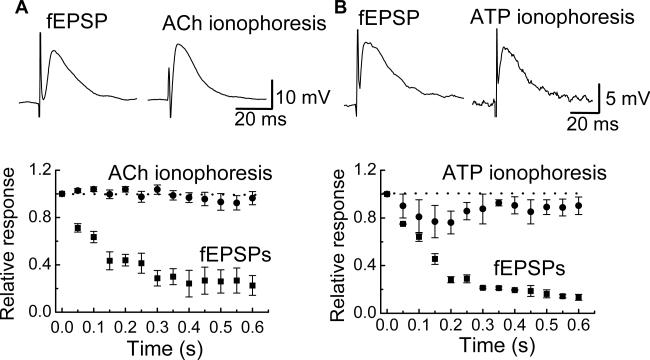
ACh and ATP ionophoresis was utilized to investigate if fEPSP rundown was due to nAChR and/or P2X receptor desensitization. ACh and ATP ionophoresis caused depolarizations that were similar in amplitude and time course to fEPSPs recorded from the same neurons (Fig. 5A). ACh and ATP were applied as trains (20 Hz) of ionophoretic pulses and fEPSPs were evoked in the same neurons using a 20 Hz stimulus train. As described above, fEPSPs declined in amplitude during the stimulus train. However, in the same neurons, neither ACh nor ATP responses declined in amplitude during a train of agonist application (Fig. 5). These data indicate that synaptic rundown is not due to postsynaptic receptor desensitization.
Fig. 5.
Postsynaptic desensitization does not contribute to synaptic rundown. (A) ACh applied by ionophoresis caused a depolarization that mimicked the amplitude and time course of the fEPSP. During a 20 Hz stimulation train, the fEPSP ran down while responses caused by a 20 Hz train of ionophoretic pulses of ACh did not. Data are mean ± SEM (n = 4). (B) ATP applied by ionophoresis caused a depolarization that mimicked the fEPSP. Responses caused by a 20 Hz train of ionophoretic pulses of ATP did not run down while fEPSPs caused by a 20 Hz stimulus train did. Data are mean ± SEM (n = 4).
3.6 Rundown of fEPSPs was not due to presynaptic inhibition
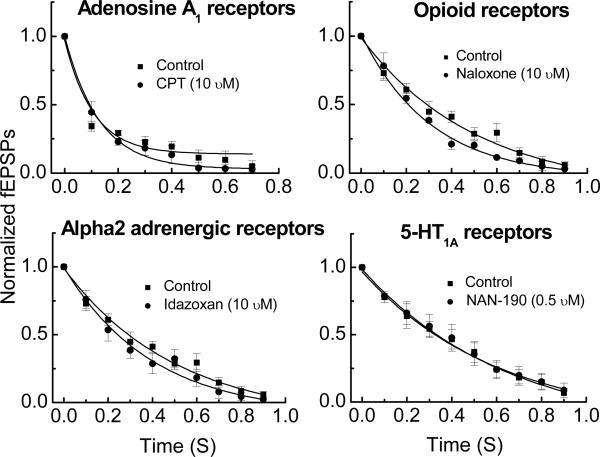
Nerve terminals in the myenteric plexus express a number of receptors that mediate presynaptic inhibition of neurotransmitter release. These receptors include muscarinic M2 receptors, 5-HT1A receptors, opioid receptors, α2 adrenergic receptors and adenosine A1 receptors. It is possible that during trains of stimulation, myenteric nerves release the endogenous agonists for one or more of these receptors and that presynaptic inhibition of ACh and/or ATP is responsible for fEPSP rundown. To test this hypothesis, fEPSP rundown was measured before and after application of antagonists for each of the receptors listed above. The exception being an antagonist for the M2 muscarinic receptor as scopolamine, which blocks all muscarinic receptor subtypes, was present in the Krebs’ solution in all studies (see Methods and Materials). We used naloxone (10 μM) to block opioid receptors, NAN-190 (0.5 μM) to block 5-HT1A receptors, idazoxan (10 μM) to block α2 adrenergic receptors, and 8-cyclopentyl-theophylline (CPT, 10 μM) to block A1 adenosine receptors. None of the antagonists tested altered fEPSP rundown during a 10 Hz stimulus train (Fig. 6).
Fig. 6.
Antagonists of presynaptic inhibitory receptors did not alter synaptic rundown. Each figure shows the mean ± SEM of successive fEPSPs normalized to the first fEPSP in the train before and after drug treatment. There were no differences between the control and drug treatment values at any time point. Data are mean ± SEM (n = 4 − 5).
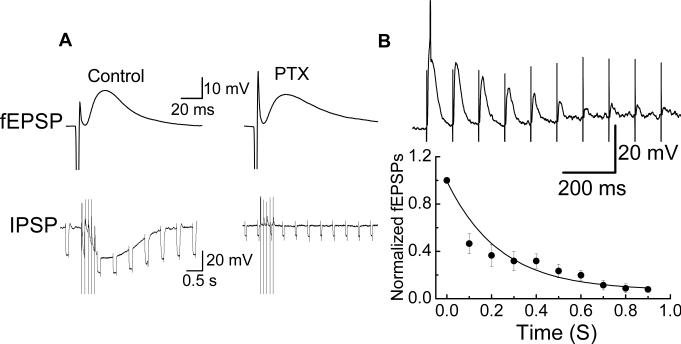
It is possible that in using the antagonists tested above we have not blocked the receptor responsible for presynaptic inhibition leading to synaptic rundown. In order to comprehensively eliminate presynaptic inhibition as a mechanism of synaptic rundown we attempted to block the intracellular signaling pathway coupled to presynaptic receptors. Most inhibitory presynaptic receptors are G-protein coupled receptors, which link to the pertussis toxin (PTX)-sensitive Gi/Go subsets of G-proteins (Zamponi, 2001). We used the amplitude of the inhibitory postsynaptic potential (IPSP) recorded from submucosal S neurons as an indicator of the effectiveness of the PTX-treatment protocol. Previous work has shown that the IPSP is mediated by norepinephrine acting α2-adrenergic receptors which couple via a PTX sensitive G-protein to activation of a potassium channel (Surprenant and North, 1988). We showed that IPSPs were blocked in PTX pretreated submucosal preparation while fEPSPs recorded from the same cells were unchanged (Fig. 7A). The same protocol was used to treat myenteric plexus prepartions with PTX. In these tissues it was found that fEPSPs still exhibited rundown that was similar to that observed under control conditions (Fig. 7B).
Fig. 7.
Pertussis toxin (PTX) did not affect fEPSP rundown. (A) PTX pretreatment abolished the IPSP but not the fEPSP in submucosal neurons. Downward deflections in the IPSP traces are voltage responses caused by hyperpolarizing current pulses. A decrease in the amplitude of these responses during the IPSP indicates a decrease in membrane resistance. (B) Successive fEPSPs recorded from myenteric neurons with and without PTX pretreatment. There were no differences between values obtained in treated and untreated tissues at any time point. Data are mean ± SEM (n = 8).
3.7 Action potential waveform was not altered during stimulus trains
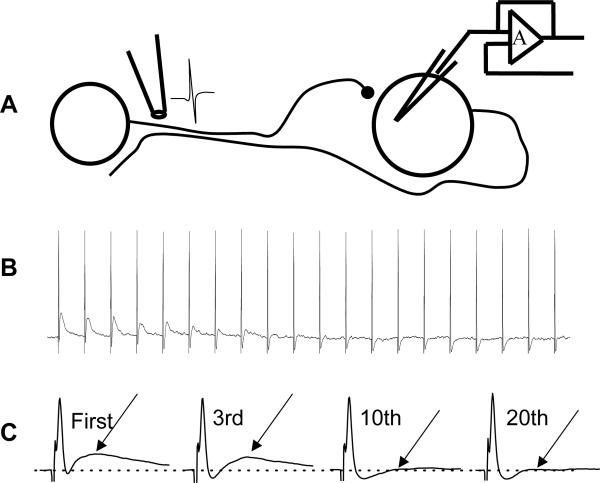
In order to test the hypothesis that action potential failureis a mechanism of fEPSP rundown we made recordings from the neurons of which the focal stimulating electrode was positioned on the axons . We have made such recordings from at least ten neurons. This allowed us to make simultaneous recordings of axonally-propagated action potentials and fEPSPs during a 10 Hz stimulus train. In these recordings it was found that antidromic action potentials were maintained throughout the stimulus train while fEPSPs declined in amplitude during the same stimulus train (Fig. 8).
Fig. 8.
Action potential failure did not contribute to synaptic rundown. (A) Diagram of experiment set-up used to evaluate action potential failure during trains of stimulation. The focal stimulating electrode was positioned on an internodal strand that contained the axon of the neuron from which intracellular recordings were obtained. The strand also contained nerve fibers providing synaptic input to the same neuron. (B) Recording of antidromic action potentials and fEPSPs elicited by a 10 Hz train of stimulation. The fEPSP declined in amplitude while the antidromic action potential followed each stimulus in the train. (C) Examples of the antidromic action potential and fEPSPs shown on an expanded time scale.
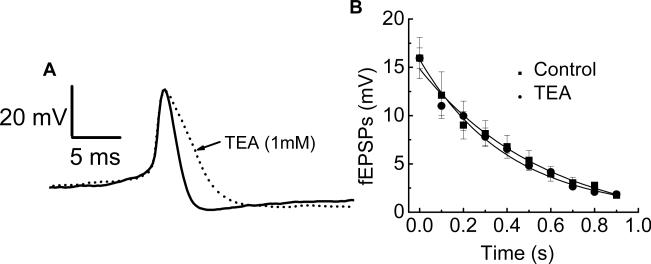
The activity of large conductance calcium-activated potassium (BK) channels in nerve terminals can modulate neurotransmitter release (Robitaille and Charlton, 1992). Calcium entering the nerve terminal during the action potential opens BK channels. The openning of BK channels hyperpolarizes the nerve terminal, which inhibits subsequent action potentials and transmitter release. We used tetraethylammonium (TEA) at a concentration (1 mM) that will block BK channels to determine if activation of these channels contributed to fEPSP rundown. It was found that TEA broadened somal action potentials triggered by intracellular depolarization. This is an indication of effectiveness in blocking potassium channels in the preparation (Fig. 9A). However, it was also found that TEA did not alter either fast synaptic rundown or the amplitude of fEPSPs in the same tissues (Fig. 9B).
Fig. 9.
TEA (1 mM) did not affect synaptic rundown. (A) TEA broadened the somal action potential indicating that it blocked K+ channels. (B) TEA did not change the time course or extent of synaptic rundown during a 10 Hz train of stimulation. Data are mean ± SEM (n = 5).
4. Discussion
4.1 Rundown of fEPSPs
In isolated segments of guinea pig ileum, it has been shown that mechanical stimulation of mucosal villi generates short bursts of fEPSPs that occurred in the frequency range of 15 − 40 (Bornstein et al. 1991). In similar experiments it was shown that if the mechanical stimulus is applied at intervals of less than 10 seconds, the amplitude of fEPSPs declined during repetitive stimulation (Smith et al., 1992). Chemical stimulation (low pH, 5-HT) of the mucosa also elicits short bursts of fEPSPs (average of 14 fEPSPs per burst) occurring at the frequencies between 5 −20 Hz (Bertrand et al., 1997). In the present study, we attempted to study the dynamics of fast synaptic excitation during short trains of electrical stimulation of presynaptic nerve fibers. The frequencies of the stimulus trains that we used mimicked the frequencies of fEPSPs evoked by the physiological stimuli described above. We found that short trains of electrical stimulation elicited fEPSPs that declined in amplitude in a frequency dependent manner in all neurons tested. Our conclusions are based on recordings from S type neurons, as these cells all received fast excitatory synaptic input, and the action potentials were not followed a long-lasting (> 1s) action potential afterhyperpolarization. Our data differ from those obtained in studies of fast synaptic input to AH-type neurons in the pig intestine where it was found that fEPSPs did not rundown when evoked by trains of stimulation at frequencies up to 20 Hz (Cornelissen et al., 2001). Similarly, in the rat descending colon, fast synaptic input to most myenteric S neurons is maintained during 10 Hz trains of stimulation (Browning and Lees 1996). These observations suggest that there are species or tissue differences in the mechanisms regulating the availability and release of stores of fast synaptic transmitters in the myenteric plexus.
4.2 Receptor desensitization did not contribute to synaptic rundown
In myenteric S neurons, most fEPSPs are mediated by ACh acting at nAChRs and ATP acting at P2X receptors (Galligan et al., 2000). Previous work had shown that nAChRs expressed by guinea pig myenteric neurons desensitize faster than enteric P2X receptors (Zhou and Galligan, 1998; Brown and Galligan, 2003). Based on this difference in desensitization rate we proposed that nAChRs and P2X receptors would make a differential contribution to the fEPSPs during trains of stimulation. Our hypothesis was that the nicotinic component would desensitize early in a train of stimulation while P2X receptors would maintain synaptic transmission as the stimulus trained continued. However, our data indicate that this does not happen, as selective blockade of nAChRs or P2X receptors did not alter the rate of fEPSP rundown during a 10 Hz train of stimulation. Mecamylaine and PPADS alone reduced approximately 35% of fEPSP while co-application of these two blockers reduced 90% of fEPSP (fig. 4). This result is consistent with the previous finding that P2X receptors and nAChRs are linked in a mutually inhibitory manner in guinea-pig myenteric neurons (Zhou and Galligan, 1998; Khakh et al., 2000). Furthermore, when ACh and ATP were applied by trains of ionophoretic pulses to mimic fEPSPs, the ionophoretic responses did not decline in amplitude. These data indicate that little desensitization occurs during the brief occupation of nAChRs or P2X receptors by either synaptically-released or ionophoretically-applied agonist. Taken together these data indicate that receptor desensitization does not make a major contribution to rundown of fast synaptic excitation during trains of electrical stimulation.
4.3 Rundown is not caused by presynaptic inhibition
Presynaptic inhibition is a prominent mechanism for modulating synaptic transmission in the ENS. α2-adrenoceptors are localized to cholinergic neurons and these receptors mediate inhibition of acetylcholine release onto myenteric S neurons (Stebbing et al., 2001; Scheibner et al., 2002). Opioid receptors are expressed in cell bodies and processes of myenteric neurons and activation of opioid receptors inhibits the electrically evoked release of ACh (Sternini, 2001). Muscarinic M2 receptors also mediate presynaptic inhibition of fast synaptic transmission in the myenteric plexus (North et al., 1985). Adenosine A1 (Christofi and Wood, 1993) and 5-HT1A (Pan and Galligan, 1994) receptors also couple to presynaptic inhibition of fast synaptic excitation in the ENS. Acetylcholine, norepinephrine, enkephalins and 5-HT-containing nerve fibers are present throughout the myenteric plexus (Costa and Furness, 1984; Costa et al., 1996; Brookes, 2001) and trains of nerve stimulation will release all of the substances. As adenosine is a breakdown product of ATP of neurally-released ATP (Westfall et al., 2002), adenosine would accumulate in the myenteric plexus during trains of nerve stimulation. Therefore, fast synaptic rundown could be due to presynaptic inhibition caused by the accumulation of endogenous agonists for presynaptic inhibitory receptors. However, our data show that fast synaptic rundown persists in the presence of muscarinic, α2 adrenergic, opioid, A1 adenosine and 5-HT1A receptor antagonists. Based on these data, we conclude that presynaptic inhibition mediated by one or more of these receptor classes is not responsible for fEPSP rundown. Although we have tested the contribution of several established presynaptic receptor classes to fEPSP rundown, there are many presynaptic receptors localized to myenteric nerve terminals. Inhibitory presynaptic receptors in the enteric nervous system belong to the family of G-protein coupled receptors which link to PTX sensitive mechanisms leading to inhibition of neurotransmitter release (Zamponi, 2001). In order to eliminate presynaptic inhibition mediated by G-protein coupled receptors, we studied fast synaptic rundown in myenteric plexus preparations that had been treated with PTX to inactivate the Gi/Go class of G-proteins. Fast synaptic rundown was identical in control and in PTX-treated tissues indicating that presynaptic inhibition does not account for fast synaptic rundown. Although PTX-treatment did not alter synaptic rundown, the treatment protocol was effective in inactivating the target G-proteins. This conclusion is based on our data showing that the α2-adrenoceptor-mediated IPSP recorded from submucosal S neurons was blocked in preparations that had been treated using our PTX incubation protocol. IPSPs in submucosal neurons are mediated by α2-adrenoceptors that couple directly to a potassium channel via a PTX sensitive G-protein (Surprenant and North, 1988).
4.4 Changes in presynaptic action potentials do not contribute synaptic rundown
A frequency-dependent decrease in nerve terminal Ca2+ influx could result in synaptic rundown. Decreased nerve terminal Ca2+ entry be due to axonal action potential failure during high frequency nerve stimulation (Cunnane and Stjarne, 1984). We made recordings from ten neurons in which fEPSPs and antidromically propagated action potentials were recorded simultaneously. These experiments showed that action potentials followed each stimulus in a 10 Hz train while fEPSPs declined in amplitude. These data indicate that action potential propagation into the nerve terminal is maintained throughout a train of stimuli and action potential failure does not contribute to fEPSP rundown.
Frequency-dependent shortening of action potential duration could contribute to synaptic rundown. This could occur either through Ca2+ channel inactivation or though a Ca2+-dependent activation of a potassium channel which would accelerate action potential repolarization (Franciolini et al., 2001). The inactivation rate of Ca2+ channels in myenteric neurons is in the range from 100 ms to 1 s (Bian et al., 2004 ). It is unlikely that Ca2+ channels accounted for the fast rundown in myenteric neurons. BK channels are a class of Ca2+-activated potassium channels expressed in nerve terminals and these channels function to limit nerve terminal action potential duration and calcium entry (Robitaille and Charlton, 1992). We used TEA to block BK channels and to increase action potential duration thereby increasing Ca2+ entry during each action potential. It was found that TEA did not alter fast synaptic rundown. We also noticed that TEA did not enhance amplitude of fEPSPs. More Ca2+ entry should trigger an increased transmitter release. Since there is no change in the sensitivity of P2X receptors and nAChRs in post synapse, we would expect the bigger fEPSPs. TEA has been found to antagonize nAChRs in various tissues by acting at BK channels (Alder et al., 1979; Blanchet and Dulon 2001). It suggested that BK channels in myenteric neurons may not play the role or the TEA effect on BK was covered by antagonizing nAChRs. As fEPSPs still ran down in present of TEA, these data indicate that BK channel-dependent shortening of the action potential and decreases in nerve terminal Ca2+ entry do not contribute to rundown of fEPSPs during trains of stimulation.
4.4 Depletion of transmitter accounts for synaptic rundown
When nerve fibers were stimulated at low frequency (0.5 Hz), fEPSP amplitude declined by 50% after the first stimulus but was maintained for the remainder of the stimulus train. There are two models that could explain these data. Firstly, there may be two separate neurotransmitter pools in myenteric nerve terminals. A readily releasable pool (RRP) is available for exocytosis during the first action potential in a train and at low stimulation frequencies, a reserve pool will partly replenish the RRP prior to subsequent stimuli (Rosenmund and Stevens, 1996; Pyle et al., 2000; Sudhof, 2000; von Gersdorff and Borst, 2001; Zucker and Regher, 2002). The release probability of the RRP is very high as a single action potential can deplete this pool. In addition, we never observed PPF whose occurrence is most prominent at low probability synapses (Thomson, 2000; Zucker and Regehr, 2002) . At stimulation frequencies ≥ 5 Hz, fEPSPs decline in amplitude because transmitter release rate exceeds the RRP refilling rate (Stevens and Tsujimoto, 1995; Rosenmund and Stevens, 1996; Dobrunz and Stevens, 1997; Lin et al., 2001). In myenteric nerve terminals the refilling rate of the RRP is relatively slow (τ = 7 s, see Fig. 3) and the slow filling rate will limit the duration and frequency at which bursts of fEPSPs can occur. The mechanism by which vesicles are replenished after short-term depletion may vary among synapses with different recovery time constants. For example, the τ for recovery is 5 s at synapses between hippocampal CA3 and CA1 pyramidal neurons (Debanne et al., 1996), 3 s at synapses between cerebellar granule and Purkinje cells, 4 s at the calyx of held in the brain stem (von Gersdorff et al., 1997), and 2.8 s at synapses in the rat superior cervical ganglion (Lin et al., 2001). The recovery rate depends on trafficking between vesicle pools and the mechanisms of vesicle movement at different synapses may be specialized to meet the particular demands of those synapses.
A second model that would account for our data involves vesicle recycling. In this scheme, the RRP is released by the first action potential in a train. At low frequencies of stimulation (0.5 Hz) some of these vesicles recycle and are then available for release prior to the next action potential. At stimulation frequencies ≥ 5 Hz the release rate exceeds the recycling rate and the RRP becomes depleted. Rapid vesicle recycling (τ ≤ 1 s) contributes to maintained transmission at synapses in the hippocampus where the recycling rate is rapid enough to maintain transmission at a 10 Hz stimulation frequency (Pyle et al., 2000; Sara et al., 2002; Fernandez-Alphonso and Ryan, 2004). These data suggest that vesicle recycling in the myenteric plexus must occur at a much slower rate than in the hippocampus. Alternatively, vesicle mobilization from a reserve pool may be the principal mechanism for synaptic recovery after rundown in the myenteric plexus. Mobilization from the reserve pool is relatively slow (Pyle et al., 2000) and this would account for synaptic rundown during trains of stimulation ≥ 5 Hz.
4.5 Summary and conclusions
Trains of electrical stimulation evoke fEPSPs that decline in amplitude in a frequency-dependent manner in the guinea-pig ileum myenteric plexus. Rundown of fEPSPs is not due to postsynaptic receptor desensitization, presynaptic inhibition mediated by G-protein coupled receptors or to changes in presynaptic action potentials. The data presented here are consistent with a model in which there are two pools of neurotransmitter in myenteric nerve endings. There is readily releasable pool of transmitter that has a high release probability; this pool is depleted following a single action potential. At a low stimulation frequency (0.5 Hz) fast synaptic transmission is maintained either by recycling of released synaptic vesicles or by replenishment of the readily releasable pool by a reserve pool of transmitter. At high frequencies of stimulation (≥ 5 Hz), fEPSPs decline in amplitude because the stimulation rate exceeds the rate at which the readily releasable pool can be restored either by recycling or by refilling from a reserve pool.
Acknowledgment
This work was supported by NIH DK57039.
References
- Adler M, Oliveira AC, Albuquerque EX, Mansour NA, Eldefrawi AT. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J. Gen. Physiol. 1979;74:129–52. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand PP, Kunze WA, Bornstein JC, Furness JB, Smith ML. Analysis of the responses of myenteric neurons in the small intestine to chemical stimulation of the mucosa. Am. J. Physiol. 1997;273:G422–35. doi: 10.1152/ajpgi.1997.273.2.G422. [DOI] [PubMed] [Google Scholar]
- Bian X, Zhou X, Galligan JJ. R-type calcium channels in myenteric neurons of guinea pig small intestine. Am. J. Physiol. 2004;287:G134–42. doi: 10.1152/ajpgi.00532.2003. [DOI] [PubMed] [Google Scholar]
- Blanchet C, Dulon D. Tetraethylammonium ions block the nicotinic cholinergic receptors of cochlear outer hair cells. Brain Res. 2001;915:11–7. doi: 10.1016/s0006-8993(01)02806-2. [DOI] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Smith TK, Trussell DC. Synaptic responses evoked by mechanical stimulation of the mucosa in morphologically characterized myenteric neurons of the guinea-pig ileum. J. Neurosci. 1991;11:505–18. doi: 10.1523/JNEUROSCI.11-02-00505.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein JC, Furness JB, Kunze WAA. Electrophysiological characterization of myenteric neurons: how do classification schemes relate? J. Auton. Nerv. Syst. 1994;48:1–15. doi: 10.1016/0165-1838(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat. Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Brown EN, Galligan JJ. Muscarinic receptors couple to modulation of nicotinic ACh receptor desensitization in myenteric neurons. Am. J. Physiol. 2003;285:G37–G44. doi: 10.1152/ajpgi.00053.2003. [DOI] [PubMed] [Google Scholar]
- Browning KN, Lees GM. Myenteric neurons of the rat descending colon: electrophysiological and correlated morphological properties. Neuroscience. 1996;73:1029–1047. doi: 10.1016/0306-4522(96)00118-2. [DOI] [PubMed] [Google Scholar]
- Christofi FL, Wood JD. Presynaptic inhibition by adenosine A1 receptors on guinea pig small intestinal myenteric neurons. Gastroenterology. 1993;104:1420–1429. doi: 10.1016/0016-5085(93)90351-c. [DOI] [PubMed] [Google Scholar]
- Cornelissen W, de Laet A, Kroese AB, van Bogaert PP, Scheuermann DW, Timmermans JP. Excitatory synaptic inputs on myenteric Dogiel type II neurones of the pig ileum. J. Comp. Neurol. 2001;432:137–54. doi: 10.1002/cne.1093. [DOI] [PubMed] [Google Scholar]
- Costa M, Furness JB. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience. 1984;13:911–919. doi: 10.1016/0306-4522(84)90105-2. [DOI] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–67. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Cunnane TC, Stjarne L. Frequency dependent intermittency and ionic basis of impulse conduction in postganglionic sympathetic fibres of guinea-pig vas deferens. Neuroscience. 1984;11:211–229. doi: 10.1016/0306-4522(84)90225-2. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guerineau NC, Gahwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J. Physiol. 1996;491:163–76. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alphonso T, Ryan TA. The kinetics of synaptic vesicle pool depletion and CNS synaptic terminals. Neuron. 2004;41:943–953. doi: 10.1016/s0896-6273(04)00113-8. [DOI] [PubMed] [Google Scholar]
- Franciolini F, Hogg R, Catacuzzeno L, Petris A, Trequattrini C, Adams DJ. Large-conductance calcium-activated potassium channels in neonatal rat intracardiac ganglion neurons. Pflugers Arch. 2001;441:629–638. doi: 10.1007/s004240000471. [DOI] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WAA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurons of the intestine. Prog. Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Furness JB, Jones C, Nurgali K, Clerc N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 2004;72:143–64. doi: 10.1016/j.pneurobio.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Galligan JJ, Bertrand P. ATP mediates fast synaptic potentials in myenteric neurons. J. Neuronsci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galligan JJ, LePard KJ, Schneider DA, Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 2000;81:97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- Galligan JJ. Ligand-gated ion channels in the enteric nervous system. Neurogastroenterol. Motil. 2002;14:611–23. doi: 10.1046/j.1365-2982.2002.00363.x. [DOI] [PubMed] [Google Scholar]
- Grafe P, Mayer CJ, Wood JD. Synaptic modulation of calcium-dependent potassium conductance in myenteric neurones in the guinea-pig. J. Physiol. 1980;305:235–248. doi: 10.1113/jphysiol.1980.sp013360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst GDS, Holman ME, Spence I. Two types of neurons in the myenteric plexus of duodenum in the guinea pig. J. Physiol. 1974;236:303–326. doi: 10.1113/jphysiol.1974.sp010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Zhou X, Sydes J, Galligan JJ, Lester HA. State-dependent cross-inhibition between transmitter-gated cation channels. Nature. 2000;406:405–10. doi: 10.1038/35019066. [DOI] [PubMed] [Google Scholar]
- Kunze WAA, Furness JB. The enteric nervous system and regulation of intestinal motility. Ann. Rev. Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Lin YQ, Graham K, Bennett MR. Depression of transmitter release at synapses in the rat superior cervical ganglion: the role of transmitter depletion. Auton. Neurosci. 2001;88:16–24. doi: 10.1016/S1566-0702(00)00287-3. [DOI] [PubMed] [Google Scholar]
- Morita K, North RA. Significance of slow synaptic potentials for transmission of excitation in guinea-pig myenteric plexus. Neuroscience. 1985;14:661–672. doi: 10.1016/0306-4522(85)90317-3. [DOI] [PubMed] [Google Scholar]
- Nishi S, North RA. Intracellular recording from the myenteric plexus of the guinea-pig ileum. J. Physiol. 1973;231:471–491. doi: 10.1113/jphysiol.1973.sp010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Slack BE, Surprenant A. Muscarinic M1 and M2 receptors mediate depolarization and presynaptic inhibition in guinea-pig enteric nervous system. J. Physiol. 1985;368:435–452. doi: 10.1113/jphysiol.1985.sp015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Galligan JJ. 5-HT1A and 5-HT4 receptors mediate inhibition and facilitation of fast synaptic transmission in enteric neurons. Am. J. Physiol. 1994;266:230–8. doi: 10.1152/ajpgi.1994.266.2.G230. [DOI] [PubMed] [Google Scholar]
- Pyle JL, Kavalali ET, Piedras-Renteria ES, Tsien RW. Rapid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–31. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Robitaille R, Charlton MP. Presynaptic calcium signals and transmitter release are modulated by calcium-activated potassium channels. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–207. doi: 10.1016/s0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Sara Y, Mozhayeva MG, Liu X, Kavalali ET. Fast vesicle recycling supports neurotransmission during sustained stimulation at hippocampal synapses. J. Neurosci. 2002;22:1608–1617. doi: 10.1523/JNEUROSCI.22-05-01608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibner J, Trendelenburg AU, Hein L, Starke K, Blandizzi C. Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice. Br. J. Pharmacol. 2002;135:697–704. doi: 10.1038/sj.bjp.0704512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Bornstein JC, Furness JB. Convergence of reflex pathways excited by distension and mechanical stimulation of the mucosa onto the same myenteric neurons of the guinea pig small intestine. J. Neurosci. 1992;12:1502–10. doi: 10.1523/JNEUROSCI.12-04-01502.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing M, Johnson P, Vremec M, Bornstein J. Role of alpha(2)-adrenoceptors in the sympathetic inhibition of motility reflexes of guinea-pig ileum. J. Physiol. 2001;534:465–78. doi: 10.1111/j.1469-7793.2001.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C. Receptors and transmission in the brain-gut axis: potential for novel therapies. III. Mu-opioid receptors in the enteric nervous system. Am. J. Physiol. Gastrointest Liver Physiol. 2001;28:G8–15. doi: 10.1152/ajpgi.2001.281.1.G8. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Tsujimoto T. Estimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc. Natl. Acad. Sci. U. S. A. 1995;92:846–9. doi: 10.1073/pnas.92.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle revisited. Neuron. 2000;28:317–20. doi: 10.1016/s0896-6273(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Surprenant A, North RA. Mechanism of synaptic inhibition by noradrenaline acting at alpha 2-adrenoceptors. Proc. R. Soc. Lond. B. Biol. Sci. 1988;234:85–114. doi: 10.1098/rspb.1988.0039. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends in Neuroscience. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Schneggenburger R, Weis S, Neher E. Presynaptic depression at a calyx synapse: the small contribution of metabotropic glutamate receptors. J. Neurosci. 1997;17:8137–46. doi: 10.1523/JNEUROSCI.17-21-08137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat. Rev. Neurosci. 2002;3(1):53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J. Pharmacol. Exp. Ther. 2002;303:439–444. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J. Pharmacol. Exp. Ther. 2002;303(2):439–44. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- Wood JD, Kirchgessner A. Slow excitatory metabotropic signal transmission in the enteric nervous system. Neurogastroenterol. Motil. 2004;16(Suppl 1):71–80. doi: 10.1111/j.1743-3150.2004.00479.x. [DOI] [PubMed] [Google Scholar]
- Zamponi GW. Determinants of G protein inhibition of presynaptic calcium channels. Cell Biochem. Biophys. 2001;34:79–94. doi: 10.1385/CBB:34:1:79. [DOI] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. P2X purinoceptors in cultureed myenteric neurons of guinea-pig small intestine. J. Physiol. 1996;496:719–729. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Galligan JJ. Non-additive interaction between nicotinic chholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. J. Physiol. 1998;513:685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Ren J, Brown E, Schneider D, Caraballo-Lopez Y, Galligan JJ. Pharmacological properties of nicotinic acetylcholine receptors expressed by guinea pig small intestinal myenteric neurons. J. Pharmacol. Exp. Ther. 2002;302:889–97. doi: 10.1124/jpet.102.033548. [DOI] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Ann. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]