Abstract
Reactivation of herpes simplex virus type 1 from neuronal latency is a common and potentially devastating cause of disease worldwide. CD8+ T cells can completely inhibit HSV reactivation in mice, with IFN-γ affording a portion of this protection. Here, we found that CD8+ T cell lytic granules are also required for the maintenance of neuronal latency both in vivo and in ex vivo ganglia cultures, and that their directed release to the junction with neurons in latently infected ganglia did not induce neuronal apoptosis. We describe a non-lethal mechanism of viral inactivation in which the lytic granule component, granzyme B, degrades the herpes simplex virus type 1 immediate early protein, ICP4, which is essential for further viral gene expression.
Several lines of evidence support a role for CD8+ T cells in controlling herpes simplex virus type 1 (HSV-1) latency. CD8+ T cells, many expressing granzyme B (GrB), are found juxtaposed to HSV-1 latently infected sensory neurons of both humans (1–4) and mice (5–8). In C57BL/6 mice, CD8+ T cells specific for the immunodominant HSV-1 glycoprotein B498–505 epitope (gB-CD8) polarize their T cell receptor (TCR) to junctions with neurons in situ forming apparent immunologic synapses (9). Murine gB-CD8 can block HSV-1 reactivation from latency in vivo and in ex vivo ganglia cultures in an MHC-dependent manner (9–11). Because HSV-1 establishes latency solely within ganglionic neurons (12, 13), we hypothesize that some latently infected neurons directly present viral antigens to HSV-specific CD8+ T cells during attempted reactivation, which is subsequently quelled by CD8+ T cell effector functions.
CD8+ T cells can use IFN-γ to block HSV-1 reactivation in some, but not all latently infected sensory neurons (14, 15). HSV-1 reactivation is suppressed by CD8+ T cells in neurons that are refractory to IFN-γ through an as yet undefined mechanism. Lytic granules represent an important CD8+ T cell effector mechanism, but their use is generally lethal to targeted cells. Indeed, GrB-expressing gB-CD8 from latently infected trigeminal ganglia (TG) polarized and released their lytic granules toward susceptible fibroblasts leading to apoptosis (Fig. S1; see note 27). Thus, we investigated whether gBCD8 used lytic granules during immunosurveillance of latently infected neurons, and if they induced neuronal apoptosis.
GrB+ gB-CD8 expanded from latently infected TG of wild type (WT) mice (Fig. S2) were added to cultures of dispersed TG in which reactivated HSV-1 had spread to surrounding fibroblasts. Most fibroblasts targeted by gB-CD8 showed active caspase staining in punctate, multifocal, or diffuse patterns (Fig. S3A&C), consistent with early, intermediate, and late stages of apoptosis, respectively (16). Conversely, none of the gBCD8-targeted neurons showed caspase activation (Fig. S3B&C). Thus, either CD8+ T cells do not release lytic granules towards neurons or lytic granule release does not activate the caspase system of neurons.
To distinguish between these possibilities, we first documented CD8+ T cell polarization of GrB toward junctions with neurons in latently infected TG in situ (Fig. 1A) and ex vivo (Fig. 1B), suggesting ongoing use of directed lytic granule release by CD8+ T cells during immunosurveillance of latently infected ganglia. Histologic studies of HSV-1 latently infected human (1–4) and murine (5–8) ganglia have failed to detect morphologic signs of apoptosis in neurons in direct contact with activated CD8+ T cells. To directly investigate whether neurons are refractory to lytic granule-mediated apoptosis, WT GrB-expressing gB-CD8 were added to dispersed latently infected TG directly ex vivo when HSV-1 is confined to neurons. Of 13 documented neuron/CD8+ T cell interactions exhibiting lytic granule release, none of the targeted neurons exhibited activated caspases (Fig. 1C), whereas neuronal caspases could be activated by ethanol treatment (Fig. 1D). CD8+ T cells contacting non-neuronal cells or not contacting any cell showed no lytic granule release (Fig. 1E). The selective resistance of neurons to apoptosis induction by CD8+ T cell lytic granules might be due to the anti-apoptotic activity attributed to HSV-1 latency-associated transcripts (17).
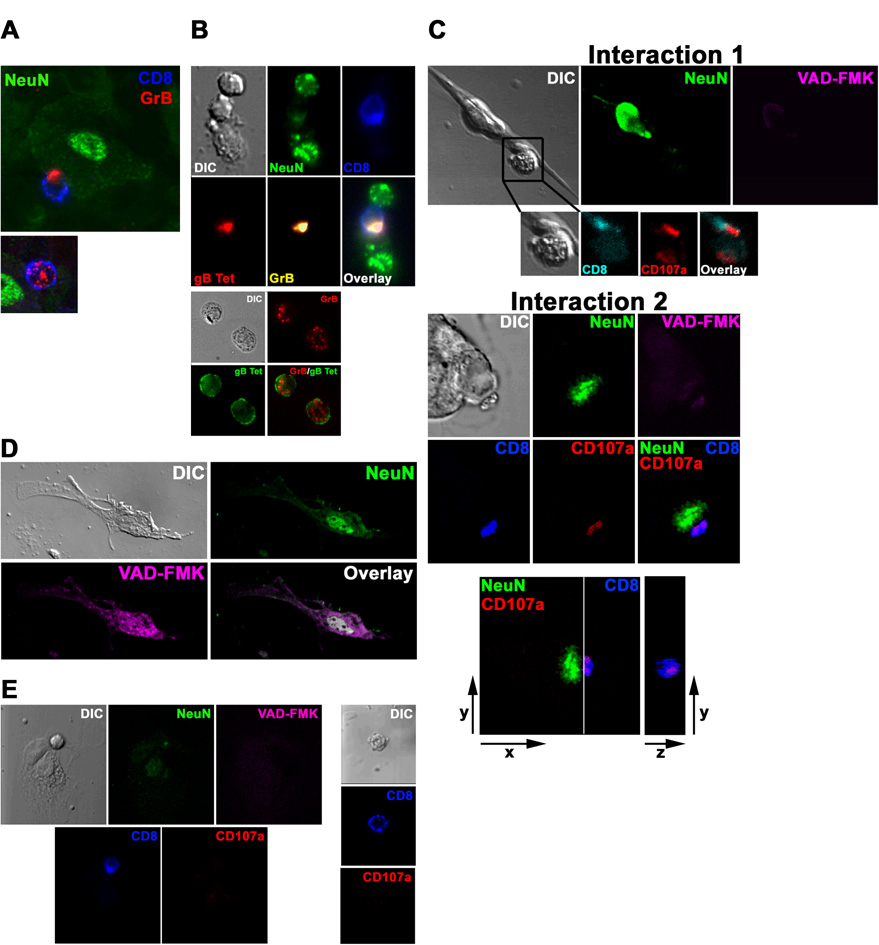
Figure 1. CD8+ T cells release lytic granules toward neurons within HSV-1 latently infected ganglia without activating neuronal caspases.
(A) In situ confocal images of intact latently infected WT TG stained with antibodies to CD8α, GrB, and NeuN (neuronal nucleus). Top: CD8+ T cell with GrB polarized to the junction with a NeuN+ neuron. Bottom: Most CD8+ T cells have GrB dispersed throughout the cell. (B) Imaging of ex vivo cultures of latently infected WT TG. Top: CD8+ T cell in contact with two neurons polarizes TCR and GrB toward lower neuron only. Bottom: CD8+ T cells not contacting targets show no TCR or GrB polarization. (C–E) Imaging of ex vivo latently infected WT TG cultured 24–48 hrs with WT gB-CD8, which prevent reactivation from latency. (C) Two representative interactions between NeuN+ neurons and CD8+ T cells with CD107a polarized toward the junction with a neuron lacking activated caspases (VAD-FMK−). Interaction 2, bottom panel: the plane demarcated by the line between the cells (left) is shown en face (right) demonstrating an apparent secretory domain of an immunological synapse. (D) 10% ethanol induced neuronal caspase activation. (E) CD8+ T cells contacting NeuN− non-infected fibroblast-like cells (left) or not contacting cells (right) lack surface CD107a expression. Data are representative of 11 TG from three separate experiments.
The use of lytic granules in maintaining HSV-1 latency in vivo was assessed by infecting the corneas of WT mice or mice deficient in the lytic granule components perforin (Pfn−/−) or GrB (GrB−/−). All three strains cleared virus from infected corneas and TG with similar kinetics (Fig. S4) and initially retained a similar latent viral load in the TG (Fig. 2A). However, latency was unstable in Pfn−/− and GrB−/− TG as indicated by a significant increase in the number of viral genome copies compared to WT TG at 14 dpi (Fig. 2A). The viral load returned to WT levels in GrB−/− mice by 20 days post-infection (dpi) and in Pfn−/− mice by 34–36 dpi suggesting additional mechanisms, such as INF-γ, in blocking HSV-1 reactivation. HSV-1 reactivation frequency correlates directly with viral load and inversely with CD8+ T cell numbers in ex vivo TG cultures (18). At >30 dpi, these parameters became equivalent in WT, Pfn−/−, and GrB−/− TG (Fig. 2A & S5), yet cultures of Pfn−/− and GrB−/− TG reactivated to a significantly greater extent than WT TG (Fig. 2B) further establishing a role for Pfn and GrB in inhibiting HSV-1 reactivation. To assess the relative roles of lytic granules and IFN-γ in blocking HSV-1 reactivation, dispersed latently infected WT or Pfn−/− TG were cultured in medium alone or medium supplemented with recombinant IFN-γ (rIFN-γ) or neutralizing antibodies against IFN-γ or CD8α (Fig. 2C). The extent of reactivation in Pfn−/− TG was further elevated by neutralization of IFN-γ to levels similar to CD8-neutralized WT TG. Addition of rIFN-γ decreased the level of reactivation in Pfn−/− TG, although it remained higher than WT TG containing endogenous CD8+ T cells. Thus, both lytic granules and IFN-γ are important in immune control of HSV-1 reactivation from latency.
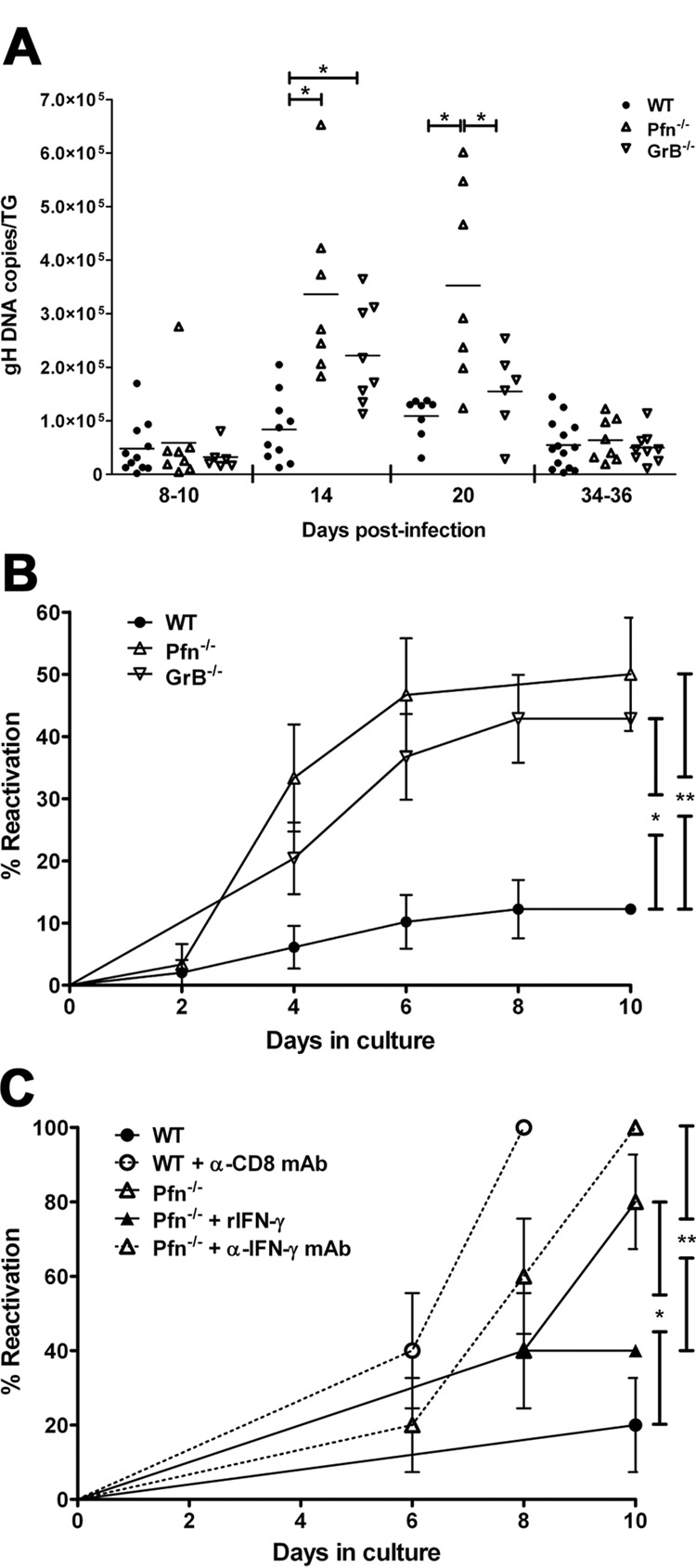
Figure 2. Pfn and GrB are required to maintain HSV-1 neuronal latency in vivo and in ex vivo ganglia cultures.
(A) DNA was extracted from individual TG at designated times and HSV-1 genome copies was determined by quantitative real-time PCR (horizontal bar = mean). Data pooled from at least two independent experiments per time point. * p≤0.05 as calculated by ANOVA with Bonferroni post-test. (B) 34–41 dpi TG were dispersed and cultured at one-fifth TG equivalents per well. HSV-1 reactivation was indicated by the presence of infectious virus in serially sampled supernatants as assessed by plaque assay. Pooled data from three independent experiments presented as mean ± SEM. * p=0.0009; ** p=0.0002 as calculated by survival curve analysis (Log-rank test). (C) 14 dpi TG were dispersed and cultured at 1 TG per well in medium alone or in medium supplemented with 1000 U/ml recombinant IFN-γ (rIFN-γ), 100 μg/ml anti-CD8α monoclonal antibody (α-CD8α mAb), or 20 μg/ml anti-IFN-γ monoclonal antibody (α-IFN-γ mAb). Reactivation was assessed, analyzed, and presented as in (B). n=10 TG/condition. Data representative of two independent experiments. * p=0.0108; ** p=0.0049.
Direct evidence that gB-CD8 use lytic granules to inhibit HSV-1 reactivation came from the observation that 2.5 fold more GrB−/− and ≥ 5 fold more Pfn−/− gB-CD8 were required to block HSV-1 reactivation in cultures of pooled, CD8-depleted WT TG (Table 1). Thus, gB-CD8 use lytic granules to block HSV-1 reactivation, and GrB is an important, but not the only, lytic granule component involved. An additional contribution 6 from GrA is likely because this granzyme inhibits the interneuronal spread of HSV-1 within infected ganglia (19).
Table 1. Pfn−/− & GrB−/− gB-CD8 are less efficient than WT gB-CD8 at inhibiting HSV-1 reactivation in a common pool of latently infected neurons.
HSV-1 reactivation detected by plaque assay of supernatants from dispersed 34–44 dpi WT TG depleted of endogenous CD8+ T cells and cultured at one-fifth TG per well with the indicated number and type of gB-CD8 from culture initiation. +, reactivation detected; −, no reactivation detected; X, condition not tested. Data pooled from four independent experiments (n=15–35 cultures per condition).
| # gB-CD8 per well | Type of gB-CD8 | ||
|---|---|---|---|
| WT | Pfn−/− | GrB−/− | |
| 2×103 | + | × | × |
| 2×104 | − | + | + |
| 4×104 | − | + | + |
| 5×104 | − | + | − |
| 1×105 | − | + | − |
We hypothesized that GrB might inhibit the viral life cycle without killing the host neuron by directly cleaving an essential HSV-1 protein similar to GrH cleavage of the adenovirus DNA-binding protein (20). The GraBCas bioinformatics program (21) identified two distinct GrB cleavage sites in ICP4 (Fig. S6), an HSV-1 IE protein that is essential for efficient viral transcription beyond the α genes (22). Levels of ICP4 were reduced in HSV-1-infected syngenic fibroblasts following exposure to WT but not Pfn−/− or GrB−/− gB-CD8 (Fig. 3A&B). GrB-mediated degradation of ICP4 was directly established by exposing lysates of 293T cells transfected to express ICP4, lysates of fibroblasts infected with HSV-1, or ICP4 immunoprecipitated from HSV-1-infected fibroblasts to recombinant human GrB (Fig. 3C–F). Demonstration of GrB and ICP4 colocalization within infected cells and the exact cellular compartment where cleavage occurs require further investigation.
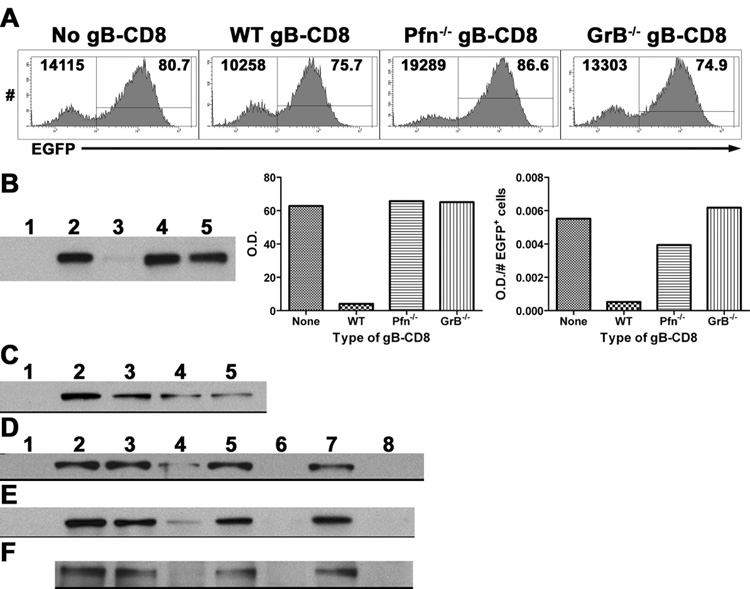
Figure 3. GrB cleaves the essential HSV-1 IE protein ICP4.
(A&B) B6WT3 fibroblasts were infected with recombinant HSV-1 expressing EGFP from the ICP0 promoter for 1 hr, washed, and exposed to no, WT, Pfn−/−, or GrB−/− gB-CD8 for 5 hrs. (A) Histograms from flow analysis of recovered cells show total fibroblasts (CD8−) recovered from cultures (top left) and percent infected (EGFP+; top right). (B) Lysates of recovered cells were subjected to Western blot for ICP4 (Lane 1: Noninfected; Lanes 2–5: Infected with No, WT, Pfn−/−, or GrB−/− gB-CD8, respectively). Bar graphs show optical density (O.D.) readings that were not adjusted (middle) or adjusted for the number of infected fibroblasts recovered from each culture (right). (C) Lysates from 293T cells that were either non-transfected (lane 1) or transfected with an ICP4-expressing plasmid (lanes 2–5) and exposed to different concentrations of recombinant GrB (lanes 1–5: 0, 0, 25, 50, 100 nM GrB, respectively) for 1 hr at 37° C. Lysates from 293T cells transfected with an ICP4-expressing plasmid (D), lysates from B6WT3 cells infected with HSV-1 (E), or ICP4 immunoprecipitated from HSV-1-infected fibroblasts (F) were exposed to 0 (lanes 1–3, 5, 7) or 100 nM GrB (lanes 4, 6, 8) for varying times at 37° C (lanes 1–2: 0 hr; 3–4: 1 hr; 5–6: 2 hrs; 7–8: 3 hrs). Lane 1 contains non-transfected 293T cell lysate (D) or noninfected fibroblast lysate (E).
Understanding the mechanisms that control HSV-1 latency is of paramount importance because HSV-induced pathology is associated with viral shedding following reactivation from latency in sensory ganglia. Viral microRNAs expressed during latency can inhibit production of multiple HSV-1 immediate early proteins, including ICP4 (23). Such mechanisms, in addition to epigenetic modifications of the viral genome (24), probably contribute to the stable latency that appears to exist in the vast majority of latently infected neurons. We propose that at any given time, some neurons escape these control mechanisms and require protection from HSV-specific CD8+ T cells (25). It is these neurons that might represent those most likely to reactivate in vivo.
Our findings resolve the paradox that potentially cytotoxic CD8+ T cells surround apparently healthy HSV-1 latently infected neurons in ganglia of both mice and humans. CD8+ T cell lytic granules can block the HSV-1 life cycle through a non-lytic mechanism, and GrB can directly cleave ICP4, a viral protein required for efficient transcription of early and late viral genes. The fact that gB (a gene product expressed at low levels before viral DNA synthesis) is detectable by gB-CD8 within 2 hours of infection (26) would permit ICP4 degradation very early in the viral life cycle during both lytic infection and reactivation from latency. Blocking reactivation at this early point would prevent replication of viral DNA, consistent with our observation of a dramatic increase in viral genome copy number in TG of GrB−/− or Pfn−/− mice. This mechanism might be particularly efficient during attempted HSV-1 reactivation events where ICP4 expression has escaped repression by viral microRNAs and host neuron epigenetic modifications. Thus, we propose a tripartite relationship in which HSV-1 latency is maintained through the activity of the virus, the host neuron, and the contiguous CD8+ T cells permitting viral persistence with neuronal survival (Fig. S7).
Supplementary Material
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS. Published version available at http://www.sciencemag.org/cgi/content/full/322/5899/268
References and Notes
- 1.Theil D, et al. Am J Pathol. 2003;163:2179. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hufner K, et al. J Neuropathol Exp Neurol. 2006;65:1022. doi: 10.1097/01.jnen.0000235852.92963.bf. [DOI] [PubMed] [Google Scholar]
- 3.Verjans GM, et al. Proc Natl Acad Sci U S A. 2007;104:3496. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derfuss T, et al. Brain Pathol. 2007;17:389. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons A, Tscharke DC. J Exp Med. 1992;175:1337. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantin EM, Hinton DR, Chen J, Openshaw H. J Virol. 1995;69:4898. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimeld C, et al. J Neuroimmunol. 1995;61:7. doi: 10.1016/0165-5728(95)00068-d. [DOI] [PubMed] [Google Scholar]
- 8.Liu T, Tang Q, Hendricks RL. J Virol. 1996;70:264. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Immunity. 2003;18:593. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu T, et al. J Exp Med. 2000;191:1459. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. J Immunol. 2007;179:322. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croen KD, et al. N Engl J Med. 1987;317:1427. doi: 10.1056/NEJM198712033172302. [DOI] [PubMed] [Google Scholar]
- 13.Stroop WG, Schaefer DC. Acta Neuropathol (Berl) 1987;74:124. doi: 10.1007/BF00692842. [DOI] [PubMed] [Google Scholar]
- 14.Liu T, Khanna KM, Carriere BN, Hendricks RL. J Virol. 2001;75:11178. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decman V, Kinchington PR, Harvey SA, Hendricks RL. J Virol. 2005;79:10339. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telford WG, Komoriya A, Packard BZ. Cytometry. 2002;47:81. doi: 10.1002/cyto.10052. [DOI] [PubMed] [Google Scholar]
- 17.Perng GC, et al. Science. 2000;287:1500. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino Y, Pesnicak L, Cohen JI, Straus SE. J Virol. 2007;81:8157. doi: 10.1128/JVI.00474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pereira RA, Simon MM, Simmons A. J Virol. 2000;74:1029. doi: 10.1128/jvi.74.2.1029-1032.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade F, et al. EMBO J. 2007;26:2148. doi: 10.1038/sj.emboj.7601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Backes C, et al. Nucleic Acids Res. 2005;33:W208. doi: 10.1093/nar/gki433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeLuca NA, McCarthy AM, Schaffer PA. J Virol. 1985;56:558. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umbach JL, et al. Nature. 2008;454:780. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knipe DM, Cliffe A. Nat Rev Microbiol. 2008;6:211. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 25.Sheridan BS, Knickelbein JE, Hendricks RL. Expert Opin Biol Ther. 2007;7:1323. doi: 10.1517/14712598.7.9.1323. [DOI] [PubMed] [Google Scholar]
- 26.Mueller SN, et al. J Virol. 2003;77:2445. doi: 10.1128/JVI.77.4.2445-2451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Materials and methods are available as supporting material on Science Online
- 28.We thank K. Lathrop and J. Karlsson for assistance with microscopy and preparation of figures and N. Zurowski for assistance with flow cytometry. The authors have no conflicting financial interests. This work was supported by NIH grants F30NS061471 (JEK), R01EY05945 (RLH), R01EY015291 (PRK), and P30EY08098 (RLH); a Research to Prevent Blindness Medical Student Eye Research Fellowship (JEK); and unrestricted grants from Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh (RLH).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.