Abstract
Objectives
We describe treatment failure rates by antibiotic duration for prosthetic joint infection (PJI) managed with debridement, antibiotics and implant retention (DAIR).
Methods
We retrospectively collected data from all the cases of PJI that were managed with DAIR over a 5 year period. Surgical debridement, microbiological sampling, early intravenous antibiotics and prolonged oral follow-on antibiotics were used.
Results
One hundred and twelve cases of PJI were identified. Twenty infections (18%) recurred during a mean follow-up of 2.3 years. The mean duration of antibiotic use was 1.5 years. Failure was more common after arthroscopic debridement, for previously revised joints and for Staphylococcus aureus infection. There were 12 failures after stopping antibiotics and 8 while on antibiotics [hazard ratio (HR) = 4.3, 95% confidence interval (CI) 1.4–12.8, P = 0.01]. However, during the first 3 months of follow-up, there were eight failures after stopping antibiotics and two while on antibiotics (HR = 7.0, 95% CI 1.5–33, P = 0.015). The duration of antibiotic therapy prior to stopping did not predict outcome.
Conclusions
PJI may be managed by DAIR. The risk of failure with this strategy rises after stopping oral antibiotics, but lengthening antibiotic therapy may simply postpone, rather than prevent, failure.
Keywords: prosthetic, infection, management
Introduction
Arthroplasty is one of the most cost-effective healthcare interventions described,1–3 but prosthetic joint infection (PJI) presents a major challenge to patients, physicians and funding agencies. PJI complicates up to 2.5%4 of the estimated 600 000 primary arthroplasties5 performed annually in the USA. Established PJI usually proves intractable to antibiotics alone, which led to the development of one- or two-stage surgical exchange revision protocols to achieve satisfactory outcomes.6 Two-stage exchange, requiring two surgical procedures as a minimum, may present the patient and surgeon with significant attendant surgical and peri-operative risk including a substantial period of reduced mobility. Removal of a soundly fixed prosthesis with any bone cement may also result in degradation of the bone stock and peri-operative fracture. This makes the approach of debridement, antibiotics and implant retention (DAIR) attractive, especially in the elderly or infirm.7 There is evidence, in animal8,9 and human10–16 studies, that combinations of a fluoroquinolone and rifampicin are effective treatments in device-related staphylococcal infections.
Variable success rates (14%–100%) are reported for DAIR10–36 with a range of factors identified as conferring a poor prognosis including delay to debridement, age of implant, presence of a sinus, implant loosening, arthroscopy and Staphylococcus aureus. Other factors, including the choice and duration of both intravenous (iv) and oral antibiotics, are less well defined. In this study, we report the outcome in 112 patients managed with DAIR. We analysed these data to describe the impact of the length of oral antibiotic use on failure rates.
Methods
This was a retrospective series of all cases of PJI managed with our DAIR protocol in the Bone Infection Unit of the Nuffield Orthopaedic Centre, Oxford, UK, between 1 January 1998 and 30 April 2003. Cases were managed by infectious disease physicians and orthopaedic surgeons in a multi-disciplinary team. We established a registry and included all cases of PJI using multiple data sources: histopathology and outpatient parenteral antimicrobial therapy (OPAT) databases; hospital activity coding databases; systematically hand-searched diagnostic details listed in outpatient clinic letters; and prospective capture of patients attending follow-up outpatient appointments or readmitted to our unit. The use of multiple data sources appeared to result in the capture of all cases during this period, since all cases were represented in at least two data sources.
Following a review of the case notes of all patients in the registry, we excluded patients whose primary management of infection was revision surgery or suppressive antibiotic therapy without debridement, or whose only debridement and retention procedure was carried out at a referring hospital.
A single dedicated researcher (S. M.) extracted data from case notes of included patients for entry into a Microsoft Access database. The senior investigators (I. B., B. L. A. and A. B.) each validated data from a different random set of these case notes (totalling 10% of the case notes) by independent extraction of the dataset and reconciliation of the two extractions. Differences were resolved by group discussion (S. M., A. B., B. L. A. and I. B.) and all affirmed the accuracy of the data extracted by the primary data abstractor (S. M.). Differences in data were resolved and the data locked before any preliminary analysis took place, but researchers were not blinded to outcomes.
Case definition
Cases were defined as those having a clinical syndrome of arthroplasty infection triggering the DAIR protocol (any of persistent wound inflammation, wound discharge or intra-operative purulence in the context of a soundly fixed implant) and at least one culture-positive deep peri-prosthetic sample and/or histology of peri-prosthetic tissues indicative of infection. Patients with clinical or radiographic evidence of joint loosening were not managed with DAIR; for them, staged revision was the standard of care. Patients were not excluded based on their previous history of joint infection or on the duration of their symptoms. Multiple samples were taken for microbiological culture as described previously.37 The histological diagnosis was made on the basis of the degree of infiltration by neutrophil polymorphonuclear leucocytes as outlined in previous studies.38,39
Definition of treatment failure
We defined treatment failure as: (i) infection recurrence with positive cultures from peri-prosthetic samples or an aspirate; (ii) wound or sinus drainage recurring or persisting for 3 months beyond the index debridement procedure; or (iii) a requirement for revision surgery (irrespective of the indication). Repeated debridements were not considered treatment failure, and the monitoring period for treatment failure began after the last debridement of the acute episode.
Data collection
We collected data from the patient notes on patient demographics, co-morbidities (diabetes, renal failure, immunosuppression, rheumatoid arthritis, malignancy and smoking), the dates of primary surgery, symptom onset, presentation and debridement; clinical features at presentation and surgical findings; nature of implant; number of debridements; microbiology; antibiotic choice, duration and side effects; and outcomes. We categorized infections by organism, and by age of implant rather than exogenous versus haematogenous, since these first two variables were directly observed and known in all cases, and less open to interpretation. The data were then anonymized. There was no research-related contact with patients, and informed consent was not required. All activity was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Clinical management
During the period of retrospective study, protocols guiding operative sampling, definitions of infection and antibiotic use were used by the multi-disciplinary clinical teams involved in patient care, and overseen by the infection physician.
Antibiotic management
Empirical antibiotics were avoided prior to DAIR. If antibiotics had been initiated elsewhere, surgery was delayed, if clinically safe, to achieve a minimum 48 h antibiotic-free interval prior to microbiological sampling. Following intra-operative sampling, whilst debridement continued, patients received empirical iv vancomycin 1 g (continued as 1 g initially 12 hourly) plus meropenem 500 mg (continued as 500 mg 8 hourly).40 Therapy was rationalized once definitive culture results were obtained from the laboratory. Meropenem was discontinued at 48 h if no aerobic Gram-negative pathogens requiring treatment with a carbapenem had been cultured.
Intravenous antibiotic therapy was continued for 6 weeks with a β-lactam or glycopeptide, since organisms may more readily acquire resistance to our preferred oral regimens (rifampicin and quinolones). The risk of this is greatest at the start of therapy when there is a high bioburden. If patients were suitable for OPAT, the preferred treatment was with ceftriaxone (1 g daily) for most susceptible pathogens or with teicoplanin (400 mg daily following loading, adjusted by levels) for coagulase-negative staphylococci and methicillin-resistant Gram-positive organisms. Patients with negative cultures were treated with glycopeptides.41 We treated Gram-negative organisms that were resistant to cephalosporins or associated with inducible resistance to cephalosporins with a carbapenem. Patients with side effects were switched to alternative agents where possible. For staphylococci, we preferred oral follow-on with a fluoroquinolone/rifampicin combination (typically ciprofloxacin 500 mg twice daily plus rifampicin 300 mg twice daily). If the organism was resistant, or the drugs poorly tolerated, we used combinations of doxycycline, fusidic acid, rifampicin, clindamycin or amoxicillin for oral follow-on therapy. If the susceptibility profiles permitted, we used single oral agents for streptococci and Gram-negative organisms. For polymicrobial infections, antibiotic regimens with activity against all cultured pathogens were used. The duration of oral therapy was specified to patients and primary care physicians as a minimum of 12 months. However, because of the uncertainty on optimal duration, treatment decisions were made case by case, considering drug side effects, the potential risks of revision surgery in the event of failure, quality of life and patient preference. This resulted in a range of durations used in our clinical practice.
Surgical management
The surgical strategy for DAIR commenced with excision of the wound margins followed by removal of necrotic soft tissue, debris, haematoma or collections of pus from around the prosthesis. Intra-operative samples were taken at arthrotomy from multiple samples of tissue, haematoma and pus from the vicinity of the prosthesis for culture and histology. Each sample was obtained with separate instruments and placed into separate containers for processing as described previously.37 The prosthesis was assessed for integrity of the cement–bone or prosthesis–bone interfaces and if firmly fixed and mechanically sound it was retained. Any modular prosthesis components were exchanged when an open debridement was performed, and any loose bone graft was removed. The exposed tissue surfaces were irrigated with aqueous chlorhexidine using pulsed lavage. Wounds were always closed primarily over drains, which were removed at 48 h or when drainage ceased. For knee and ankle replacements, arthroscopic washout was sometimes used with sampling of fluid and tissue carried out via multiple portals followed by high-flow irrigation.
Analysis
We used STATA version 10 (Stata Corp., College Station, TX, USA) to run Cox survival analyses. Data were censored from follow-up after recurrence of infection or when lost to follow-up. The endpoint for treatment failure was as defined above. Univariate analysis was conducted on factors relevant to presentation or initial treatment and, subsequently, multivariate analysis was conducted on factors that were significant or of borderline significance (P < 0.1) on univariate analysis.
We compared the risk of treatment failure on oral antibiotics with the risk off antibiotics. To do this, we analysed the failure rates of subjects taking oral antibiotics, on stopping antibiotics and (as a control for the effect of time elapsed) at day 500 after debridement without reference to antibiotic therapy. We used the robust cluster estimator to adjust P values and confidence intervals (CIs) to account for this.42 These analyses were adjusted for the factors found to predict failure significantly on multivariate analysis. In these analyses, we included the first 250 days only, so as to ensure comparable levels of follow-up in patients stopping antibiotics. A test of proportional hazards using Schoenfeld's residuals was used to examine for a significant change in hazard ratios (HRs) over time.43
Results
Characteristics of the cohort
One hundred and twelve cases of PJI (52 hips, 51 knees and 9 other total joint replacements) were identified. Nearly half of the patients treated with the DAIR protocol were 70 years of age or older (Table 1). There was a high incidence of co-morbidity, with more than half the patients having at least one co-morbid condition.
Table 1.
HRs from survival analysis (Cox regression) for the association of individual factors with failure
| Variable/category | n | Number failing | Hazard ratio | 95% CI | P |
|---|---|---|---|---|---|
| Gender | |||||
| female | 50 | 10 | 1 | ||
| male | 62 | 10 | 0.7 | 0.30–1.73 | 0.47 |
| Age (years) | |||||
| <40 | 2 | 0 | — | ||
| 40–49 | 2 | 1 | — | ||
| 50–59 | 22 | 4 | 1 | ||
| 60–69 | 34 | 5 | 0.8 | 0.21–3 | 0.75 |
| 70–79 | 35 | 7 | 1.2 | 0.35–4.1 | 0.86 |
| 80–89 | 17 | 3 | 1.1 | 0.26–5.13 | 0.92 |
| Co-morbidity | |||||
| none | 45 | 4 | 1 | ||
| one or more | 66 | 15 | 2.7 | 0.91–8.3 | 0.07 |
| unknown | 1 | 1 | |||
| Time from implant to debridement (days) | |||||
| <90 | 77 | 9 | 1 | ||
| ≥90 | 35 | 11 | 3.0 | 1.2–7.2 | 0.016 |
| Time from presentation to debridement (days) | |||||
| <3 | 71 | 9 | 1 | ||
| 3–14 | 25 | 6 | 0.77 | 0.24–2.5 | 0.67 |
| >14 | 16 | 5 | 0.36 | 0.12–1.0 | 0.07 |
| Arthroplasty | |||||
| primary implant | 86 | 11 | 1 | ||
| revised implant | 26 | 9 | 2.6 | 1.1–6.3 | 0.031 |
| Surgical debridement | |||||
| open debridement | 97 | 12 | 1 | ||
| arthroscopy | 15 | 8 | 5.4 | 2.2–13 | <0.0005 |
| Joint | |||||
| hip | 52 | 7 | 1 | ||
| knee | 51 | 13 | 0.47 | 0.19–1.18 | 0.10 |
| ankle | 3 | 0 | — | ||
| shoulder | 2 | 0 | — | ||
| elbow | 4 | 0 | — | ||
| Surgical findings | |||||
| no pus at surgery | 54 | 8 | 1 | ||
| pus at surgery | 48 | 1 | 1.5 | 0.59–3.65 | 0.41 |
| unknown | 10 | 11 | — | ||
| Number of debridements | |||||
| single procedure | 88 | 14 | 1 | ||
| multiple procedures | 24 | 6 | 1.8 | 0.68–4.7 | 0.24 |
| Microbiologya | |||||
| MRSA | 9 | 3 | 2.0 | 0.59–7 | 0.26 |
| MSSA | 39 | 10 | 1.8 | 0.71–4.4 | 0.21 |
| CoNS | 26 | 5 | 1.0 | 0.36–2.8 | 0.99 |
| S. aureus | 47 | 13 | 2.6 | 0.97–6.9 | 0.052 |
| Intravenous antibiotics | |||||
| <28 days | 26 | 8 | 1 | ||
| ≥28 days | 86 | 12 | 0.41 | 0.16–0.99 | 0.050 |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; CoNS, coagulase-negative staphylococci.
aMultiple organisms can be isolated from one patient, hence the sum of n is >112.
Sixty-nine percent had arthroplasties that were <90 days old, and 63% were treated surgically within 3 days of presentation. S. aureus was frequently isolated from surgical specimens (42%), but methicillin-resistant S. aureus (MRSA) was rare (8%).
Eighty-seven percent of the patients were treated with an open debridement and 13% with an arthroscopic washout. Some patients required multiple procedures (21%).
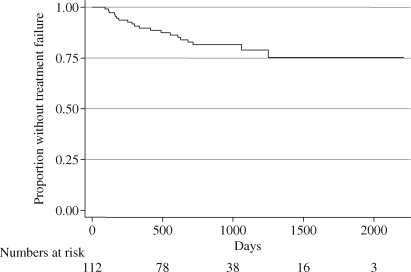
We observed 20 treatment failures (18%) during a mean follow-up of 2.3 years (Figure 1); 89%, 81% and 78% of joints had not failed at 1, 2 and 3 years, respectively.
Figure 1.
Kaplan–Meier plot of time to treatment failure for all patients, showing all follow-up data available.
The mean subsequent duration of oral antibiotic use was 1.5 years. Eighty-four percent received rifampicin, 59% ciprofloxacin and 59% received both. Tetracyclines (20%), β-lactams (13%), fusidic acid (6%) and clindamycin (4%) were also used. Among patients with staphylococcal disease, 94% received rifampicin, 70% ciprofloxacin and 70% both. Twenty-three percent of the patients received <4 weeks of iv antibiotics. For 91% of these shortened courses, a side effect was responsible. These were a rash (40%), diarrhoea (26%), nausea (10%), hepatitis (10%) and presumed drug fever (10%).
S. aureus, previous revision and arthroscopic washouts predicted treatment failure
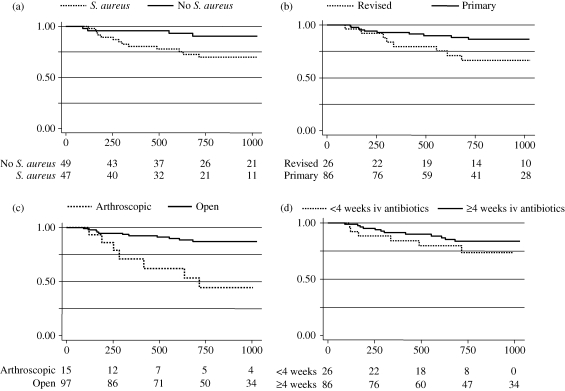
We used univariate analysis (Table 1) to select factors for a multivariate model (Table 2). Both MRSA and methicillin-susceptible S. aureus infection were individually associated with non-significant increases in the risk of treatment failure, but the combined group with S. aureus infection had an increased risk of treatment failure of borderline significance, which was included in the multivariate model (HR = 2.9, 95% CI 1.0–8.4, P = 0.05; Figure 2a). Only three patients (3%) were culture-negative. Previously revised joints were associated with a significant risk of treatment failure (HR = 3.1, 95% CI = 1.2–8.3, P = 0.008; Figure 2b), as were joints treated with arthroscopic washouts (HR = 4.2, 95% CI 1.5–12.5, P = 0.008; Figure 2c).
Table 2.
Multiple Cox regression model of significant factors from univariate analysis
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Implant to debridement ≥90 days | 1.1 | 0.31–3.8 | 0.89 |
| Intravenous antibiotics ≥28 days | 0.49 | 0.18–1.37 | 0.18 |
| Arthroscopy versus open | 4.2 | 1.5–12.5 | 0.008 |
| S. aureus | 2.9 | 1.0–8.4 | 0.050 |
| Revised versus primary arthroplasty | 3.1 | 1.2–8.3 | 0.008 |
| Presence of co-morbidity | 1.81 | 0.55–5.9 | 0.32 |
Goodness of fit: log likelihood = −59.6, χ2 = 17.2, P = 0.0006.
Figure 2.
Kaplan–Meier plots for time to failure are shown for (a) the presence or absence of S. aureus infection, (b) primary versus previously revised implant, (c) arthroscopic versus open debridement and (d) length of intravenous (iv) antibiotic use. The x-axes show days since DAIR and the y-axes show the proportion without treatment failure. The numbers at risk at each timepoint are shown beneath each plot.
Joint replacements presenting at 90 days or longer post-implantation were associated with treatment failure on univariate analysis, but not on multivariate analysis (HR = 1.1, 95% CI 0.31–3.8, P = 0.89). Arthroscopic washouts were more often performed in arthroplasties that were ≥90 days old, and once arthroscopic washout was included as a factor, age of implant did not influence outcome. The effect of an arthroscopic washout was significant even when analysis was restricted to joint replacements <90 days old (HR = 18, 95% CI 1.7–200, P = 0.018). We used 90 days to classify on the basis of time to presentation based on previous literature,36 but similar results were obtained using the alternative 30 day cut-off.44 Hip infection appeared to be associated with a reduced risk of recurrent infection on univariate analysis, but this effect was no longer apparent after adjusting for the effect of arthroscopic washouts, which are only done for knees (adjusted HR = 0.9, 95% CI 0.29–2.8, P = 0.8).
An iv antibiotic course lasting ≥28 days was associated with halving the risk of treatment failure, which was of borderline statistical significance in a univariate model (Figure 2d), but was not significant in the multivariate model. An iv antibiotic course longer than 42 days was associated with a non-significantly worse outcome (HR = 1.3, 95% CI 0.5–3.3), but is strongly confounded since a duration longer than specified by the protocol was only used where oral therapy was not possible, or where repeated debridements were undertaken.
There was a trend towards better outcomes in patients with a longer time between presentation and debridement (Table 1) on univariate analysis. However, the significance was marginal and we had no data on the reasons for delay to debridement, and so did not include this variable in further models because of the potential for bias (i.e. that a delay was more likely with less severe disease).
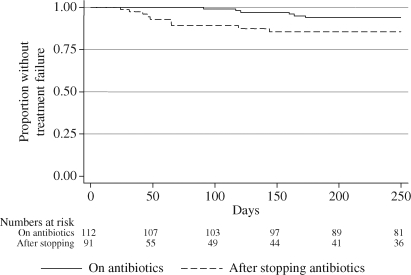
The risk of treatment failure rises after stopping antibiotics
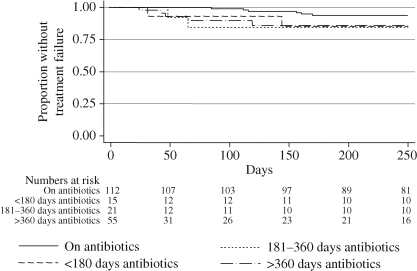
We examined two rates of failure; those seen in all patients treated with the DAIR protocol, and those seen after oral antibiotics were stopped (resetting the day of stopping oral antibiotics to day 0 to plot a new survival curve). The risk of treatment failure increased more than 4-fold after stopping oral antibiotics (Figure 3). The majority of the recorded failures were during the first 4 months after stopping antibiotics. A test of Schoenfeld's residuals for changing proportional hazards43 was positive (P = 0.015), indicating a significant change in the relative risk of failure over time. The 4-fold rise in hazard on stopping antibiotics therefore represents an early period of high risk and a later period of lower risk. An HR estimated over only the first 3 months was 7.0 (95% CI 1.5–33, P = 0.015) and the HR over the next 3 months was 1.4 (95% CI 0.1–19, P = 0.79). There was no trend of lower failure rates with longer antibiotic use (Figure 4).
Figure 3.
Kaplan–Meier plot of time to treatment failure for patients on oral antibiotics (HR = 1) and patients stopping oral antibiotics (where day of stopping is day 0, HR = 4.3, 95% CI 1.4–12.8, P = 0.01).
Figure 4.
Kaplan–Meier plot of time to treatment failure for patients at the start of the DAIR protocol and patients stopping oral antibiotics (where day of stopping is day 0), divided according to the length of use of oral antibiotics prior to stopping: <180 days oral suppression, HR = 3.7 (95% CI 0.7–18), P = 0.11; 181–360 days oral suppression, HR = 9.1 (95% CI 0.9–90), P = 0.058; and >360 days oral suppression, HR = 5.1 (95% CI 1.4–19), P = 0.013.
Length of follow-up does not explain the increased risk of failure on stopping antibiotics
We reasoned that the increased risk of failure on stopping antibiotics might be due to the greater time that had elapsed since DAIR was undertaken. To explore this possibility, we examined the rate of failure in all patients who were still under follow-up after 500 days (resetting day 500 as day 0). The risk of failure in the cohort still under follow-up from day 500 was similar to the risk from day 0 (HR = 1.4, 95% CI 0.5–3.9, P = 0.47), suggesting that the increased rates of failure seen on stopping antibiotics were not confounded by the greater time that had elapsed since DAIR.
Discussion
This is the largest study to date that describes the antibiotic and surgical treatments of infected arthroplasties with DAIR.18 We achieved successful salvage of the infected arthroplasty at 1, 2 and 3 years of 89%, 81% and 78%, respectively. It is difficult to compare these outcomes with those obtained elsewhere without data on the subgroups of patients selected for DAIR rather than other treatment strategies.
However, we do clearly show that the rate of failure increased 4-fold after stopping antibiotics. Despite the increased risk of the recurrence of infection, this only affected a minority of patients stopping antibiotics. This suggests that the combination of debridement and prolonged antibiotics results in a cure of infection for many patients, but a suppression of infection in a subgroup who then relapse once antibiotics are stopped. The length of antibiotic prescribing before stopping antibiotics did not appear to alter the outcome, and most failures occurred in the first 4 months after stopping antibiotics (despite a median follow-up time of a year after stopping). The resulting change in the HR over time was significant (P = 0.015). This predicts that the survival plots in Figure 3 will eventually meet, but there was insufficient data to test this. One might conclude that most patients cured of PJI by DAIR are cured early on, and that prolonged antibiotic therapy does not prevent treatment failures in those who are not cured, but merely postpones them. However, there were few patients receiving <6 months of antibiotic treatment, so these data cannot be used to support shortening oral antibiotic courses below 6 months. Life-long antibiotics might simply postpone, rather than prevent, treatment failure, but this may be all that is required for older patients with limited life expectancy. For patients in whom further surgery might be limb- or life-threatening, postponing this outcome with indefinite antibiotic treatment is also justified.
Although we adjusted for a number of factors besides antibiotic use that might influence outcome, we cannot exclude confounding from unmeasured factors including radiographic status, functional status and inflammatory markers. It is possible that patients with a poorer prognosis used longer courses of antibiotics, and failure rates might have increased had the course been reduced in length. However, such a bias would be expected to result in patients with a better prognosis stopping antibiotics, and hence lower apparent failure rates after stopping. In fact, we observed the opposite (i.e. an increased failure rate after stopping).
We found that outcomes were worse when attempting to salvage a revision implant, when an arthroscopic washout was used or when S. aureus infection was identified. The poorer bone stock, abnormal soft tissue and meshes or bone grafts associated with revision implants may lead to poorer outcomes. Arthroscopy results in a less thorough debridement and precludes exchanging modular components. However, these results must be interpreted with caution given our retrospective observational study design. We do not know why arthroscopy was selected for a small subset of patients, and the result might be confounded, for instance by co-morbidity leading to both poorer outcomes and a decision to undertake a less invasive procedure. However, there was no evidence that affirmed co-morbidity was associated with either revision implants (P = 0.87) or arthroscopy (P = 0.67). The published literature gives conflicting results regarding the efficacy of arthroscopic washout in earlier, smaller studies,15,31,32,35 and S. aureus infection has previously been identified as an adverse factor, despite various antibiotic regimens.25,26
Our data were equivocal regarding the effect of the age of the implant (which would have indicated immediate revision in some protocols). Although in univariate analysis, older implants appeared more likely to fail following DAIR, this was not seen in multivariate analysis. Older implants were more likely to be treated with arthroscopic washout (for technical reasons, this is less often performed after recent implantation). Arthroscopic washout was the stronger factor in multivariate analysis and was still significant after restricting analysis to recent implants. Previous studies have shown a worse outcome for older implants15,19 and where debridement is delayed.14,24 However, these differences may simply reflect differences in patient selection and local practice, since in all these studies (ours included), there is limited data on the factors influencing patient selection.
In summary, we present the largest study to date on outcomes after DAIR for infected joint prostheses. These data suggest that the length of duration of antibiotic prescribing beyond 6 months is not critical to the outcome, but that patients should be warned of an increase in the risk of treatment failure, particularly during the first 3 or 4 months after stopping antibiotics. These conclusions should now be tested in prospective randomized controlled trials of prolonged versus shortened antibiotic treatment.
Funding
Specific funding was not used for this study. P. B. is funded by the Biomedical Research Centre, Oxford Radcliffe Hospitals Trust.
Transparency declarations
I. B. has received honoraria for serving on advisory boards (Pfizer) and lecture fees (Pfizer and Nordic Pharma). A. B. has received honoraria for serving on advisory boards (Pfizer and Macrochem), for speakers bureaux (Merck) and for producing sponsored non-promotional educational materials (Merck). P. M.-S. is an advisor to Wright Medical Technologies and receives royalties from the Corin Group. P. B., B. L. A., B. A., S. M. and R. G. have no conflicts of interest.
References
- 1.Lavernia CJ, Guzman JF, Gachupin-Garcia A. Cost effectiveness and quality of life in knee arthroplasty. Clin Orthop Relat Res. 1997;345:134–9. [PubMed] [Google Scholar]
- 2.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–65. [PubMed] [Google Scholar]
- 3.Ethgen O, Bruyere O, Richy F, et al. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86-A:963–74. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Phillips CB, Barrett JA, Losina E, et al. Incidence rates of dislocation, pulmonary embolism, and deep infection during the first six months after elective total hip replacement. J Bone Joint Surg Am. 2003;85-A:20–6. doi: 10.2106/00004623-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 6.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–31. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Fisman DN, Reilly DT, Karchmer AW, et al. Clinical effectiveness and cost-effectiveness of 2 management strategies for infected total hip arthroplasty in the elderly. Clin Infect Dis. 2001;32:419–30. doi: 10.1086/318502. [DOI] [PubMed] [Google Scholar]
- 8.Tshefu K, Zimmerli W, Waldvogel FA. Short-term administration of rifampin in the prevention or eradication of infection due to foreign bodies. Rev Infect Dis. 1983;5(Suppl 3):S474–80. doi: 10.1093/clinids/5.supplement_3.s474. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerli W, Frei R, Widmer AF, et al. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J Antimicrob Chemother. 1994;33:959–67. doi: 10.1093/jac/33.5.959. [DOI] [PubMed] [Google Scholar]
- 10.Barberan J, Aguilar L, Carroquino G, et al. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am J Med. 2006;119:993.e7–10. doi: 10.1016/j.amjmed.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 11.Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampicin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–41. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]
- 12.Soriano A, Garcia S, Bori G, et al. Treatment of acute post-surgical infection of joint arthroplasty. Clin Microbiol Infect. 2006;12:930–3. doi: 10.1111/j.1469-0691.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- 13.Drancourt M, Stein A, Argenson JN, et al. Oral rifampicin plus ofloxacin for treatment of Staphylococcus-infected orthopedic implants. Antimicrob Agents Chemother. 1993;37:1214–8. doi: 10.1128/aac.37.6.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tattevin P, Cremieux AC, Pottier P, et al. Prosthetic joint infection: when can prosthesis salvage be considered? Clin Infect Dis. 1999;29:292–5. doi: 10.1086/520202. [DOI] [PubMed] [Google Scholar]
- 15.Laffer RR, Graber P, Ochsner PE, et al. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12:433–9. doi: 10.1111/j.1469-0691.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 16.Berdal JE, Skramm I, Mowinckel P, et al. Use of rifampicin and ciprofloxacin combination therapy after surgical debridement in the treatment of early manifestation prosthetic joint infections. Clin Microbiol Infect. 2005;11:843–5. doi: 10.1111/j.1469-0691.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 17.Trampuz A, Cattelan C, Fluckiger U, et al. Abstracts of the Forty-fifth Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 2005. Washington, DC, USA: American Society for Microbiology; Treatment outcome of prosthetic joint infection: ten year cohort study (1992–2003) p. 322. Abstract K-883. [Google Scholar]
- 18.Marculescu CE, Berbari EF, Hanssen AD, et al. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–8. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 19.Berbari EF, Osmon DR, Duffy MC, et al. Outcome of prosthetic joint infection in patients with rheumatoid arthritis: the impact of medical and surgical therapy in 200 episodes. Clin Infect Dis. 2006;42:216–23. doi: 10.1086/498507. [DOI] [PubMed] [Google Scholar]
- 20.Crockarell JR, Hanssen AD, Osmon DR, et al. Treatment of infection with debridement and retention of the components following hip arthroplasty. J Bone Joint Surg Am. 1998;80:1306–13. doi: 10.2106/00004623-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Chiu FY, Chen CM. Surgical debridement and parenteral antibiotics in infected revision total knee arthroplasty. Clin Orthop Relat Res. 2007;461:130–5. doi: 10.1097/BLO.0b013e318063e7f3. [DOI] [PubMed] [Google Scholar]
- 22.Burger RR, Basch T, Hopson CN. Implant salvage in infected total knee arthroplasty. Clin Orthop Relat Res. 1991;273:105–12. [PubMed] [Google Scholar]
- 23.Rao N, Crossett LS, Sinha RK, et al. Long-term suppression of infection in total joint arthroplasty. Clin Orthop Relat Res. 2003;414:55–60. doi: 10.1097/01.blo.0000087321.60612.cf. [DOI] [PubMed] [Google Scholar]
- 24.Brandt CM, Sistrunk WW, Duffy MC, et al. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24:914–9. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 25.Deirmengian C, Greenbaum J, Lotke PA, et al. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty. 2003;18:22–6. doi: 10.1016/s0883-5403(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 26.Schoifet SD, Morrey BF. Treatment of infection after total knee arthroplasty by debridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–90. [PubMed] [Google Scholar]
- 27.Mont MA, Waldman B, Banerjee C, et al. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–33. doi: 10.1016/s0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 28.Teeny SM, Dorr L, Murata G, et al. Treatment of infected total knee arthroplasty. Irrigation and debridement versus two-stage reimplantation. J Arthroplasty. 1990;5:35–9. doi: 10.1016/s0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 29.Aboltins CA, Page MA, Buising KL, et al. Treatment of staphylococcal prosthetic joint infections with debridement, prosthesis retention and oral rifampicin and fusidic acid. Clin Microbiol Infect. 2007;13:586–91. doi: 10.1111/j.1469-0691.2007.01691.x. [DOI] [PubMed] [Google Scholar]
- 30.Segreti J, Nelson JA, Trenholme GM. Prolonged suppressive antibiotic therapy for infected orthopedic prostheses. Clin Infect Dis. 1998;27:711–3. doi: 10.1086/514951. [DOI] [PubMed] [Google Scholar]
- 31.Waldman BJ, Hostin E, Mont MA, et al. Infected total knee arthroplasty treated by arthroscopic irrigation and debridement. J Arthroplasty. 2000;15:430–6. doi: 10.1054/arth.2000.4637. [DOI] [PubMed] [Google Scholar]
- 32.Dixon P, Parish EN, Cross MJ. Arthroscopic debridement in the treatment of the infected total knee replacement. J Bone Joint Surg Br. 2004;86:39–42. [PubMed] [Google Scholar]
- 33.Choong PF, Dowsey MM, Carr D, et al. Risk factors associated with acute hip prosthetic joint infections and outcome of treatment with a rifampin-based regimen. Acta Orthop. 2007;78:755–65. doi: 10.1080/17453670710014527. [DOI] [PubMed] [Google Scholar]
- 34.Widmer AF, Gaechter A, Ochsner PE, et al. Antimicrobial treatment of orthopedic implant-related infections with rifampicin combinations. Clin Infect Dis. 1992;14:1251–3. doi: 10.1093/clinids/14.6.1251. [DOI] [PubMed] [Google Scholar]
- 35.Ilahi OA, Al-Habbal GA, Bocell JR, et al. Arthroscopic debridement of acute periprosthetic septic arthritis of the knee. Arthroscopy. 2005;21:303–6. doi: 10.1016/j.arthro.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Giulieri SG, Graber P, Ochsner PE, et al. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection. 2004;32:222–8. doi: 10.1007/s15010-004-4020-1. [DOI] [PubMed] [Google Scholar]
- 37.Atkins BL, Athanasou N, Deeks JJ, et al. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. The OSIRIS Collaborative Study Group. J Clin Microbiol. 1998;36:2932–9. doi: 10.1128/jcm.36.10.2932-2939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirra JM, Amstutz HC, Matos M, et al. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976;117:221–40. [PubMed] [Google Scholar]
- 39.Feldman DS, Lonner JH, Desai P, et al. The role of intraoperative frozen sections in revision total joint arthroplasty. J Bone Joint Surg Am. 1995;77:1807–13. doi: 10.2106/00004623-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 40.Moran E, Masters S, Berendt AR, et al. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J Infect. 2007;55:1–7. doi: 10.1016/j.jinf.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Matthews PC, Conlon CP, Berendt AR, et al. Outpatient parenteral antimicrobial therapy (OPAT): is it safe for selected patients to self-administer at home? A retrospective analysis of a large cohort over 13 years. J Antimicrob Chemother. 2007;60:356–62. doi: 10.1093/jac/dkm210. [DOI] [PubMed] [Google Scholar]
- 42.Schaubel DE. Variance estimation for clustered recurrent event data with a small number of clusters. Stat Med. 2005;24:3037–51. doi: 10.1002/sim.2157. [DOI] [PubMed] [Google Scholar]
- 43.Durham LK, Longini IM, Jr, Halloran ME, et al. Estimation of vaccine efficacy in the presence of waning: application to cholera vaccines. Am J Epidemiol. 1998;147:948–59. doi: 10.1093/oxfordjournals.aje.a009385. [DOI] [PubMed] [Google Scholar]
- 44.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–23. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]