Abstract
The culture of primary hepatocytes as spheroids creates an efficient 3-dimensional tissue construct for hepatic studies in vitro. Spheroids possess structural polarity and functional bile canaliculi with normal differentiated function. Thus, hepatocyte spheroids have been proposed as the cell source in a variety of diagnostic, discovery, and therapeutic applications, such as a bioartificial liver. Using a novel rocking technique to induce spheroid formation, kinetics of spheroid formation, cell-cell adhesion, gene expression and biochemical activities of rat hepatocyte spheroids were tested over 14 days of culture. Evidence was provided that the formation of spheroids occurred faster and with fewer non-adherent hepatocytes in rocked suspension culture compared to a traditional rotational system. Hepatocyte spheroids in rocked culture showed stable expression of over 80% of 242 liver-related genes including those of albumin synthesis, urea cycle, phase I and II metabolic enzymes, and clotting factors. Biochemical activity of rocked spheroid hepatocytes was superior to monolayer culture of hepatocytes on tissue culture plastic and collagen. In conclusion, spheroid formation by rocker technique was more rapid and more efficient than rotational technique. Rocker formed spheroids appear suitable for application in a bioartificial liver or as an in vitro liver tissue construct.
Keywords: liver tissue construct, bioartificial liver, custom microarray, drug metabolism, spheroid
Novel systems are needed to facilitate short and long-term culture of hepatocytes for diagnostic, discovery, and therapeutic applications (1). Traditional monolayer culture of primary hepatocytes on tissue culture plastic is problematic and has been associated with a rapid loss of differentiated function (2). While single and double layer surfaces of collagen or other biomatrix materials are associated with improved differentiated functions in vitro (3), biological surfaces can pose manufacturing hurdles and do not support high cell density culture of primary hepatocytes (exceeding 1×107 cell/mL). In contrast, spheroids, non-adherent multi-cell aggregates of greater than 40μm diameter, provide a 3-dimensional tissue construct which form spontaneously and allow suspension culture of primary hepatocytes at high cell density under oxygenated bioreactor conditions (4). Spheroid formation allows recapitulation of the cuboidal geometry of primary hepatocytes with relatively stable long-term differentiated function (5, 6). Reports of structural polarity and bile canaliculi formation by primary rat hepatocytes in spheroid aggregates provide further evidence that hepatic spheroids mimic the hepatocellular microanatomy of the liver (7).
Original observations of tissue-like aggregate formation from isolated cells was reported by Moscona in 1961 using fetal liver cells and a rotational technique (8). The descriptive term “spheroid” was coined years later by Landry in 1985 when multi-cellular aggregates were formed from isolated rat hepatocytes after 3−5 days of culture on non-adherent plastic surfaces (9). Later, spheroids were observed from primary hepatocytes after 24 hours of rotational culture with 56% of the hepatocyte inoculum forming multi-cellular aggregates of greater than 40μm in diameter (10). More recently, we reported preliminary observations that hepatocytes formed spheroids when suspended in rocked (oscillatory) motion (11). Rocking promoted mixing, oxygenation, and increased frequency of collisions between freshly isolated hepatocytes, which in turn accelerated their aggregation into clusters and the formation of spheroids.
Our preliminary report of spheroid formation under rocked conditions was limited to a 5 day culture interval using porcine hepatocytes (11). The goals of the current study were to compare the spheroid formation kinetics of rat hepatocytes in rocked vs. rotational culture and to characterize the gene expression and biochemical activity of rocker-formed rat hepatocyte spheroids over extended (14 days) duration. Spheroid formation was measured by direct observation and automated Coulter counter. In order to expand the evaluation of gene expression by hepatocyte spheroids, we utilized a custom microarray consisting of 242 liver-related genes in a wide array of cellular functions: cell adhesion, cell respiration, phase I and II metabolism, ureagenesis, glycolysis, stress response, and assorted transcription factors. Functionality of spheroids was compared to monolayer controls (tissue culture plastic, TCP; collagen monolayer, CM) using clinically relevant liver-specific biochemical assays: albumin production, ureagenesis, and cytochrome P450 activities. All studies were conducted using rat hepatocytes inoculated at an intermediate cell density (1×106 cell/mL) to allow comparisons of monolayer and spheroid geometries formed by static, rocked or rotational technique. Our results indicate that the rocked technique is a simple yet rapid and highly efficient means of forming three-dimensional, suspension-stable, hepatic tissue constructs.
Experimental Procedures
Animals were provided ad lib access to water and standard chow. All animal procedures were performed under the guidelines set forth by the Mayo Foundation Animal Care and Use Committee and are in accordance with those set forth by the National Institutes of Health. All compounds were obtained from Sigma (St. Louis, MO) unless noted otherwise.
Rat hepatocyte isolation
Hepatocytes were isolated from 250−300 g male Sprague Dawley rats (Harlan, Indianapolis, IN) by a two-step perfusion method as previously described (12). All harvests yielded hepatocytes with viability exceeding 90% by trypan-blue dye exclusion.
Spheroid and monolayer culture
Freshly isolated hepatocytes were suspended in culture medium [Williams-E medium with 10% fetal bovine serum (FBS; Mediatech, Herndon, VA), 10 U/mL penicillin G, 100 μg/mL streptomycin, 10 μg/mL insulin, 5.5 μg/mL transferrin, 5 ng/mL sodium selenite] at 1×106 cells/mL. Suspended hepatocytes were inoculated into four different culture vessels: spheroid rocker dishes (10×8×2cm); spheroid rotational flasks (6cm diameter); monolayer plates of tissue culture plastic (60mm; Corning, Corning, NY); monolayer plates of type I collagen-coated plastic (60mm Biocoat; BD Labware, Franklin Lakes, NJ). Cell density (1×106cells/mL) and plating density (1.8×105cells/cm2) were standardized by inoculating cell suspensions of 20mL per spheroid vessel and 5mL per monolayer plate. Spheroid dishes were rocked continuously at 0.25Hz while spheroid flasks were rotated on an orbital spinner. Monolayer plates were placed on a stable rack. All cultures were incubated at 37°C and 5%CO2.
Media changes
Medium was first changed at 24 hours and subsequently at weekly intervals. Dexamethasone (100ng/mL) was added on day1 and thereafter. One day prior to sampling, cultures received 10μM β-naphthoflavone (βNF), 8μM diazepam, and 5%v/v heavy deuterium-enriched ammonia gas (2.23mM; Cambridge Isotope Laboratories, Inc, Andover, MA) to test the hepatic clearance ability of these compounds. βNF was omitted from non-induced cultures. Samples were stored at −80°C.
Morphometrics of hepatocyte spheroid formation
Rocked and rotational cultures were sampled (1mL) from 4 to 48 hours. Spheroid diameter, spheroid number and free hepatocyte number were measured by phase contrast microscopy (Axiocam; Carl Zeiss, Inc., Thornwood, NY) and by automated technique using a Multisizer3 (560μm aperture; Beckman Coulter, Fullerton, CA).
Immunofluorescence microscopy
Hepatocytes were fixed in 4%paraformaldehyde, rinsed with PBS and prehybridized with 0.1%Triton and 1%goat serum. Cells were incubated in mouse anti-rat E-cadherin antibody (1:500; #610181, BD Biosciences, San Jose, CA) and secondary antibody - fluorescein-5-isothiocyanate(FITC)-labeled goat anti-mouse IgG (1:1000; Molecular Probes, Invitrogen, Carlsbad, CA). Immunofluorescence was imaged by confocal microscopy (LSM510, Carl Zeiss, Inc.).
Electron microscopy
Spheroids were fixed in Trumps solution, sectioned, and imaged by transmission electron micrscopy in the Microscopy Core Facility (Mayo Clinic, Rochester, MN).
Hepatocyte viability
Spheroid samples (300μL) were resuspended in a 50μL mixture of acridine orange and ethidium bromide stain (FluoroQuench™; One Lambda, Canoga Park, CA). Cells were imaged using fluorescent microscopy using FITC filters and AttoArc HBO 100 W excitation. Fluorescence was quantified using ImageProPlus6.0 (Media Cybernetics, Silver Springs, MD).
Calcium influence on spheroid formation
Hepatocytes were cultured in medium with or without EGTA supplementation - equimolar (31.4 mM) to calcium concentration. Hepatocytes were viewed by phase contrast microscopy after 48hrs of rocking. EGTA was also added after spheroid formation (48hrs) to assess the influence of calcium depletion on formed spheroids.
Extraction of DNA and RNA
Samples of spheroids (5mL) and hepatocytes scraped from plates were pelleted and aliquoted for DNA or RNA analysis. DNA quantification was performed using DNeasy kit (Qiagen, Valencia, CA). Total RNA was extracted immediately and stored at −80°C in RNAlater® (Qiagen, Valencia, CA) until quantification.
Custom microarray analysis
A custom microarray of 242 liver–related genes with rat-specific oligonucleotide sequences was created and analyzed by the Advanced Genomic Technology Center (Mayo Clinic, Rochester, MN). Microarray analysis was performed on spheroid samples obtained on days 2, 7, 14 and compared to fresh hepatocytes (day 0). Each sample not induced with βNF was compared to the baseline (day 0) sample, and the βNF-induced samples were compared to non-induced samples from the same day. Ten comparisons were performed for each gene sequence. Gene expression was reported as the log2 fold-change of signal intensity between compared samples.
Albumin gene expression and production of rat albumin
Rat albumin mRNA copy number was measured by a standardized quantitative competitive-reverse transcription-polymerase chain reaction (QC-RT-PCR) with capillary electrophoresis detection in the Mayo Immune Monitoring Lab Core Services as previously described (13). Concentration of rat albumin in culture media was determined by ELISA kit (Bethyl Laboratories, Montgomery, TX) using a sheep anti-rat albumin antibody.
qPCR analyses of hepatocyte gene expression
Expression of Cdh1, Nags, Ass, Asl, Otc, Arg1, Cps1, Cyp1a1, Cyo1a2, Aldh3a1, Nqo1, Gsta2, Chst10, Cyp2c, Cyp2a1 and Hmox1 genes was determined using real time RT-PCR (qPCR). Briefly, 8 or 16 ng total RNA and 50ng of primers were added to a 10ul reaction containing Quantifast SYBR green RT-PCR master mix (Qiagen). qPCR reactions were performed in 384-Well Clear Optical reaction plates (Applied Biosystems) and detection was determined using an ABI 7900 HT Fast Real Time PCR system (Applied Biosystems). Cycle Threshold (CT) values were determined using Applied Biosystems SDS 2.0 software. Fold changes in gene expression were calculating using the deltadelta CT method. Results were based on RNA obtained from four independent experiments.
Quantification of ammonia and isotopes of urea
Rates of ureagenesis were determined by the conversion of heavy ammonia (15ND3) to native and deuterium-enriched urea. Isotopes of urea were quantified with capillary gas chromatography/mass spectrometry as previously reported (14).
CYP1A activity
Cytochrome P450 1A (CYP1A) activity was assessed by the conversion of 7-ethoxyresorufin to resorufin as previously described (15). Resorufin was detected at excitation and emission wavelengths of 530 and 590 nm, respectively, using a FL600 microplate fluorescence reader (Bio-Tek Instruments, Inc, Winooski, VT).
CYP3A and CYP2C11 activity
CYP3A and CYP2C11 activities were determined from media samples supplemented with diazepam as previously described (11). Data acquisition was completed by Chrom Perfect® software (Denville, NJ).
Statistical methods
Two-way ANOVA and student's t-test were used to assess statistical significance of differences in biochemical activity and gene expression between culture conditions and time points. A Mann-Whitney rank sum test was used to compare diazepam metabolism values. A non-parametric Spearman correlation was used to compare albumin QC-RT-PCR and albumin protein data. Results were expressed as mean values ±SEM.
Gene expression was compared using log2 transforms of the median signal intensity. Data was normalized by a semi-parametric approach to correct for intensity-dependent effects (16). We used SAS© Version 9 statistical software (SAS Institute Inc., Cary, NC) for these analyses.
Results
Total hepatocyte number, hepatocyte viability, and incorporation into spheroids
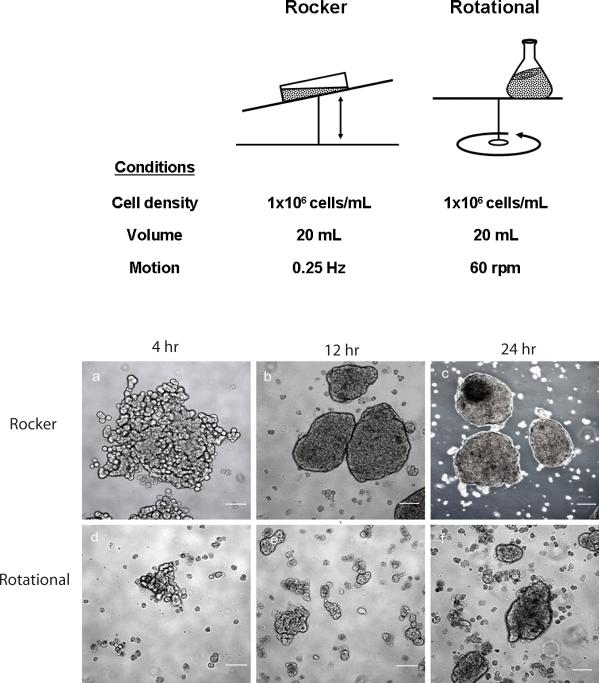
The kinetics of spheroid formation was compared under rocked and rotational conditions as summarized Fig. 1, upper panel. Measurements of spheroid size, spheroid number, hepatocyte number, and percent incorporation of hepatocytes into spheroids were obtained at the time of inoculation through 48 hours. These measurements were made in triplicate. Aggregation of hepatocytes was observed under both rocked and rotational conditions by 4 hours; however, hepatocyte aggregates were larger and more numerous in rocked cultures at all time points (Fig. 1, lower panel). Well-formed spheroids were observed throughout the rocked spheroid cultures at 12 hours. By 24 hours of rocking, 85% of inoculated hepatocytes had incorporated into well-formed spheroids of greater than 40μm diameter (Table 1). At 48 hours, only 13% of cell particles measured less than 40μm diameter by Coulter counter (Fig. 2). The majority of rocker-formed aggregates (84%) ranged in diameter of 75−200μm (Fig. 3, day 2). In contrast, only 58% of hepatocytes incorporated into spheroids under rotational conditions at 24 hours. Most unattached hepatocytes appeared dead. Rotational formed spheroids were less spherical at earlier time points.
Figure 1.
Comparison of rocked vs. rotational conditions (upper panel) along with rat hepatocyte spheroids viewed by phase microscopy during spheroid formation (lower panel). Rat hepatocytes (1×106 cells/mL) were rocked at 0.25 Hz or spun at 60 rpm to induce spheroid formation. Representative images of spheroid formation by rocked technique are provided at 4hr (a), 12hr (b), and 24hr (c) and by rotational technique at 4hr (d), 12hr (e), and 24hr (f). Scale bars equal 50μm.
Table 1.
Spheroid Formation after 24 hours - Rotational vs. Rocked Method
| Rotational (60 rpm) |
Rocked (0.25Hz) |
|
|---|---|---|
| Diameter (mean±SEM) | 90±36 μm | 145±33 μm ** |
| Percent of inoculum incorporated into spheroids | ||
| >40 μm diameter | 58% | 85% ** |
| >75 μm diameter | 52% | 80% ** |
p<0.001 vs. rotational culture (based on 1000 measurements per group, repeated in triplicate)
Figure 2.
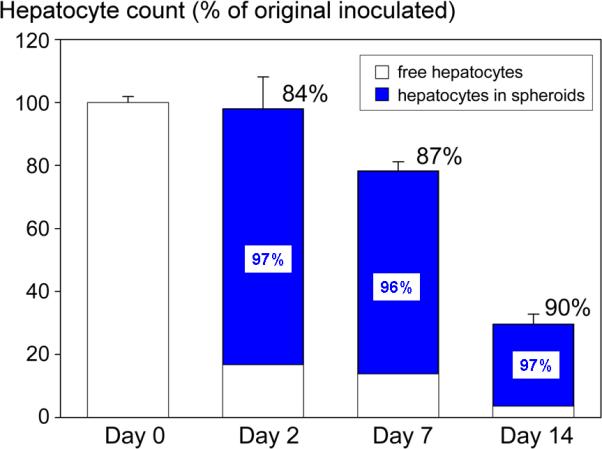
Hepatocyte count in suspension and incorporated into spheroids by rocker technique. Cell counts were measured by Multisizer 3 Beckman Coulter counter. Cells were inoculated (day 0) at 1×106 cells/mL. Bars indicate percent of free hepatocytes (white) vs. hepatocytes in spheroids (blue, >75 μm diameter) of original cells inoculated. Percent values listed above each bar in black indicate proportion of cells present in spheroids, while the percent viability of cells in spheroids is listed inside of each blue bar. Values are averages based on three independent experiments.
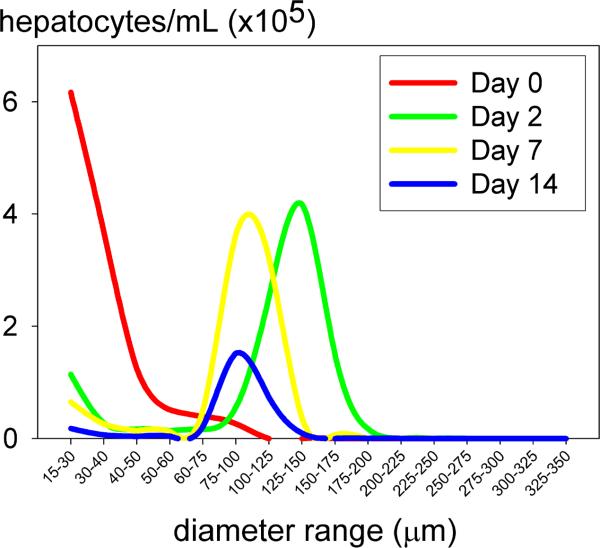
Figure 3.
Time course of spheroid size and total cells under rocked conditions. Isolated hepatocytes were originally plated at 1×106 cells/mL (equal to the area under the curve on day 0, red line). Measurements were completed by Multisizer 3 Beckman Coulter counter. Repeated in triplicate.
Spheroid number and cell number remained relatively stable after day2 during the first week of rocked culture. In contrast, average spheroid size decreased from day 2 (125−150μm) to day 7 (75−100μm) consistent with formation of mature spheroids during the first week of rocked culture (Fig. 3). Spheroid formation provided a protective benefit as over 95% of hepatocytes present in spheroids were viable at each time point. A decline in spheroid number and cell number was observed during the second week of culture. Approximately 30% of hepatocytes inoculated into rocked dishes remained in spheroids on day 14. In contrast, less than 10% of hepatocytes inoculated into rotational flasks remained in spheroids on day 14. Therefore, spheroids formed by rocker technique were used in subsequent studies.
Expression of liver-related genes in spheroids by custom microarray
We profiled gene expression of rat hepatocytes over 14 days of rocked spheroid culture. A custom microarray of 242 liver-related genes was developed for this task. We observed that the average expression of 85% (206) genes remained stable - less than 2-fold increase or 50% decrease relative to baseline - over 14 days in culture (Fig. 4). Expression increased 2-fold or greater in 11 (5%) genes and decreased 50% or more in 25 (10%) genes. A complete listing of custom microarray data can be found at http://mayoresearch.mayo.edu/mayo/research/nyberg_lab/.
Figure 4.
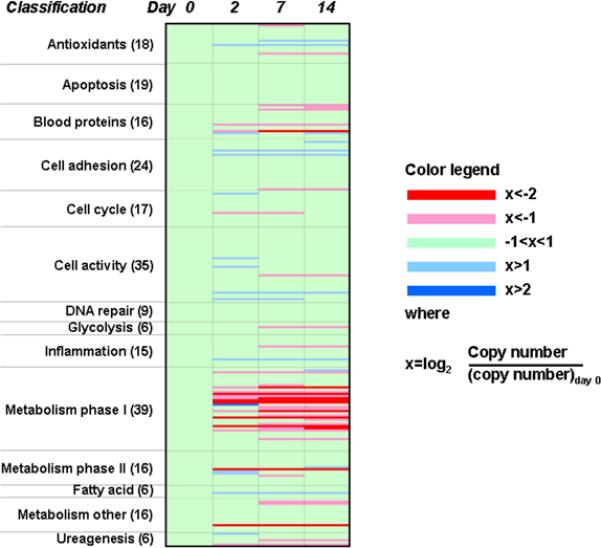
Expression of 242 liver specific genes by custom microarray in rocked spheroid culture over 14 days duration. Average expression of over 80% of these genes was stable over time (green color). A reduction in gene expression from baseline (freshly isolated rat hepatocytes) is indicated in pink or red, while an increase in expression is labeled blue. Genes of phase I metabolism showed the greatest level of fluctuation in rocked spheroid culture.
Hepatocyte cell adhesion
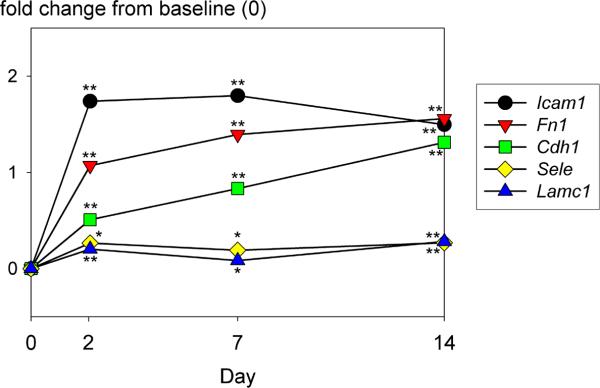
The expression of several cell adhesion genes including E-cadherin (Cdh1), intracellular adhesion molecule 1 (Icam1), and fibronectin (Fn1) remained stable in rocked spheroid culture (Fig. 5). Expression of N-cadherin (Cdh2), selectin (Sele), laminin gamma 1 (Lamc1) and six other integrin-related genes (Itga1, Itgae, Itgam, Itgb1, Itgb3, Itgb4) also remained stable. The pattern of expression of Cdh1 was validated by qPCR.
Figure 5.
Trends in the expression of cell adhesion genes during formation and extended culture of rat hepatocyte spheroids under rocker conditions. Multiple structural genes important in cell adhesion were stable or up-regulated over time. Intracellular adhesion molecule (Icam1); fibronectin (Fn1); E-cadherin (Cdh1); endothethial cell selectin (Sele); laminin gamma 1 (Lamc1). The y axis signifies the log base 2-fold change of the gene expression ratio intensity values. **p<0.001, *p<0.05 vs. baseline (isolated hepatocytes).
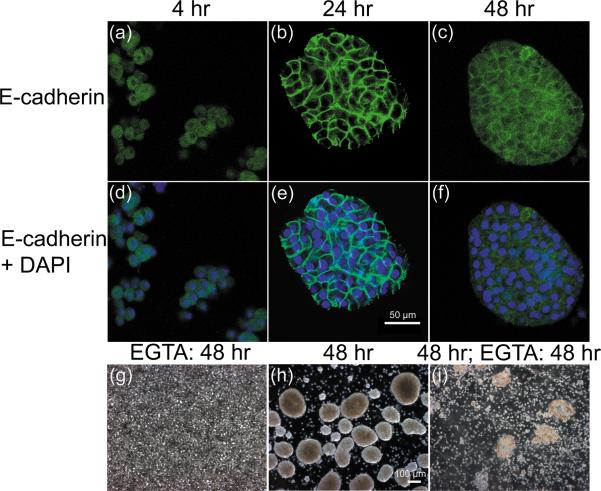
Because of the known importance of E-cadherin as an adhesive protein between epithelial cells, we studied the role of E-cadherin during spheroid formation by rocked technique. Cell samples were examined by immunohistochemical staining during initial hours of spheroid formation in culture medium and culture medium supplemented with EGTA to remove free calcium. We observed rapid spheroid formation and strong staining of E-cadherin on the surface of cells formed in the presence of free calcium (Fig. 6a-f). However, spheroids failed to form in medium supplemented with EGTA (Fig. 6g). Furthermore, formed spheroids quickly freagmented after addition of EGTA on day 2 (Fig. 6h,i). Most unattached hepatocytes appeared dead after 24 hours in calcium-free conditions confirming the importance of calcium-dependent adhesion in the formation and viability of liver tissue constructs such as spheroids. These studies suggest an essential role for E-cadherin in the prevention of cell death after separation of hepatocytes from their extracellular matrix. This form of cell death, termed anoikis, has been observed after separation of other epithelial cell types from their surrounding matrix (17).
Figure 6.
Importance of calcium-dependent E-cadherin in spheroid formation of primary rat hepatocytes. (a-f) E-cadherin was strongly expressed on the surface of hepatocytes during spheroid formation and its expression continued over 14 days as determined by microarray analysis (see Fig. 4). (a,d,g) Hepatocytes at 4 hours, (b,e,h) spheroids at 24 hours, (c,f,i) spheroids at 48 hours. Scale bar in (e) equals 50 μm and applies to all immunofluorescence images. (g-i) The importance of calcium in spheroid formation is illustrated by phase contrast microscopy. (g) No spheroids form in medium containing EGTA compared to spheroids in normal media condition (h) at 48 hours. (i) EGTA supplementation caused rapid disruption of formed spheroids within an additional 48 hours. Scale bar in (h) equals 100 μm and applies to all contrast microscopy images.
Gene expression and albumin production
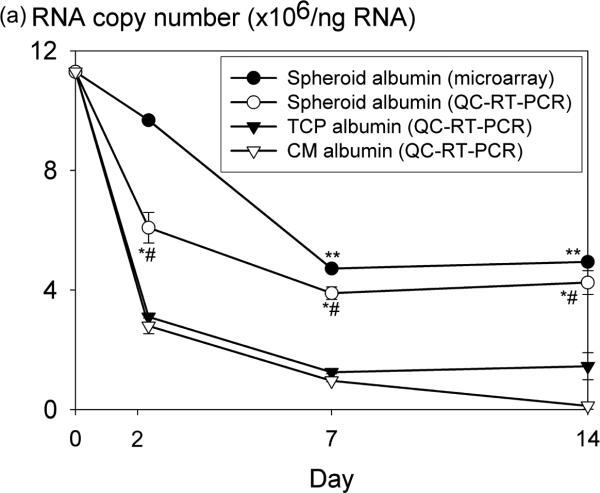
Custom gene microarray, QC-RT-PCR, and ELISA were used to assess gene expression and production of albumin under rocked spheroid and monolayer culture conditions. By custom microarray, albumin gene expression in spheroid cultures remained at 86% of baseline on day 2 and declined to 57% on day 14. This pattern of albumin gene expression was confirmed by QC-RT-PCR (Fig. 7a). Copy number of the albumin gene was significantly higher in spheroid culture compared to tissue culture plastic or collagen monolayer at all time points (p<0.05). Production of albumin protein was also significantly higher in spheroid culture compared to monolayer control cultures at all time points (Fig. 7b, p<0.05). A correlation was suggested between gene copy number and production rate of albumin by non-parametric comparison (Spearman correlation coefficient=0.56; p=0.09).
Figure 7.

Albumin protein and mRNA measurements of rat hepatocytes. (a) Microarray and quantitative competitive reverse transcriptase-polymerase chain reaction (QC-RT-PCR) values. RT-PCR baseline value indicates RNA measurements taken from individual rat hepatocytes at day 0 (before inoculation). *p<0.05 Spheroids vs. TCP, #p<0.05 Spheroids vs. CM. **p<0.0001 vs. day 0 hepatocytes (microarray). Spheroid microarray vs. QC-RT-PCR: Spearman correlation coefficient=0.804; p<0.001. (b) ELISA albumin protein measurements. n=10 samples analyzed per data point. *p<0.05 Spheroids vs. TCP, #p<0.05 Spheroids vs. CM.
Urea cycle
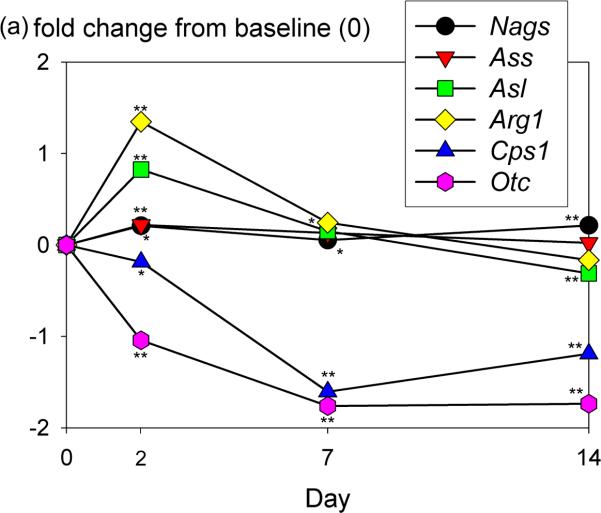
Six genes of the urea cycle were included on the custom array (Fig. 8a): n-acetylglutamate synthase (Nags), arginino succynate synthase (Ass), argino succynate lyase (Asl), arginase 1 (Arg1), carbamoyl-phosphate synthetase 1 (Cps1), ornithine transcarbamolase (Otc). Cps1 and Otc encode for proteins which catalyze the initial steps of the urea cycle within the inner membrane of the mitochondria. Nags encodes for an inner mitochondrial protein. Arg, Ass, Asl express cytoplasmic proteins of the urea cycle. The expression of three urea cycle genes (Ass, Asl, Nags) remained stable during 14 days of rocked spheroid culture; Arg1 was up-regulated to 250% at day 2 and declined gradually to 89% of baseline on day 14; Cps1 and Otc showed a mean reduction of 55% and 36% of baseline, respectively. Expression of these six genes was also measured by qPCR. Patterns of expression of all six genes were similar by microarray and qPCR. However, greater reductions in expression of Cps1 and Otc were determined by qPCR (8-fold and 25-fold decrease on day 14, respectively).
Figure 8.
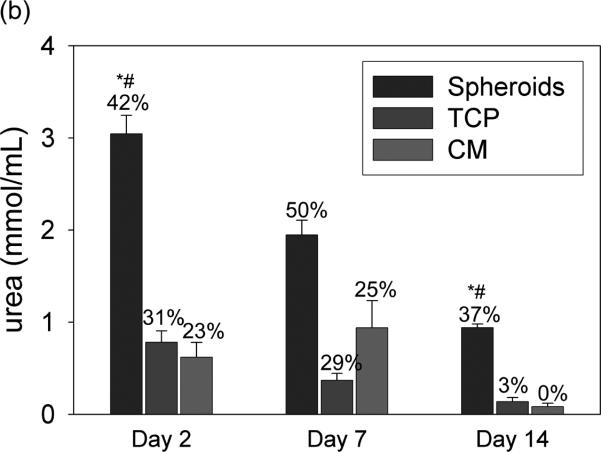
Ureagenesis measurements in rat hepatocytes. (a) Gene expression measurements of urea cycle enzymes by custom microarray. The y axis signifies the log base 2-fold change of the gene expression ratio intensity values. **p<0.001, *p<0.05 vs. baseline (isolated hepatocytes). (b) Heavy ammonia (0.5% v/v) was added to culture media and levels of deuterium-enriched urea were determined by GC-MS on sampled collected at 24 hours. Percentages indicate proportion of total urea labeled with deuterium (sum of all isotopes) in each sample. n=8 samples analyzed per data point. *p<0.0001 rocked spheroids vs. TCP, #p<0.0001 rocked spheroids vs. CM.
Rocker-formed spheroids demonstrated significant levels of ureagenesis at all time points based on conversion of heavy ammonia to heavy urea (Fig. 8b). Total ureagenesis was greater in rocked spheroid cultures than monolayer control conditions at all time points. The ratio of heavy urea to total urea produced by spheroid hepatocytes was also greater than monolayer controls at all time points.. The decline in ureagenesis over time paralleled the decline in expression of Cps1 and Otc.
Phase I Metabolism
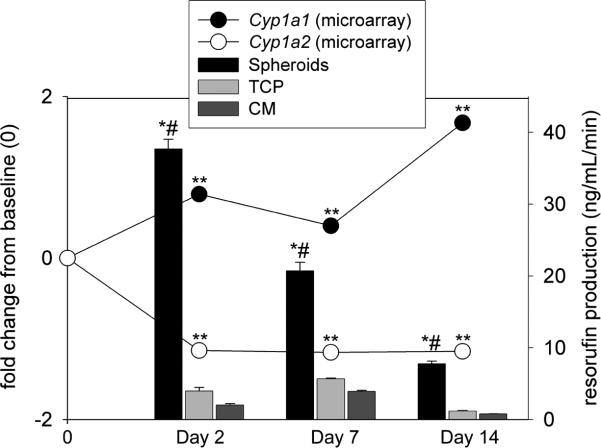
Thirty-nine genes involved in phase I metabolism were assessed by microarray in rocked spheroid culture. These genes showed the greatest variability in expression of any group tested (Figure 4). Average expression of 19 genes (49%) remained stable. One phase I gene [Cyp1a1 (Fig. 9)] was up-regulated over time (without addition of βNF) by both custom microarray and qPCR, while the gene expression of Cyp1a2 declined; eighteen other phase I genes were down-regulated less than 50% of baseline including 3 isoforms of Cyp3a, 5 isoforms of Cyp2c, 4 isoforms of Cyp2d, and Cyp2e1. Cyp1a2, Cyp2a1, Cyp2a2, and Cyp2c11 were also down regulated by qPCR.
Figure 9.
Cyp1a1 and Cyp1a2 gene expression were measured in cultures of rocked spheroids and monolayer conditions by custom microarray (line graph, left axis). **p<0.0001 vs. day 0 hepatocytes. CYP1A1/1A2 activities were measured by the appearance of resorufin in cultures of rat hepatocytes over 14 days (bar graph, right axis). Cultures were exposed to 7-ethoxyresorufin for 60 min after 24 hour induction with β-naphthoflavone. Production of resorufin was fluorescently detected at excitation and emission wavelengths of 530 and 590 nm, respectively. n=24. *p<0.0001 rocked spheroids vs. TCP, #p<0.0001 rocked spheroids vs. CM.
Biochemical activities of multiple CYP450 enzymes were determined by addition of test substrates to hepatocyte cultures. 7-ethoxyresorufin conversion to resorufin (CYP1A1, CYP1A2) was highest in spheroids compared to monolayer conditions on all days (p<0.05, Fig. 9). Collective activity of CYP3A and CYP2C11 was determined by biotransformation of diazepam to oxazepam. Oxazepam concentrations were significantly higher in spheroids (4.35±0.98 μM) compared to both monolayer culture conditions after day 2 (TCP: 1.42±0.35 μM; CM: 1.21±0.34 μM; p<0.001) on day 1. Production of oxazepam peaked in spheroid culture on day 7. This trend paralleled the expression of Cyp3a genes determined by microarray.
Phase II Metabolism/Conjugation
Expression of 14 of 16 (88%) phase II metabolism genes, including six sulfotransferases and all five UDP-glucuronosyl transferases (Udpgt), remained stable during 14 days of spheroid culture. Sulfotransferase 1B (Sult1b) was up-regulated while sulfotransferase-estrogen-preferring (Ste) was down-regulated.
Gene Induction by βNF
The influence of βNF on gene expression of spheroid hepatocytes was quantified by microarray data at each time point. Nine genes summarized in Table 2 were induced by βNF. The expression of most inducible genes remained high throughout the culture period. Six genes (Cyp1a1, Cyp1a2, Cyp2a, Aldh3a1, Nqo1, Hmox) were also examined by qPCR which confirmed there induction by βNF.
Table 2.
Rat genes induced by β-naphthoflavone (βNF) in rocked spheroid conditions.
|
βNF induction ratioa |
|||
|---|---|---|---|
| Day 2 |
Day 7 |
Day 14 |
|
| Cyp1a1 | 13.2 | 15.1 | 13.0 |
| Aldh3a1 | 4.0 | 6.9 | 10.2 |
| Nqo1 | 3.0 | 4.9 | 4.8 |
| Gsta2 | 4.6 | 4.6 | 3.0 |
| Chst10 | 2.2 | 3.6 | 3.2 |
| Cyp2c | 1.4 | 2.1 | 2.7 |
| Cyp2a1 | 1.8 | 1.7 | 1.9 |
| Cyp1a2 | 2.5 | 1.9 | 1.6 |
| Hmox1 | *1.1 | 1.5 | 1.9 |
Induction ratio is the level of expression of induced vs. non-induced spheroid cultures at each time point. The induction ratio was significant at a level of p<0.0001.
indicates induction ratio was not significant, p>0.05
Antioxidants
Expression of 15 of 18 antioxidant genes (83%) remained stable during 14 days of culture. Two antioxidant genes, glutathione synthetase (Gss) and glutathione-S-transferase Mu 1 (Gstm1), showed increased expression by an average greater than 2-fold over 14 days. Glutathione reductase (Gsr), glutathione-S-transferase theta 2 (Gstt2), and microsomal glutathione S-transferase 1 (Mgst1) showed a decline in expression.
Coagulation factors
Expression of 5 of 7 (71%) clotting factor genes remained stable during 14 days of culture. Thrombin and factor X showed an average 2-fold decrease from baseline over 14 days.
Discussion
The most important observation from the current study is that spheroid formation occurs rapidly and most efficiently by rocker technique. Rapid aggregation of hepatocytes into spheroids by rocked technique was associated with fewer dead cells and improved spheroid morphology compared to the traditional rotational technique. These observations are significant and predict that a higher dose of primary hepatocytes is possible in a spheroid reservoir configuration compared to prior designs of bioartificial livers (18, 19)]. Rocker-formed spheroids may also prove to be an alternative method for preparation and maintenance of hepatocytes prior to cellular transplantation (20). Transplantation of hepatocyte spheroids has been reported previously (21). Studies are planned to test whether rocker-formed spheroids are suitable for therapeutic purposes such as hepatocyte transplantation.
The influence of spheroid size on hepatocyte function was previously studied by Glickis et al (22), who predicted that the diffusion of oxygen would not be rate limiting and central hepatocytes would not become hypoxic if spheroid diameter was on the order of 100μm. Our observations support those of Glickis in that we found optimal spheroid size to be approximately 100−150um based on viability and mitochondrial membrane potential of rocked spheroid hepatocytes (unpublished observations). We determined mitochondrial membrane potential of hepatocytes by tetramethylrhodamine ethyl ester perchlorate (TMRE) staining (23).
The formation of spheroids by rocker technique draws focus on the anchorage dependency of epithelial cells such as hepatocytes and the importance of cell-cell contact and adhesion to extracellular matrix in maintaining differentiated phenotype. In turn, hepatocyte spheroids formed by rocker technique preserved differentiated function better than classic monolayer culture techniques. Alternative techniques exist for culture of hepatocytes and creation of in vitro liver tissue constructs, including collagen sandwich (24) and hepatocyte co-culture (6, 25). Comparisons to these more elaborate, labor intensive techniques will be helpful in establishing the role of the rocked spheroid technique in experimental biology of liver tissue constructs.
Further optimization of the rocked hepatocyte culture system may also be required as a progressive decline in cell number was observed over the 2-week study interval. The decline in both metabolic activity and protein production paralleled the decline in cell number, despite stable gene expression on a viable cell basis. The reason for cell loss from the spheroid system has not yet been determined. Some of the spheroid losses occurred during changes of media, while other losses occurred by adhesion of spheroids to the walls of the bioreactor and along the liquid-air interface where surface tension was greatest. Cell death between time points was another possibility because hepatocytes appeared viable in intact spheroids when examined by vital staining and transmission electron microscopy (data not shown) during the 14 day culture period.
In conclusion, we offer a thorough analysis of the biochemical functions and liver-specific gene expression of rat hepatocyte spheroids formed by rocker technique over 14 days in culture. Over 85% of hepatocytes were incorporated into spheroids by rocker technique after 24 hours compared to 58% by conventional rotational technique. Cell loss occurred in all culture systems studied; however, the rate of cell loss was lower and biochemical activity was significantly greater at all time points in rocked spheroid cultures compared to control conditions. Custom microarray studies confirmed stable and inducible expression of many liver-related genes in spheroids formed by rocked technique. Further optimization of this system for application as a bioartificial liver is warranted.
Acknowledgments
Special thanks to Nancy Borson, Trace Christensen, Harris Khan, Chris Kolbert, Perry Loken, Vernadette Simon and Jim Tarara for their expert technical assistance. Thanks to Diane Grill for statistical assistance.
Funding provided by NIH-R01-DK56733, Discovery Translation Award – Mayo Foundation
Abbreviations
- Aldh3a1
aldehyde dehydrogenase 3A1
- Arg1
arginase 1
- Asl
arginino succynate lyase
- Ass
arginino succynate synthase
- Ahr
aryl hydrocarbon receptor
- Arnt
aryl hydrocarbon receptor nuclear translocator
- βNF
β-naphthoflavone
- Cps1
carbamoylphosphate-synthetase 1
- Chst10
carbohydrate sulfotransferase 10
- CM
collagen monolayer
- Cdk4
cyclin dependent kinase 4
- Ccnd1
cyclin D1
- CYP1a1
cytochrome P450 1A1
- ELISA
enzyme-linked immunosorbent assay
- Fn1
fibronectin 1
- Gsta2
glutathione S transferase A2
- Hmox1
hemoxygenase-1
- Icam1
intracellular adhesion molecule 1
- Itga1
integrin alpha 1
- Lamc1
laminin gamma 1
- Nags
N-acetylglutamate synthase
- Nqo1
NADPH quinone oxidoreductase 1
- Nrf2
nuclear factor erythroid 2-related factor
- Otc
ornithine transcarbamolase
- QC-RT-PCR
Quantitative Competitive-Reverse Transcriptase-Polymerase Chain Reaction
- Sele
selectin (endothelial cell)
- TCP
tissue culture plastic
- Udpgt
UDP-glucuronosyltransferase
References
- 1.Khetani S, Bhatia S. Microscale culture of human liver cells for drug development. Nature Biotechnology. 2008;26:120–126. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- 2.Enat R, Jefferson DM, Ruiz-Opazo N, Gatmaitan Z, Leinwand LA, Reid LM. Hepatocyte proliferation in vitro: its dependence on the use of serum-free hormonally defined medium and substrata of extracellular matrix. Proc Natl Acad Sci U S A. 1984;81:1411–1415. doi: 10.1073/pnas.81.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang S, Nagrath D, Chen PC, Berthiaume F, Yarmush ML. Three-Dimensional Primary Hepatocyte Culture in Synthetic Self-Assembling Peptide Hydrogel. Tissue Eng. 2008 doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 4.Sakai Y, Naruse K, Nagashima I, Tetsuichior M, Suzuki M. A new bioartificial liver using porcine hepatocyte spheroids in high-cell-density suspension perfusion culture: in virto performance in synthesized culture medium and in 100% human plasma. Cell Transplantation. 1999;8:531–541. doi: 10.1177/096368979900800508. [DOI] [PubMed] [Google Scholar]
- 5.Peshwa M, Su F, Sharp H, Cerra F, W-S H. Mechanistics of formation and ultrastructural evaluation of hepatocyte spheroids. In Vitro Cell Dev Biol. 1996;32:197–203. doi: 10.1007/BF02722946. [DOI] [PubMed] [Google Scholar]
- 6.Lu H-F, Chua K-N, Zhang P-C, Lim W-S, Ramakrishna S, Leong KW, Mao H-Q. Three-dimaensional co-culture of rat hepatocyte spheroids and NIH/3T3 fibroblasts enhances hepatocyte functional maintenance. Acta Biomaterialia. 2005;1:399–410. doi: 10.1016/j.actbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- 8.Moscona A. Rotation-mediated histogenietic aggregation of dissociated cells. Exp Cell Res. 1961;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- 9.Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: Histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakai Y, Naruse K, Nagashima I, Muto T, Suzuki M. Large-scale preparation and function of porcine hepatocyte spheroids. Int J Artif Organs. 1996;19:294–301. [PubMed] [Google Scholar]
- 11.Nyberg S, Hardin J, Amiot B, Argikar U, Remmel RP, Rinaldo P. Rapid, large-scale formation of porcine hepatocyte spheroids in a novel spheroid reservoir bioartificial liver. Liver Transplantation. 2005;11:901–910. doi: 10.1002/lt.20446. [DOI] [PubMed] [Google Scholar]
- 12.Seglen P. Preparation of isolated rat liver cells. Methods in Cell Biology. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 13.Borson N, Strausbauch M, Wettstein P, Oda R, Johnston S, Landers J. Direct quantitation of RNA transcripts by competitive single-tube RT-PCR and capillary electrophoresis. BioTechniques. 1998;25:130–137. doi: 10.2144/98251rr01. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldo P. Organic acids. In: Blau N, Duran N, Gibson K, editors. Laboratory guide to the methods in Biochemical Genetics. Springer-Heidelberg; 2008. pp. 137–170. [Google Scholar]
- 15.Donato M, Gomez-Lechon M, Castell J. A microassay for measuring cytochrome P4501A1 and P450IIB1 activities in intact human and rat hepatocytes cultured on 96-well plates. Analytical Biochem. 1993;213:29–33. doi: 10.1006/abio.1993.1381. [DOI] [PubMed] [Google Scholar]
- 16.Eckel JE, Gennings C, Therneau TM, Burgoon LD, Boverhof DR, Zacharewski TR. Normalization of two-channel microarray experiments: a semiparametric approach. Bioinformatics. 2005;21:1078–1083. doi: 10.1093/bioinformatics/bti105. [DOI] [PubMed] [Google Scholar]
- 17.Fouquet S, Lugo-Martinez VH, Faussat AM, Renaud F, Cardot P, Chambaz J, Pincon-Raymond M, et al. Early loss of E-cadherin from cell-cell contacts is involved in the onset of Anoikis in enterocytes. J Biol Chem. 2004;279:43061–43069. doi: 10.1074/jbc.M405095200. [DOI] [PubMed] [Google Scholar]
- 18.Strain A, Neuberger J. A bioartificial liver - state of the art. Science. 2002;295:1005–1009. doi: 10.1126/science.1068660. [DOI] [PubMed] [Google Scholar]
- 19.Demetriou A, Brown R, Busuttil R, Fair J, McGuire B, Rosenthal P, Schulte J, et al. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660–670. doi: 10.1097/01.sla.0000124298.74199.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strom S, Bruzzone P, Cai E, Lehmann T, Mitamura K, Miki T. Hepatocyte Transplantation:clinical experience and potential for future use. Cell Transplantation. 2006;15:S105–110. doi: 10.3727/000000006783982395. [DOI] [PubMed] [Google Scholar]
- 21.Saito S, Sakagami K, Koide N, Takasu S, Oiwa T, Orita K. Transplantation of spheroidal aggregate cultured hepatocytes into the rat spleen. Transplantation Proceedings. 1989;21:2374–2377. [PubMed] [Google Scholar]
- 22.Glicklis R, Merchuk JC, Cohen S. Modeling mass transfer in hepatocyte spheroids via cell viability, spheroid size, and hepatocellular functions. Biotechnol Bioeng. 2004;86:672–680. doi: 10.1002/bit.20086. [DOI] [PubMed] [Google Scholar]
- 23.Yagi T, Hardin J, Miyoshi H, Gores G, Nyberg S. Caspase inhibition reduces apoptotic death of cryopreserved porcine hepatocytes. Hepatology. 2001;33:1432–1440. doi: 10.1053/jhep.2001.24560. [DOI] [PubMed] [Google Scholar]
- 24.Dunn J, Thompkins R, Yarmush M. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;116:1043–1053. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalopoulos G, Bowen W, Zajac V, Beer-Stolz D, Watkins S, Kostrubsky V, Strom S. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology. 1999;29:90–100. doi: 10.1002/hep.510290149. [DOI] [PubMed] [Google Scholar]