Abstract
Monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary columns have been prepared via either thermally or photochemically initiated polymerization of the corresponding monomers and the repeatability of their preparation has been explored. Three separate batches of five columns each were prepared using thermal and photochemical initiation for a total of thirty columns. All thirty capillary columns were tested in liquid chromatography-electrospray ionization-mass spectrometry mode for the separation of a model mixture of three proteins - ribonuclease A, cytochrome c and myoglobin. Excellent repeatability of retention times was observed for the proteins as evidenced by relative standard deviation (RSD) values of less than 1.5%. Somewhat broader variations with RSD values of up to 10% were observed for the pressure drop in the columns. The stability of retention times was also monitored using a single monolithic column and no significant shifts in either retention times or back pressure was observed in a series of almost 2200 consecutive protein separations.
Keywords: nanoHPLC, Monolithic columns, Column technology, Capillary, Mass spectrometry, Proteins, Repeatability, Reproducibility, Validation
1. Introduction
Since their invention in the early 1990s, rigid porous polymer-based monolithic columns have shown their unique suitability for the rapid separations of large molecules such as proteins [1–5], nucleic acids [6–8], and synthetic polymers [9–11]. The original analytical scale size columns were supplemented with their capillary counterparts in the early 2000s [5,12–19]. These sub-millimeter internal diameter columns facilitate direct splitless coupling with electrospray ionization mass spectrometry (ESI-MS) [20–23].
The major advantages of capillary columns containing porous polymer-based monoliths include a wide range of available chemistries, high porosity, low resistance to hydraulic flow, fast mass transfer, and almost no limitation in column diameter and length due to the simplicity of the in-situ preparation process from liquid precursors. All of these features combine to enable excellent separations to be achieved at a high flow rate [24].
To date, and despite the enormous significance for users, only a few studies related to the stability and repeatability of monolithic columns are found in the literature [18,25,26]. The most comprehensive study was published in 2002 by Kele and Guiochon [27]. Using a variety of small molecules, they measured the chromatographic and hydrodynamic properties of six silica-based Chromolith Performance C18 reversed phase columns obtained directly from the manufacturer. They found that repeatability of retention times, peak symmetry, column efficiency, and column permeability was good. For example, relative standard deviation (RSD) values for relative retention varied between 0.04% and 10.8% depending on the type of analyte. Since each commercial silica-based column is considered a separate batch, this study could not compare column-to-column repeatability within a single batch.
In contrast, a thorough repeatability study concerning polymer-based monolithic capillary columns has never been published. Therefore, we decided to test three batches of monolithic capillary columns prepared in-house by both thermally and photochemically initiated polymerization with monitoring of two key properties: (i) retention times for proteins and (ii) back pressure. These variables are closely related to chromatographic and porous properties, respectively. Since each batch included five columns, we could investigate both intra-batch and inter-batch variations. In addition, we also tested the long-term stability of the monolithic columns.
Our approach is adapted from a type of validation procedure largely used in the pharmaceutical industry for quality control of drugs and described in guidelines published by International Conference on the Harmonization of the Technical Requirements of Pharmaceuticals for Human Use (ICH) and US Food and Drug Administration (FDA) [28] and requirements of the Société Française des Sciences et Techniques Pharmaceutiques (SFSTP) [29–31]. Therefore, we shall not use the term reproducibility which is frequently used in the literature to characterize the ability to replicate column synthesis but would best be reserved to describe the ability to prepare equally performing columns in different locations. To avoid any confusion and to follow the spirit of the above-mentioned guidelines, we shall use the term repeatability.
2. Experimental
2.1. Chemicals
Ethylene dimethacrylate (EDMA), butyl methacrylate (BuMA), 1-propanol, 1,4-butanediol, azobisisobutyronitrile (AIBN) and 3-(trimethoxysilyl)propyl methacrylate were purchased from Aldrich (Milwaukee, WI, USA). EDMA and BuMA were distilled under reduced pressure. Acetone, ethanol and HPLC grade acetonitrile were from Fischer Scientific (Pittsburgh, PA, USA). Chromasolv water (MS purity grade), sodium hydroxide, hydrochloric acid, acetic acid, formic acid, ribonuclease A, cytochrome c, and myoglobin were obtained from Sigma (St. Louis, MO, USA).
2.2. Preparation of monolithic capillary columns
Polyimide and PTFE coated 100 μm I.D. fused silica capillaries were purchased from Polymicro Technologies (Phoenix, AZ, USA). The capillaries were rinsed with acetone, water, 200 mmol/L sodium hydroxide, water, 200 mmol/L HCl, and ethanol. Then, a 20% (w/w) solution of 3-(trimethoxysilyl)propyl methacrylate in ethanol at pH 5 adjusted using acetic acid was pumped through each capillary for 1 h at a flow rate of 0.25 μL/min using a syringe pump (KdScientific, New Hope, PA, USA). Then, the capillaries were rinsed with acetone, and finally dried in a stream of nitrogen flowing though the capillaries for at least 24 hours at room temperature.
The surface-vinylized capillaries were filled with a polymerization mixture comprising 24% BuMA, 16% EDMA, 34% 1-propanol, 26% 1,4-butanediol, and 0.4% AIBN (all w/w). After sealing the ends of the capillaries with a piece of rubber, the monoliths were prepared using thermal or photochemical initiation. The former was carried out by immersing the capillaries in a water bath kept at 50°C for 72 h, while the photoinitiated polymerizations in the PTFE coated UV transparent capillaries required irradiation in a Spectrolinker UV crosslinker (Fisher Scientific, Pittsburgh, PA, USA), using a wavelength of 365 nm at 10 J/cm2 with an exposure time of 50 min. Although the instrument does not enable active cooling, the temperature in the Spectrolinker did not exceed 30°C thus effectively excluding thermal initiation. After removing the seals, the monolithic capillary columns were washed with acetonitrile at a flow rate of 0.25 μL/min for 5–10 minutes using the LC pumping system until a stable back pressure was reached. When not in use, the columns were stored in a 1:1 water-acetonitrile mixture with their extremities immersed in vials containing water.
2.3. Chromatographic conditions
The mobile phases used throughout the experiments were 2% (v/v) formic acid in 98:2 water-acetonitrile mixture (mobile phase A) and 2% (v/v) formic acid in acetonitrile (mobile phase B). A typical gradient started from 100% mobile phase A to 50:50% A in B in 4 min, with constant mobile phase until 5.8 min, and then progressing to 100% B in 6 min. The flow rate was 4 μL/min.
The protein test mixture contained 1 μmol/L ribonuclease A, 500 nmol/L cytochrome c, and 150 nmol/L myoglobin dissolved in water. The mixture was freshly prepared at least every 4 days and kept at 15°C in the sample rack. Injection volume was 2 μL for a protein content of 2 pmol ribonuclease A, 1 pmol cytochrome c, and 300 fmol myoglobin.
2.4. Instrumentation
Chromatographic experiments were performed with a nanoAcquity UPLC system (Waters, Milford, MA, USA), enabling splitless flow rates 0.2–100 μL/min. The built-in autosampler had a 2 μL sample loop. Detection was carried out using a time-of-flight mass spectrometer (Micromass LCT, Manchester, UK) with a Picoview nanospray interface fitted with a distal coated silica tip 3 cm long and 10 μm I.D. (New Objective, Woburn, MA, USA). The capillary tip was placed ca. 5 mm from the MS entrance at an angle of about 45°. In order to get a stable spray, the capillary voltage value has to be adjusted daily in the range of 1650–1800 V. Data were acquired between 700 and 2000 Thompson.
3. Results and discussion
3.1. Separation conditions
For this study, we prepared a series of thirty 20 cm long monolithic columns with 100 μm I.D. using the same polymerization mixture with thermal initiation for fifteen of the columns and photochemical initiation for the remaining fifteen. AIBN was selected as an initiator since it can be used with both types of polymerization. Because porous polymer-based monolithic columns are best suited for the separation of large molecules, we optimized the test separation conditions to be used throughout the repeatability study for a model mixture of three proteins - ribonuclease A, cytochrome c and myoglobin. These proteins were selected since their aqueous solution is stable for more than 4 days at 15°C avoiding preparation of fresh solutions too often. This is an important issue in experiments involving thousands of injections.
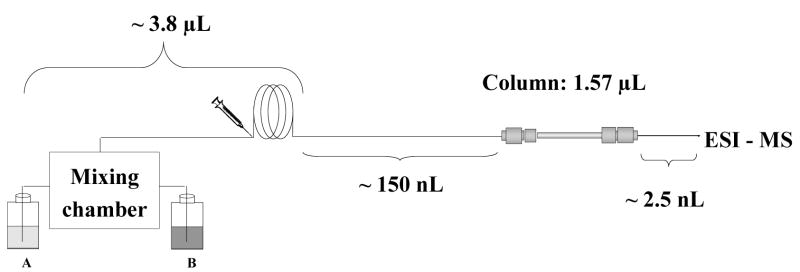
The typical flow rate affording the best efficiency in the separation of this protein mixture with a conventional 4.6 mm I.D. monolithic column is about 1 mL/min; therefore a simple calculation suggests that equivalent linear velocity would require a flow rate of ca. 500 nL/min in a 100 μm I.D. capillary. While such a low flow rate is achievable using the current advanced chromatographs, the relatively large external pre-column volumes typical of these instruments make such flow rates inconvenient for separations in gradient mode since the retention times grow significantly. For example, the volume between solvent mixer and column inlet (dwell volume) in our nanoAcquity UPLC system shown schematically in Fig. 1 is about 4 μL. At a flow rate of 500 nL/min, the time necessary for the start of the gradient to reach the column (dwell time) would be 8 min. Taking into account analysis time and column equilibration time at initial mobile phase composition, the throughput would be less than one analysis per hour.
Fig. 1.
Schematic view of distribution of extra-column volumes in LC system used in this study.
In contrast to columns packed with particulates, the mass transport of analytes within the porous structure of the monolithic stationary phase and thus the column efficiency shows little dependency on the flow rate. In addition, the high permeability of the monoliths enables the use of high flow rates at relatively low back pressures that are easily tolerated by modern HPLC systems. These properties are considered to be the major advantages of monolithic columns. They also provide a solution to the large dwell volume of our chromatographic instrument. In order to maintain a reasonably short analysis times - typically less than 10 min including column equilibration - we have to use a flow rate of 4 μL/min, which is significantly higher than the optimal flow rate mentioned above. . Despite this increase in flow velocity, the separations shown in Fig. 2 are very good.
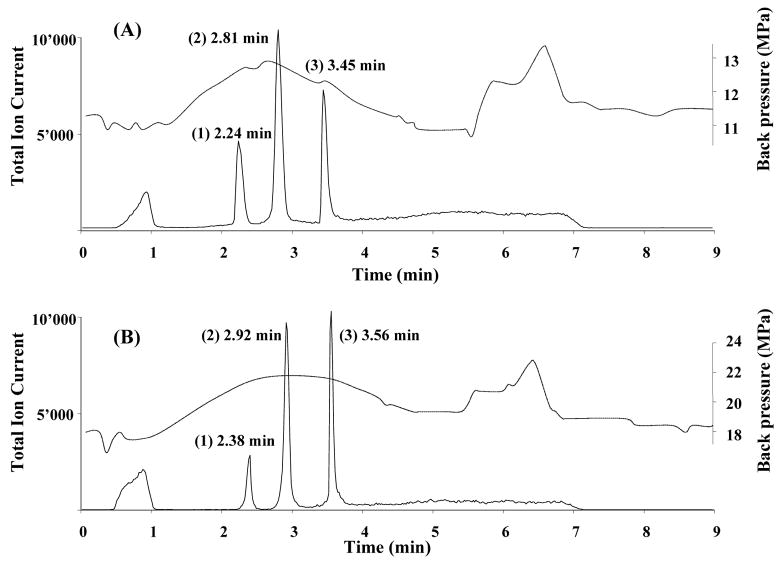
Fig. 2.
Chromatographic separation of three model proteins using monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column. Conditions: column 20 cm × 100 μm I.D. prepared by thermally (A) and photochemically (B) initiated polymerization; mobile phase A 2% formic acid in 98:2 water:acetonitrile mixture, mobile phase B 2% formic acid in acetonitrile; gradient from 100% A to 50% B in A in 4 min; flow rate 4 μL/min. Peaks: (1) ribonuclease A (2 pmol), (2) cytochrome c (1 pmol), and (3) myoglobin (0.3 pmol). Dashed line represents the overall back pressure in the system.
3.2. Comparison of thermally and photochemically polymerized monolithic columns
As indicated above, identical separation conditions were used to separate the test sample of three proteins using all thirty methacrylate-based monolithic columns prepared from the same monomer mixture via both thermally and photochemically initiated polymerization. Fig. 2 shows typical chromatograms that clearly demonstrate the very similar retention behavior of both types of columns with only a small increase (ca. 0.1–0.2 min) in the range of retention times observed with the photoinitiated columns. This finding is not surprising since both types of columns were prepared using the same polymerization mixture and therefore their chemistry is effectively the same despite the difference in temperature used in photoinitiated and thermally initiated process. Fig. 2 also presents back pressure data for the chromatographic system; this data combines the back pressure exhibited by the instrument itself with the flow resistance of the column. A simple experiment with an empty capillary allows an estimation of the contribution of the former to be made affording values ranging from 6.5 to 8.0 MPa under the gradient conditions used. Close examination reveals that, in contrast to retention, there is a significant difference in back pressures between measurements made for thermally and photochemically polymerized columns. While the peak back pressure measured with thermally polymerized columns is about 13.5 MPa, it reaches up to 23.0 MPa for the latter. This significant disparity reflects a difference in pore structure between the two types of monoliths with the higher back pressure measured for the photopolymerized monolith suggesting that its through pores are of size smaller than that for the thermally polymerized monolith [17]. Unfortunately, it would be very difficult to measure the actual pore sizes since the amount of monolithic material in the capillary is not sufficient for determination using standard methods such as mercury-intrusion porosimetry. The use of inverse size exclusion chromatography demonstrated for silica-based monoliths containing an array of micropores [32, 33] is not suitable for the determination of pore sizes in the micrometer size range that is typical of polymer-based monoliths [2, 8].
While the same polymerization mixture was used for the preparation of the two types of capillary columns, it must be pointed out that other conditions such as time and temperature of polymerization are different. Therefore, photopolymerization is carried out at a temperature not exceeding 30 °C, while the thermally initiated reaction proceeds at 50°C. This difference in polymerization temperature affects the solvency and viscosity of the porogenic solvents and therefore their function as porogens. To minimize this effect, we chose a relatively low temperature of 50 °C for the thermally initiated polymerization that is only slightly higher then the temperature used during the photoinitiated process. The photopolymerization is also much faster and we used a standard polymerization time of 50 min in contrast to the 72 h required to obtain the monolith via thermally initiated polymerization. The shorter time used in the photopolymerization reflects the more rapid formation of free radicals in the system. This effect is similar to a polymerization that would be thermally initiated at a higher temperature. Indeed, we demonstrated several years ago that an increase in polymerization temperature leads to the formation of monoliths with smaller pores [34]. Although such was not the aim of this study, it is possible to vary the composition of the polymerization mixture to obtain monoliths with similar porous properties. However, changes in composition would no doubt also alter the retention characteristics of the monolithic columns and thus direct comparisons would not be accurate.
3.3. Stability of monolithic columns
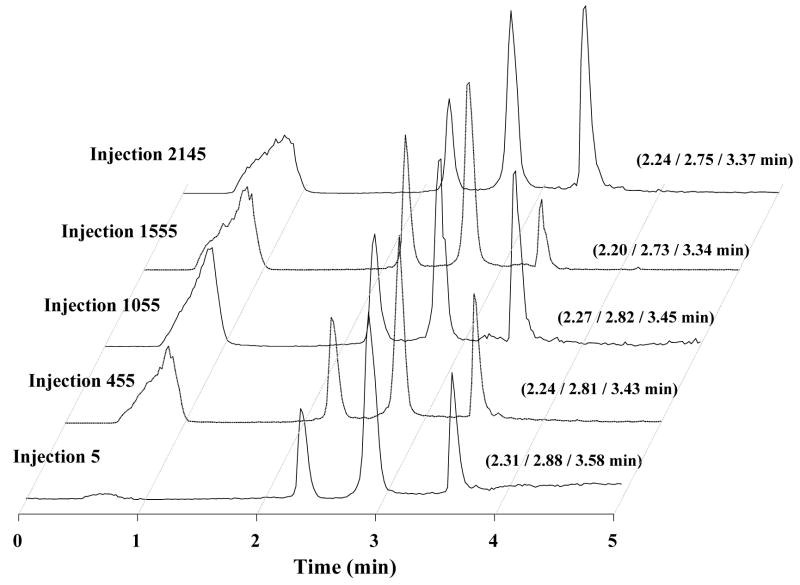
This property, important for chromatographic columns in general, was assessed for both thermally and photochemically initiated monoliths using repeated injections of the protein mixture in highly acidic mobile phase (pH 2) while monitoring the retention times of each separated component. Fig. 3, showing chromatograms randomly picked every 500 or so injections in a series of over 2000 injections in a monolithic column prepared via thermal polymerization, clearly documents the good repeatability of retention times. In order to perceive any possible shift in retention times, we analyzed the first 15 chromatograms after every 150 successive injections to calculate the mean values. Confidence intervals c.i. were calculated using the following equation:
| (1) |
where, t(df;0.05) corresponds to the Student value for a certain degree of freedom df at a risk of 5%, s2 is the variance, and n is the number of data points for each c.i. In our case, values 2.14 and 15, respectively, were used for t(14;0.05) and n. Fig. 4 presents the results obtained for all three proteins.
Fig. 3.
Chromatograms illustrating stability of monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column during multiple successive injection of a mixture of ribonuclease A (peak 1), cytochrome c (peak 2), and myoglobin (peak 3). For experimental conditions see Fig. 2.
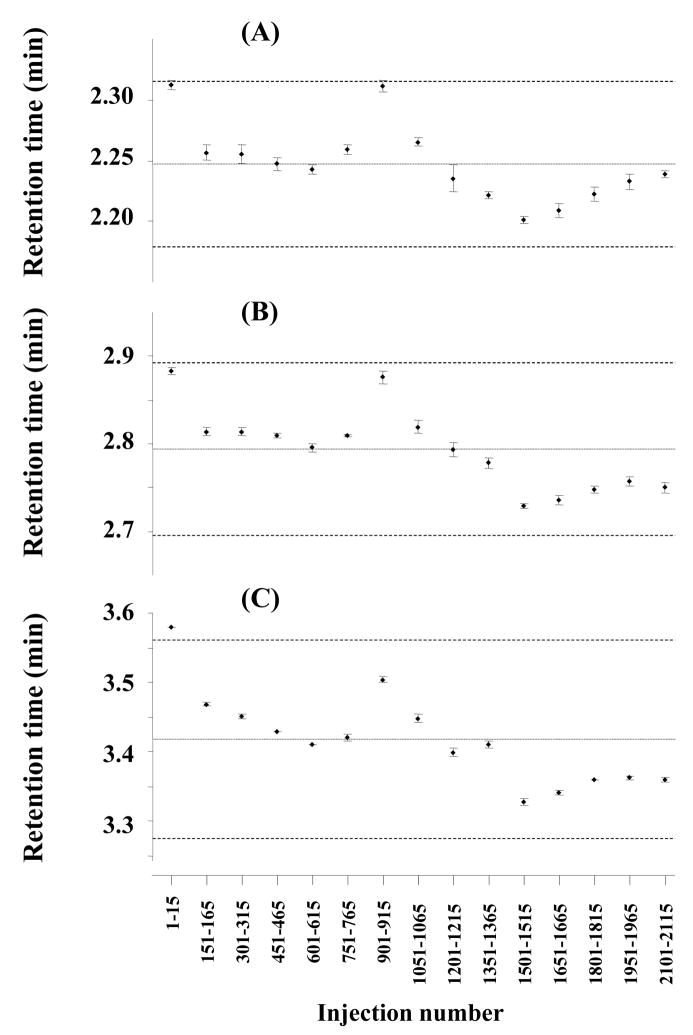
Fig. 4.
Variation in retention times observed for the separations of ribonuclease A (A), cytochrome c (B), and myoglobin (C) during 2200 injections using monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column prepared via thermally initiated polymerization. Each data point represents the mean of 15 successive injections shown below and its confidence interval at a risk of 5%. Evaluated separations: [1–15], [151–165], [301–315], [451–465], [601–615], [751–765], [901–915], [1051–1065], [1201–1215], [1351–1365], [1501–1515], [1651–1665], [1801–1815], [1951–1965], and [2101–2115]. Means, variances, and confidence intervals are reported in Table 1. For experimental conditions see Fig. 2.
Each point shown in Fig. 4 corresponds to the mean and confidence interval of retention time obtained from 15 successive injections. The very narrow confidence interval of less than 0.01 min with almost all data within the confidence limits suggests that the arithmetic means do not exhibit statistically significant differences and demonstrates the very good stability of retention times in successive analyses. In contrast, the dispersion increases if instead of 15 all 2200 injections are taken into account. This interval is confined by the broken lines in Fig. 4 and reported in Table 1. Considering that these chromatographic runs were carried out over a period of one month using columns that could not be thermostated and the temperature of the laboratory was not controlled using fresh mobile phases and protein solutions prepared every four days, the larger confidence interval of retention times of 0.2–0.3 min is not likely to result from the deterioration of the monolithic column. In fact, such performance decay would lead a continuous change in retention time while Fig. 4 shows a random distribution of the means. Furthermore, no significant changes in back pressure were monitored and the shape and values of the back pressure did not differ from those shown in Fig. 2 for all of the 2200 separations. Therefore, our monolithic columns prepared via thermally initiated polymerization can safely be considered to be perfectly stable. In addition, no clogging with the proteins or decomposition of the stationary phase could be observed even after so many separations. Table 1 reports means and statistical deviation for retention times of all three proteins over time. The relative standard deviations (RSD) remain small and do not exceed 2%.
Table 1.
Retention times of three model proteins monitored over one month on a single monolithic column prepared using thermally initiated polymerization.
| Ribonuclease A | Cytochrome c | Myoglobin | |
|---|---|---|---|
| Mean, min (n = 15) | 2.25 | 2.79 | 3.42 |
| Inferior/superior limit at 5% risk, min | 2.18 – 2.32 | 2.70 – 2.89 | 3.28 – 3.56 |
| Variance, min2 | 1.0×10−3 | 2.1×10−3 | 4.5×10−3 |
| RSD, % (n = 15) | 1.4 | 1.7 | 2.0 |
Conditions: monolithic capillary column 20 cm × 100 μm I.D.; mobile phase A 2% formic acid in 98:2 water-acetonitrile mixture, mobile phase B 2% formic acid in acetonitrile; gradient from 100% A to 50% B in A in 4 min; flow rate 4 μL/min. Initial data were retrieved from chromatogram clusters shown in Fig. 4.
Similarly, stable retention times of tested proteins featuring a RSD less than 2%, were observed even after 500 subsequent separations for a monolithic column prepared via photopolymerization. However, a continuous increase in the back pressure was observed during these separations ultimately leading to pressure exceeding the 34.5 MPa limit tolerated by our chromatographic system. As the photopolymerization approach leads to a smaller overall pore size profile, clogging of the smaller through pores is more likely to occur ultimately leading to a clear deterioration of the column permeability.
3.4. Repeatability of the preparation
We tested the repeatability of the column preparation process on two levels: (i) intra-batch (column-to-column) and (ii) inter-batch (batch-to-batch). Overall, we prepared three separate batches of five columns for each of the polymerization processes (thermally or photochemically initiated) for a total of thirty columns. A new polymerization mixture was prepared for each of the six batches. Two parameters were selected to assess the repeatability of the fabrication process: (i) the retention times of proteins separated in the gradient mode and (ii) the back pressure across the capillary column using the injection mobile phase consisting of 2% formic acid in 98:2 water-acetonitrile mixture. We measured the back pressure at various flow rates in the range 1–8 μL/min and found a linear increase in back pressure with flow rate (data not shown) similar to that reported previously [17,35]. We choose a flow rate of 4 μL/min for our repeatability study since that flow rate was also used for the measurements of repeatability of retention characteristics.
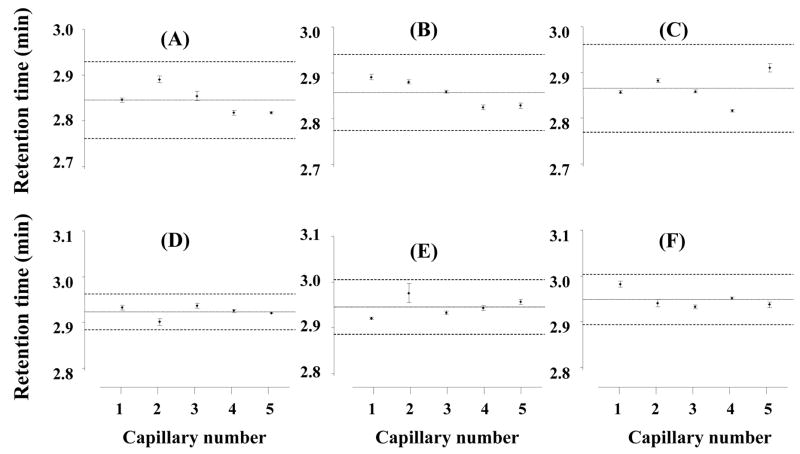
Fifteen successive injections and separations of the protein mixture were carried out using each column and means, variances, confidence interval and RSD values, all shown in Table 2, were calculated from the retention times. The RSD values are generally very low ranging from 0.8 to 1.4% for columns prepared using thermal polymerization and from 0.5 to 0.7% for photopolymerized columns. As for the stability study, fresh mobile phase preparation and fluctuation in room temperature appear to be the main reasons for the slight column-to-column changes. Statistically identical variances of retention times confirmed by Fisher tests of variances shown in Tables 1 and 2 confirm the good repeatability of the column fabrication process. As shown in Fig. 5 and confirmed by the ANOVA test, batch-to-batch repeatability is also very good.
Table 2.
Retention times for three model proteins using 30 different monolithic columns prepared via thermally and photochemically initiated polymerization.
| Thermal initiation | Photochemical initiation | |||||
|---|---|---|---|---|---|---|
| Ribonuclease A | Cytochrome c | Myoglobin | Ribonuclease A | Cytochrome c | Myoglobin | |
| Mean, min (n = 15) | 2.31 | 2.86 | 3.51 | 2.39 | 2.94 | 3.58 |
| Inferior/superior limit at 5% risk, min | 2.27 – 2.35 | 2.79 – 2.92 | 3.40 – 3.62 | 2.36 – 2.43 | 2.89 – 2.98 | 3.54 – 3.62 |
| Variance, min2 | 3.0×10−4 | 9.3×10−4 | 2.6×10−3 | 2.6×10−4 | 4.4×10−4 | 3.8×10−4 |
| RSD, % (n = 15) | 0.8 | 1.1 | 1.4 | 0.7 | 0.7 | 0.5 |
Conditions: monolithic capillary column 20 cm × 100 μm I.D.; mobile phase A 2% formic acid in 98:2 water-acetonitrile mixture, mobile phase B 2% formic acid in acetonitrile; gradient from 100% A to 50% B in A in 4 min; flow rate 4 μL/min.
Fig. 5.
Batch-to-batch variation of retention times for cytochrome c using 20 cm × 100 μm I.D. monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column prepared using thermally (A, B, and C for batches 1–3, respectively) and photochemically (D, E, and F for batches 1–3, respectively) initiated polymerization. Each batch consisted of 5 columns and 15 separations were carried out with each column. For experimental conditions see Fig. 2.
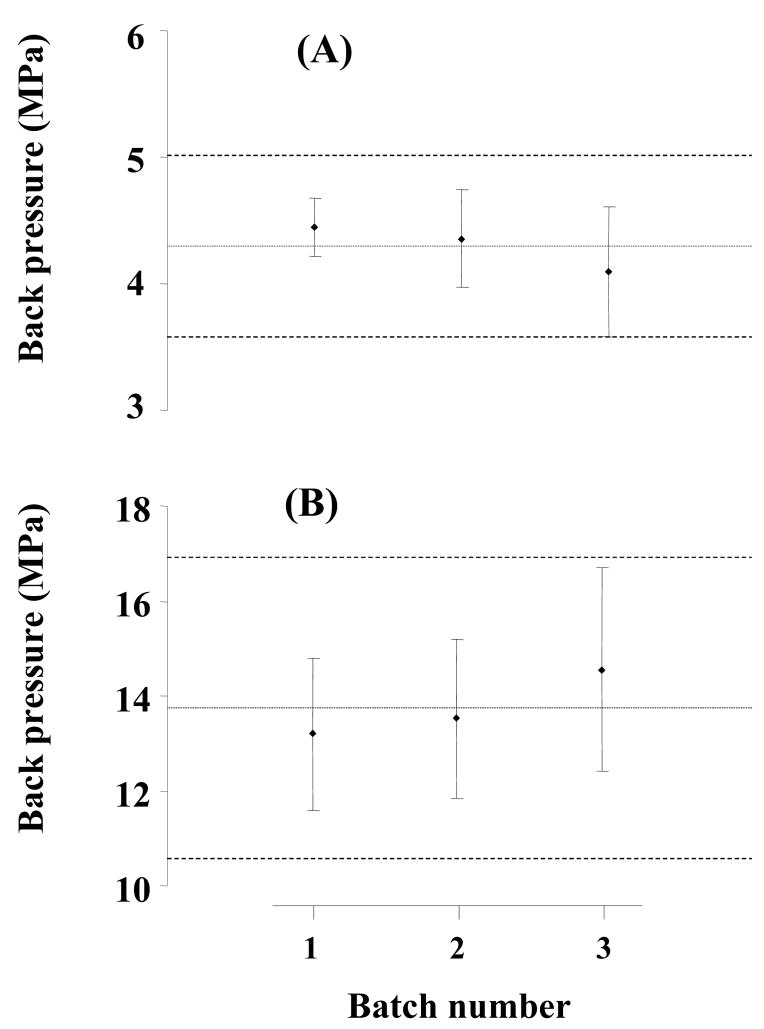
Stability of a column and repeatability of retention times during separations are critical in the evaluation of chromatographic properties. However, these parameters alone do not completely characterize the repeatability of the preparation of monolithic columns. For example, Fig. 2 illustrates that only minor changes of 0.1 min are observed for retention times in monolithic capillary columns prepared using both thermal and photoinitiated polymerization. However, a significant difference is monitored for permeability to flow. While the thermally polymerized column exhibits a back pressure of 13.5 MPa, this value almost doubles to 23.0 MPa for a monolithic column prepared using photopolymerization. Therefore, we selected back pressure in addition to retention as another metrics to describe differences between all of the columns prepared in this study. Table 3 and Fig. 6 show that the variations in back pressure for all six series of columns prepared are characterized by RSD values ranging from 4 to 12% (Table 3). Interestingly, the batch-to-batch values are very similar to those found for column-to-column variations shown in Fig. 6 and also confirmed by the ANOVA test. Therefore, all 15 columns within each batch (i.e. the thermally or photopolymerized series) can be considered as equivalent, and the overall mean for the columns is characterized by RSD values of 7.8 and 10.8% for the thermal and photopolymerized series, respectively. These values are similar to those found for standard packed chromatographic columns.
Table 3.
Back pressure for 30 different monolithic columns prepared via thermally and photochemically initiated polymerization.
| Thermal initiation | Photochemical initiation | |||||
|---|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | Batch 1 | Batch 2 | Batch 3 | |
| Mean, MPa (n = 5) | 4.45 | 4.36 | 4.09 | 13.19 | 13.52 | 14.56 |
| Inferior/superior limit at 5% risk, MPa | 4.21 – 4.68 | 3.97 – 4.75 | 3.58 – 4.60 | 11.59 – 14.79 | 11.85 – 15.20 | 12.43 – 16.69 |
| Variance, MPa2 | 0.036 | 0.098 | 0.171 | 1.658 | 1.815 | 2.937 |
| RSD, % (n = 5) | 4.2 % | 7.2 % | 10.1 % | 9.8 % | 10.0 % | 11.8 % |
| Total mean with confidence interval, and RSD (n = 15) | 4.30 ± 0.72 MPa (RSD: 7.8 %) | 13.76 ± 3.18 MPa (RSD: 10.8 %) | ||||
Conditions: monolithic capillary column 20 cm × 100 μm I.D.; mobile phase 2% formic acid in 98:2 water-acetonitrile mixture; flow rate 4 μL/min.
Fig. 6.
Batch-to-batch repeatability of back pressure for 20 cm × 100 μm I.D. monolithic poly(butyl methacrylate-co-ethylene dimethacrylate) capillary column. Each batch consisted of 5 columns.
4. Conclusions
This study clearly demonstrates that the preparation of methacrylate-based monolithic capillary columns is highly repeatable whether preparation is achieved by thermal or photochemical initiation. Only minimal column-to-column and batch-to-batch differences in retention characteristics of these monolithic columns were observed in the LC-ESI-MS separations of proteins. Retention did not change even after more than 2200 protein separations. Since the quality of separation is little affected by flow velocity, these columns can be used at high flow rates without compromising resolution. This aspect enables a significant acceleration of the separations and enables high throughput analyses. All these properties are promising for a broader application of porous polymer monoliths in high-end separations such as those typical of proteomic or genomic research. The simplicity of the in-situ preparation process also makes the monolithic columns excellent candidates for use in miniaturized devices such as narrow diameter capillaries and microfluidic chips.
Acknowledgments
Support of L.G. by a grant of the National Institute of General Medical Sciences, National Institutes of Health (GM44885) is gratefully acknowledged. Work at the Molecular Foundry was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, of the U.S. Department of Energy under Contract No. DE-AC02-05CH11231.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Svec F, Fréchet JMJ. Anal Chem. 1992;54:820. [Google Scholar]
- 2.Wang Q, Svec F, Fréchet JMJ. Anal Chem. 1993;65:2243. doi: 10.1021/ac00065a013. [DOI] [PubMed] [Google Scholar]
- 3.Josic D, Buchacher A, Jungbauer A. J Chromatogr B. 2001;752:191. doi: 10.1016/s0378-4347(00)00499-0. [DOI] [PubMed] [Google Scholar]
- 4.Svec F. J Sep Sci. 2004;27:1419. doi: 10.1002/jssc.200401825. [DOI] [PubMed] [Google Scholar]
- 5.Gu B, Armenta JM, Lee ML. J Chromatogr A. 2005;1079:382. doi: 10.1016/j.chroma.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 6.Sykora D, Svec F, Fréchet JMJ. J Chromatogr A. 1999;852:297. doi: 10.1016/s0021-9673(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 7.Premstaller A, Oberacher H, Huber CG. Anal Chem. 2000;72:4386. doi: 10.1021/ac000283d. [DOI] [PubMed] [Google Scholar]
- 8.Oberacher H, Premstaller A, Huber CG. J Chromatogr A. 2004;1030:201. doi: 10.1016/j.chroma.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Petro M, Svec F, Fréchet JMJ. J Chromatogr A. 1996;752:59. doi: 10.1016/s0021-9673(96)00510-9. [DOI] [PubMed] [Google Scholar]
- 10.Petro M, Svec F, Gitsov I, Fréchet JMJ. Anal Chem. 1996;68:315. doi: 10.1021/ac950726r. [DOI] [PubMed] [Google Scholar]
- 11.Janco M, Sykora D, Svec F, Fréchet JMJ, Schweer J, Holm R. J Polym Sci Polymer Chem. 2000;38:2767. [Google Scholar]
- 12.Premstaller A, Oberacher H, Walcher W, Timperio AM, Zolla L, Chervet JP, Cavusoglu N, van Dorsselaer A, Huber CG. Anal Chem. 2001;73:2390. doi: 10.1021/ac010046q. [DOI] [PubMed] [Google Scholar]
- 13.Walcher W, Oberacher H, Troiani S, Holzl G, Oefner PJ, Zolla L, Huber CG. J Chromatogr B. 2002;782:111. doi: 10.1016/s1570-0232(02)00667-0. [DOI] [PubMed] [Google Scholar]
- 14.Mayr B, Hoelzl G, Eder K, Buchmeiser MR, Huber CG. Anal Chem. 2002;74:6080. doi: 10.1021/ac025919a. [DOI] [PubMed] [Google Scholar]
- 15.Huang X, Zhang S, Schultz GA, Henion JD. Anal Chem. 2002;74:2336. doi: 10.1021/ac011202w. [DOI] [PubMed] [Google Scholar]
- 16.Ivanov AR, Zang L, Karger BL. Anal Chem. 2003;75:5306. doi: 10.1021/ac030163g. [DOI] [PubMed] [Google Scholar]
- 17.Lee D, Svec F, Fréchet JMJ. J Chromatogr A. 2004;1051:53. doi: 10.1016/j.chroma.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 18.Gatschelhofer C, Magnes C, Pieber TR, Buchmeiser MR, Sinner FM. J Chromatogr A. 2005;1090:81. doi: 10.1016/j.chroma.2005.06.098. [DOI] [PubMed] [Google Scholar]
- 19.Courtois J, Bystrom E, Irgum K. Polymer. 2006;47:2603. [Google Scholar]
- 20.Tsuda T. Chromatography. 2000;21:1. [Google Scholar]
- 21.Hancock WS, Wu SL, Shieh P. Proteomics. 2002;2:352. doi: 10.1002/1615-9861(200204)2:4<352::AID-PROT352>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Shen YF, Smith RD. Electrophoresis. 2002;23:3106. doi: 10.1002/1522-2683(200209)23:18<3106::AID-ELPS3106>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Wang H, Hanash S. J Chromatogr B. 2003;787:11. doi: 10.1016/s1570-0232(02)00335-5. [DOI] [PubMed] [Google Scholar]
- 24.Svec F, Geiser L. LC-GC N Am. 2006;24:22. [Google Scholar]
- 25.Andersen T, Nguyen QNT, Trones R, Greibrokk T. Analyst. 2004;129:191. doi: 10.1039/b314548d. [DOI] [PubMed] [Google Scholar]
- 26.Jiang T, Jiskra J, Claessens HA, Cramers CA. J Chromatogr A. 2001;923:215. doi: 10.1016/s0021-9673(01)00982-7. [DOI] [PubMed] [Google Scholar]
- 27.Kele M, Guiochon G. J Chromatogr A. 2002;960:19. [Google Scholar]
- 28.International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use (ICH) . 1994 http://www.fda.gov/cder/guidance.
- 29.Caporal-Gautier J, Nivet JM, Algranti P, Guilloteau M, Histe M, Lallier M, N’Guyen-Huu JJ, Russeto R. S T P Pharma Pratiques. 1992;2:205. [Google Scholar]
- 30.Caporal-Gautier J, Nivet JM, Algranti P, Guilloteau M, Histe M, Lallier M, N’Guyen-Huu JJ, Russeto R. S T P Pharma Pratiques. 1992;2:227. [Google Scholar]
- 31.Hubert P, N’Guyen-Huu JJ, Boulanger B, Chapuzet E, Chiap P, Cohen N, Compagnon PA, Dewe W, Feinberg M, Lallier M, Laurentie M, Mercier N, Muzard G, Nivet JM, Valat L. S T P Pharma Pratiques. 2003;13:101. [Google Scholar]
- 32.Al Bokari M, Cherrak D, Guiochon G. J Chromatogr A. 2002;975:275. doi: 10.1016/s0021-9673(02)01271-2. [DOI] [PubMed] [Google Scholar]
- 33.Lubda D, Lindner W, Quaglia M, Hohenesche C, Unger KK. J Chromatogr A. 2005;1083:14. doi: 10.1016/j.chroma.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 34.Svec F, Fréchet JMJ. Macromolecules. 1995;28:7580. [Google Scholar]
- 35.Viklund C, Svec F, Fréchet JMJ, Irgum K. Chem Mater. 1996;8:744. [Google Scholar]