Abstract
OBJECTIVES
To determine whether an objective measure of daytime movement is associated with better cognitive function in women in their 80s.
DESIGN, SETTING AND PARTICIPANTS
Cross-sectional study of 2,736 older women without evidence of dementia participating in a study of health and aging.
MEASUREMENTS
Daytime movement was assessed with actigraphy—a watch-like device that objectively quantifies accelerometer motion—over a mean of 3.0 ± 0.8 days. Cognitive function was measured by the Trail-Making Test, Part B (Trails B) and the Mini-Mental State Examination (MMSE). Cognitive impairment was defined as performing > 1.5 standard deviations (SD) worse than the mean on a given test.
RESULTS
Women had a mean age of 83 ± 4 years; 10% were African American. After adjustment for age, race and education, women in the highest versus lowest movement quartiles had better mean cognitive test scores (20 seconds [0.3 SD] faster on Trails B and 0.3 [0.2 SD] points higher on MMSE, both p<0.001) and were less likely to be cognitively impaired (Odds Ratio [95% Confidence Interval]: 0.61 [0.41 to 0.92] for Trails B and 0.68 [0.44 to 1.07] for MMSE). Associations were similar in different subgroups and were independent of self-reported walking, medical comorbidities, physical function and other healthy behaviors.
CONCLUSION
Daytime movement as measured objectively using actigraphy was associated with better cognitive function and lower odds of cognitive impairment in women in their 80s. Additional studies are needed to clarify the direction of the association and to explore potential mechanisms.
Keywords: movement, exercise, activity, cognition, risk factor
INTRODUCTION
Several observational studies in humans have found that elders who are more ‘active’ have higher levels of cognitive function and a lower risk of cognitive impairment and dementia.(1-8) However, these studies have generally relied upon self-report to determine activity levels, which raises concern about reporting or recall bias. In addition, questionnaires tend to ask about a limited number of specific activities and may not adequately capture the total amount of activity that elders engage in on a daily basis. Finally, most prior studies have not included large numbers of subjects in their 80s, who are currently the fastest growing segment of our population.(9)
Actigraphy is a simple technique for objectively quantifying a person's wrist movements. It consists of a watch-like device that is worn on the wrist and provides a continuous measure of accelerometer motion. Actigraphy has historically been used to quantify sleep/wake activity,(10-12) but can also be used to measure movements during the day.(13-15) Because the actigraph is worn on the wrist, it captures a wide range of activities that may include physical (e.g., moving arms while walking or gardening), mental (e.g., playing bridge), and social (e.g., volunteer work) as well as other daily activities (e.g., shopping, cooking, cleaning) and non-specific activities such as fidgeting. It therefore provides an integrated, objective measure of a person's total daytime movements.
We have previously found that actigraphically-measured nighttime movement that results in sleep disturbance is associated with increased risk of cognitive impairment.(16) The objective of our current study was to determine whether actigraphically-measured daytime movement is associated with better cognitive function and lower risk of cognitive impairment in women in their 80s.
METHODS
Participants
The study population included women participating in the Study of Osteoporotic Fractures (SOF).(17) SOF is a multicenter, prospective, observational study of women who were age 65 years or older at enrollment and were recruited from four metropolitan areas in the U.S.: Baltimore, MD; Minneapolis, MN; Portland, OR and Monongahela Valley, PA. At the baseline visit, women were excluded if they were unable to walk without help or had a previous bilateral hip replacement. The SOF study enrolled 9704 women from September 1986 to October 1988. The initial study population included primarily white women due to their high incidence of hip fractures; an additional 662 African American women were enrolled from February 1997 to February 1998 (total N = 10,366).
A total of 4,727 women (84% of active survivors) participated in visit 8, which took place between January 2002 and February 2004. Of these, 1,051 participated by self-administered questionnaire only, 233 did not have cognitive tests performed, 437 did not have any actigraphy data, 119 did not have adequate actigraphy data to calculate mean daytime movement and 151 were excluded due to evidence of possible dementia (defined as self-report of a dementia diagnosis, Mini Mental State Examination score < 24, or use of dementia medication). Thus our final sample consisted of 2,736 women without evidence of dementia who had both cognitive function and actigraphy data. Women included in our analysis were younger; had more education, fewer functional difficulties and lower depressive symptom scores; walked more; and were less likely to have a history of stroke or hypertension compared to those who were excluded. However, the groups did not differ by race or history of most chronic diseases (diabetes, cardiovascular disease, cancer, arthritis or chronic lung disease).
The institutional review boards at each clinic site approved the study, and written informed consent was obtained from all participants.
Daytime Movement
Daytime movement was measured using watch-like actigraphs (Sleepwatch-O®, Ambulatory Monitoring Inc, Ardsley, NY), which women wore on their non-dominant wrists. The actigraphs recorded activity as accelerometer motion gathered continuously and summarized over 1-minute epochs. Data were collected in three modes: zero crossings (ZCM), digital integration (DIM, also known as proportional integration mode), and time above threshold (TAT).(18) The DIM mode was used in this analysis because it is recommended by the manufacturer for estimating energy expenditure and vigor of motion.(18) The DIM mode computes movement as counts per minute based on an area under the curve analysis that takes into account both intensity and frequency of movement. Since the focus of this study was daytime movement, we excluded movement that occurred when subjects reported in sleep diaries that they were in bed at night; periods of daytime inactivity (e.g., scored as napping by the DIM algorithm or recorded as naps in diaries) were not excluded. Further details of the actigraphy data collection and scoring have been described previously.(19) Analyses were based on the mean number of daytime movement counts per minute over all of the days on which the actigraph was worn. We also assessed self-reported walking activity by asking subjects whether they walked for exercise and the number of blocks walked per day for exercise or as part of their normal routine.
Cognition
Two cognitive function tests, the Trail Making Test Part B (Trails B) and the Mini-Mental State Exam (MMSE), were administered by trained clinic staff. Trails B is a timed test that measures attention, sequencing, visual scanning and executive function.(20) A faster time for completion (in seconds) represents better cognitive functioning. The MMSE is a global measurement of cognitive function, with components for orientation, concentration, language, praxis, and immediate and delayed memory. MMSE scores may range from 0−30, with higher scores representing better cognitive functioning.(21) To examine clinically meaningful differences in cognitive function, we also classified subjects as having evidence of cognitive impairment if their cognitive test scores were more than 1.5 standard deviations worse than the mean on a given test (Trails B > 266 seconds, MMSE < 26 points).
Other Measurements
Trained interviewers administered a detailed questionnaire that included questions about age, race, education, alcohol intake during the previous 30 days, current smoking status, and history of comorbid health conditions (cancer, stroke, diabetes mellitus, hypertension, chronic obstructive pulmonary disease [COPD], cardiovascular disease [CVD], and arthritis). COPD was defined as self-report of having ever been diagnosed with chronic bronchitis, asthma, emphysema or COPD. CVD was defined as self-report of having ever been diagnosed with myocardial infarction, angina, congestive heart failure or other heart disease. Overall health compared to other women was self-rated as excellent, good, fair, poor or very poor. The Geriatric Depression Scale (GDS) was used to assess depressive symptoms, with high depressive symptoms defined using the standard cutpoint of 6 or more.(22) Number of functional limitations was determined by asking subjects whether they had difficulty with any of six instrumental activities of daily living (IADL), which included walking two or three blocks on level ground, climbing up 10 steps without resting, preparing meals, doing heavy housework, shopping for groceries or clothes and walking down 10 steps.(23, 24) Body weight and height were measured,(25) and body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
Subject characteristics and cognitive test scores were summarized by daytime movement quartile using means or medians for continuous variables and percents for categorical variables. P-values for differences between daytime movement quartiles were calculated using analysis of variance for normally distributed continuous data, Kruskal Wallis tests for skewed data and Chi-square tests for categorical data.
The Loess method was used to examine whether the association between daytime movement and cognitive function was approximately linear. This method graphically determines the shape of the function that best fits a scatterplot of two continuous variables.(26) We also formally tested for non-linearity in our linear regression models by including quadratic terms for daytime movement (i.e., examining mean cognitive test scores as a function of age, daytime movement and daytime movement squared). Because the Loess plots suggested non-linearity with a change in slope at approximately the median, and the quadratic terms were statistically significant for both Trails B (p<0.001) and MMSE (p=0.02), we performed all subsequent analyses with daytime movement categorized into quartiles.
Linear regression models and the least squared means procedure were used to examine relationships between daytime movement quartile and mean cognitive test scores. Both Trails B and MMSE scores were log-transformed to meet normality assumptions for the models and then back-transformed for display of results. Logistic regression models were used to examine relationships between daytime movement quartile and odds of cognitive impairment with results presented as odds ratios (OR) and 95% confidence intervals (CI).
We performed unadjusted linear and logistic regression analyses and also performed a series of adjusted models. Model 1 included age, race and education. We selected these factors for our primary model because they have been strongly associated with poor cognitive function in prior studies and cannot be changed by past or present daytime movement; therefore, we felt that these models were unlikely to be ‘overadjusted’ by factors that might lie on the causal pathway or be correlated with factors on the causal pathway.
In Model 2, we determined the extent to which the association between daytime movement and cognitive function was due to self-reported walking by further adjusting for the total number of blocks a woman reported walking per day. We also tested for interactions between daytime movement and whether or not subject walked for exercise.
Finally, in Model 3, we explored the factors that may have been underlying the association between daytime movement and cognitive function by further adjusting for factors that were significantly associated with poor cognitive function or increased risk of cognitive impairment after adjustment for age and could potentially change as a result of past or present daytime movement. These included depression, comorbid medical conditions (stroke, hypertension, diabetes), number of IADL impairments, self-rated health, BMI, smoking, and alcohol consumption. We also tested for interactions between these factors and daytime movement quartile.
All analyses were performed using SAS software, version 9.1 (SAS Institute, INC., Cary, NC).
RESULTS
Overall, women had a mean ± standard deviation (SD) age of 83 ± 4 years and 13 ± 3 years of education; 10% were African American. Women reported being out of bed for 15.2 ± 1.3 hours per day and wore the actigraphs for a mean of 3.0 ± 0.8 24-hour days. The mean ± SD (range) number of actigraphically-measured daytime counts per minute was 3608 ± 826 (1022 to 6790). The mean ± SD cognitive test scores were 154 ± 75 for Trails B and 28.1 ± 1.5 points for MMSE.
Women who moved more during the day were more likely to be younger; be African American; lack a history of stroke, diabetes, hypertension, COPD, CVD, or arthritis; be non-smokers; drink more alcohol; have lower depressive symptoms; have fewer IADL impairments; have a lower BMI; and to report good/excellent self-reported health (Table 1). Daytime movement was not associated with education or history of cancer.
Table 1.
Characteristics of 2,736 Older Nondemented Women by Daytime Movement Quartile
| |
Daytime Movement |
|
|||
|---|---|---|---|---|---|
| Characteristic: | Quartile 1 <3037 (n=684) | Quartile 2 3037 to <3607 (n=684) | Quartile 3 3607 to <4150 (n=684) | Quartile 4 ≥ 4150 (n=684) | p-value* |
| Age, yrs, mean ± SD | 84.1 ± 3.9 | 83.7 ± 3.5 | 83.2 ± 3.6 | 82.6 ± 3.5 | <0.001 |
| Education, yrs, mean ± SD | 13.0 ± 2.6 | 12.9 ± 2.6 | 12.9 ± 2.6 | 12.8 ± 2.8 | 0.46 |
| African American, N (%) | 64 (9.4) | 53 (7.8) | 71 (10.4) | 86 (12.6) | 0.03 |
| Cancer, N (%) | 155 (22.7) | 160 (23.4) | 163 (23.9) | 141 (20.6) | 0.49 |
| Stroke, N (%) | 120 (17.5) | 85 (12.5) | 67 (9.8) | 61 (8.9) | <0.001 |
| Diabetes, N (%) | 112 (16.4) | 82 (12.0) | 58 (8.5) | 47 (6.9) | <0.001 |
| Hypertension, N (%) | 450 (65.8) | 429 (62.8) | 413 (60.5) | 364 (53.3) | <0.001 |
| COPD, N (%) | 110 (16.1) | 79 (11.6) | 70 (10.3) | 83 (12.2) | 0.008 |
| Cardiovascular Disease, N (%) | 253 (37.0) | 222 (32.5) | 227 (33.2) | 180 (26.4) | <0.001 |
| Arthiritis, N (%) | 330 (48.3) | 302 (44.2) | 291 (42.6) | 262 (38.4) | 0.003 |
| Current smoker, N (%) | 27 (4.0) | 17 (2.5) | 17 (2.5) | 9 (1.3) | 0.023 |
| Average drinks per day in the last 30 days | 0.4 ± 0.7 | 0.5 ± 0.7 | 0.6 ± 0.7 | 0.6 ± 0.8 | <0.001 |
| High depressive symptoms (GDS>6), N(%) | 109 (15.9) | 74 (10.8) | 67 (9.8) | 45 (6.6) | <0.001 |
| Number of IADL impairments (0 to 6), mean ± SD | 2.2 ± 2.1 | 1.5 ± 1.8 | 1.2 ± 1.6 | 0.8 ± 1.3 | <0.001 |
| Body mass index, k/m2, mean ± SD | 28.7 ± 5.6 | 27.2 ± 4.9 | 26.6 ± 4.5 | 26.1 ± 4.3 | <0.001 |
| Good/excellent self-reported health, N (%) | 459 (67.1) | 511 (74.8) | 540 (79.1) | 574 (84.0) | <0.001 |
Daytime activity was measured as mean counts per minute. COPD, chronic obstructive pulmonary disease; GDS, Geriatric Depression Scale, IADL, instrumental activities of daily living.
p-values are based on analysis of variance for normally distributed continuous data, Kruskall-Wallis tests for skewed continuous data, and χ2 tests for categorical data.
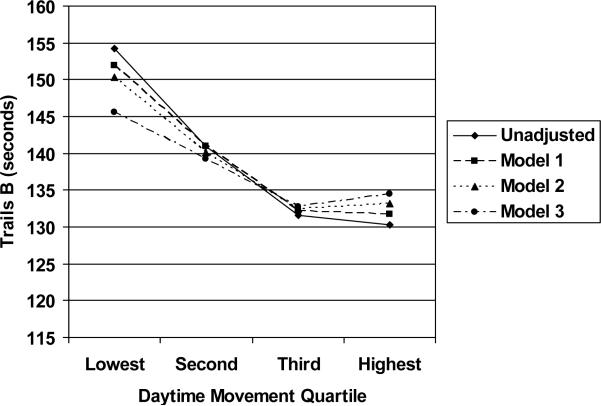
After adjustment for age, race and education, women in the highest movement quartile were 20 seconds (0.27 SD) faster on Trails B (Figure 1, Model 1) and scored 0.3 points (0.20 SD) higher on the MMSE compared to those in the lowest quartile (both p<0.001). In addition, their odds of cognitive impairment were reduced by 39% on Trails B and 32% on the MMSE (Table 2).
Figure 1.
Trails B Scores by Daytime Movement Quartile in Unadjusted and Adjusted Analyses. Mean scores on the Trail-Making Test, Part B (Trails B) were significantly lower (faster) in the second, third and highest movement quartiles compared to the lowest movement quartile in unadjusted and adjusted analyses. Model 1: adjusted for age, race and education. Model 2: adjusted for Model 1 variables plus blocks walked per day. Model 3: adjusted for Model 2 variables plus body mass index, self-rated health, high depressive symptoms, alcohol consumption, diabetes, hypertension, stroke, number of instrumental activities of daily living difficulties, and current smoking.
Table 2.
Daytime Movement Quartile and Cognitive Impairment
| Trails B |
MMSE |
|||||
|---|---|---|---|---|---|---|
| Daytime Movement | Number (%) Impaired | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Number (%) Impaired | Unadjusted OR (95% CI) | Adjusted* OR (95% CI) |
| Quartile 1 | 68 (10.5) | 1.00 (ref) | 1.00 (ref) | 54 (8.1) | 1.00 (ref) | 1.00 (ref) |
| Quartile 2 | 60 (9.2) | 0.86 (0.60 to 1.24) | 0.90 (0.62 to 1.30) | 44 (6.6) | 0.81 (0.54 to 1.22) | 0.84 (0.55 to 1.28) |
| Quartile 3 | 33 (5.0) | 0.45 (0.29 to 0.70) | 0.46 (0.30 to 0.72) | 43 (6.3) | 0.77 (0.51 to 1.17) | 0.81 (0.53 to 1.24) |
| Quartile 4 | 43 (6.5) | 0.60 (0.40 to 0.89) | 0.61 (0.41 to 0.92) | 36 (5.4) | 0.64 (0.42 to 0.997) | 0.68 (0.44 to 1.07) |
Higher scores reflect worse (slower) performance on Trails B and better performance on MMSE. MMSE, Mini-Mental State Examination; OR, Odds Ratio; CI, Confidence Interval.
Adjusted for age, race and education.
Objectively measured daytime movement was moderately correlated with the total number of blocks a woman walked per day for exercise or as part of her normal routine (r=0.26, p<0.001). When analyses were further adjusted for blocks walked per day, daytime movement remained independently associated with better cognitive function on both Trails B (17 seconds [0.23 SD] faster for highest vs. lowest movement quartile, p<0.001, Figure 1, Model 2) and MMSE (0.2 points [0.13 SD] better in highest vs. lowest movement quartiles, p=0.005) as well as lower odds of cognitive impairment on Trails B (OR [95% CI] = 0.50 [0.32 to 0.78] for third vs. lowest quartiles and 0.70 [0.46 to 1.07] for highest vs. lowest quartile) but was no longer significantly associated with lower odds of cognitive impairment on the MMSE. There was no evidence of interaction between daytime movement and walking; the effects of daytime movement were similar in subjects who did or did not walk for exercise (Figure 2B).
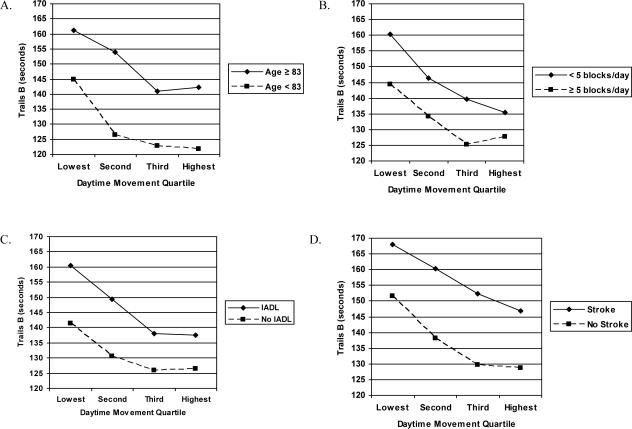
Figure 2.
Trails B Scores by Daytime Movement Quartile in Sub-Group Analyses. The association between daytime movement and mean Trails B scores was independent of the effects of other covariates and was similar in all subgroups examined, including analyses stratified by median age (Figure 2a), median number of blocks walked per day (Figure 2b), difficulty performing instrumental activities of daily living (IADL) (Figure 2c) and history of stroke (Figure 2d). All analyses are unadjusted.
Finally, we explored the factors that might mediate the association between daytime movement and cognitive function. The association between daytime movement and Trails B scores was reduced in magnitude by about one-third by adjustment for additional covariates, although it remained statistically significant (Figure 1, Model 3). Number of IADL impairments accounted for most of the reduction in the association between daytime movement and Trails B scores; other contributory factors included history of stroke or diabetes and alcohol consumption. There was no evidence of interaction between daytime movement and these or any other factors (Figures 2A-2D). The association between daytime movement and MMSE scores was similar in magnitude but of borderline statistical significance after additional covariate adjustment (0.2 points [0.13 SD] better in highest vs. lowest movement quartiles, p=0.07).
DISCUSSION
In this study of more than 2,700 non-demented women in their 80's, objectively measured daytime wrist movement was associated with higher levels of cognitive function and lower risk of cognitive impairment. This association between daytime movement and cognitive function was independent of age, race, education, medical comorbidities, physical function and other healthy behaviors, and it was similar across different subgroups of the study population. Although objectively measured daytime movement and self-reported walking were correlated, both measures were independently associated with better cognitive function and lower risk of cognitive impairment in these older women.
We also found that the association between daytime wrist movement and cognitive function was stronger for Trails B than for MMSE. This is probably explained by ceiling effects and the lack of variability in MMSE scores (range 24−30), which make it difficult to detect small differences in cognition with this test. In addition, prior studies have found that the effects of physical activity are most pronounced in the frontal lobes and, therefore, are best detected with measures of executive function such as Trails B.(27, 28)
The results of this study provide objective evidence to support our prior finding that women in this study population who reported engaging in more physical activity in their 60's and 70's were less likely to experience cognitive decline over 6 to 8 years of follow-up.(29) Our current findings build upon this by using an objective measure of daytime movement and by showing that the association between daytime movement and cognitive function was present as these women entered their 80's.
Our findings also are complimentary to other observational studies in humans, which have found that elders who report leading more ‘active’ lifestyles have higher levels of cognitive function and lower risks of cognitive impairment and dementia.(1-8, 30-32) Some of these studies have found that mental activities may be most important,(2-4) while others have found independent effects for physical, mental and social activities.(1, 5, 6) Together, these studies have provided support for the cognitive reserve hypothesis,(33) which holds that leading a more active lifestyle may help to build a buffer against the clinical manifestations of diseases such as dementia. Our study provides objective evidence to support this hypothesis, although longitudinal studies will be required to clarify whether more daytime movement leads to better cognitive function or vice versa.
There are several potential mechanisms by which more daytime movement could result in better cognitive function. Our analysis pointed to self-reported difficulty performing IADL—such as walking, climbing, cooking, cleaning and shopping—as a potential mediator. Adjustment for IADL difficulty attenuated our findings by about one-third; however, the association between daytime movement and cognitive function remained statistically significant and was similar in women who did and did not report IADL difficulty. Taken together, these findings suggests that difficulty performing IADL activities may explain some, but not all, of the association between daytime movement and cognitive function. However, it also remains possible that our IADL measure did not fully capture participants’ true functional capacity and that the relationship we observed between daytime movement and cognitive function may have been attributable to residual confounding.
It also is possible that daytime movement enhances cognitive function through a vascular mechanism. It is well-established that physical activity reduces the risk of vascular diseases and vascular risk factors such as hypertension, diabetes and stroke(34) which, in turn, are associated with increased risk of dementia.(35-37) However, as with our analysis of IADL, we found that the association between daytime movement and cognitive function was similar in subjects with and without these conditions, suggesting that they also may explain some, but not all, of the association.
There is growing evidence from animal studies that greater activity may lead directly to enhanced neuronal health and function, including reduced brain damage following injury, angiogenesis (formation of new blood vessels), neurogenesis (formation of new neurons), synaptogenesis (formation of new synapses) and increased levels of neurotrophic factors.(38) In one study, mice that were raised in an ‘enriched’ environment—which involved cages with more animals (social activity), colorful toys and tunnels (mental activity) and a running wheel (physical activity)—experienced enhanced neurogenesis and synaptic plasticity in the dentate gyrus of the hippocampus and learned to perform a water maze task more quickly.(39) In another study using a transgenic mouse model, mice raised in an enriched environment experienced decreased deposition of amyloid-β, which is a pathological hallmark of Alzheimer's disease.(40) It is possible that, through these and other mechanisms, greater activity may enable elders to build a cognitive reserve that enhances current cognitive function and lowers risk or delays onset of cognitive impairment and dementia.(33)
Strengths of our study include the large study population of non-demented women in their 80s. Few studies of cognitive function have focused specifically on this age group, which is currently the fastest growing segment of our population.(9) In addition, we used actigraphy to objectively measure women's daytime movements, rather than relying on self-report. This is important, because it enabled us to minimize recall bias and to incorporate those activities that may not be adequately captured in questionnaires (e.g., movements around the house).
This study also has several limitations. First, it is cross-sectional, so we cannot determine the direction of the association between daytime movement and cognitive function. Second, the study population was restricted to women; additional studies should determine whether a similar association is present in men. Third, the cognitive test battery was somewhat limited; future studies should include a broader range of cognitive tests. Fourth, because the actigraph is worn on the wrist, it may not accurately capture lower body movements, which might be better measured by a device worn at the waist or leg. Finally, actigraphy does not provide information about the effects of specific activities; rather, it provides an integrated measure of daytime wrist movement that captures a wide range of activities. Future studies should determine which specific daytime activities are most strongly correlated with actigraphically-measured daytime movement.
CONCLUSION
In summary, we found that non-demented women in their 80s who moved more during the day had better cognitive function and were less likely to be cognitively impaired, and that this association was consistent across all subgroups and independent of self-reported walking activities, medical comorbidities, physical function and other healthy behaviors. Additional studies are needed to clarify the direction of the association and to further explore potential mechanisms.
Acknowledgements
The Study of Osteoporotic Fractures was funded through grants from the National Institutes of Health (AG05407, AR35582, AG05394, AR35584, AR35583, AG08415). Dr. Barnes is supported by a Career Development Award from the National Institutes of Health (K01 AG024069). Dr. Yaffe was supported in part by an anonymous foundation for this work. This study was presented at the American Academy of Neurology meeting in Boston, MA on May 2, 2007 and at the American Geriatrics Society meeting in San Francisco, CA on November 19, 2007.
APPENDIX
Investigators in the Study of Osteoporotic Fractures Research Group: San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): SR Cummings (principal investigator), MC Nevitt (co-investigator), DC Bauer (co-investigator), DM Black (co-investigator), KL Stone (co-investigator), W Browner (co-investigator), R Benard, T Blackwell, PM Cawthon, L Concepcion, M Dockrell, S Ewing, C Fox, R Fullman, SL Harrison, M Jaime-Chavez, L Lui, L Palermo, M Rahorst, D Robertson, C Schambach, R Scott, C Yeung, J Ziarno
University of Maryland: MC Hochberg (principal investigator), L Makell (clinic coordinator), MA Walsh, B Whitkop.
University of Minnesota: KE Ensrud (principal investigator), S Diem (co-investigator), M Homan (co-investigator), D King (Program Coordinator), N Michels (Clinic Director), S Fillhouer (Clinic Coordinator), C Bird, D Blanks, C Burckhardt, F Imker-Witte, K Jacobson, D King, K Knauth, N Nelson, M Slindee.
University of Pittsburgh: JA Cauley (principal investigator), LH Kuller (co-principal investigator), JM Zmuda (co-investigator), L Harper (project director), L Buck (clinic coordinator), C Bashada, W Bush, D Cusick, A Flaugh, A Githens, M Gorecki, D Moore, M Nasim, C Newman, N Watson.
The Kaiser Permanente Center for Health Research, Portland, Oregon: T Hillier (principal investigator), E Harris (co-investigator), E Orwoll (co-investigator), K Vesco (co-investigator), J Van Marter (project director), M Rix (clinic coordinator), A MacFarlane, K Pedula, J Rizzo, K Snider, T Suvalcu-constantin, J Wallace.
Footnotes
Sponsor's Role: None.
CONFLICT OF INTEREST
- Deborah E. Barnes: None
- Terri Blackwell: None
- Katie L. Stone: None
- Suzanne E. Goldman: None
- Teresa Hillier: None
- Kristine Yaffe: None
REFERENCES
- 1.Scarmeas N, Levy G, Tang MX, Manly J, Stern Y. Influence of leisure activity on the incidence of Alzheimer's disease. Neurology. 2001;57:2236–2242. doi: 10.1212/wnl.57.12.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003;348:2508–2516. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RS, Bennett DA, Bienias JL, et al. Cognitive activity and incident AD in a population-based sample of older persons. Neurology. 2002;59:1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Mendes De Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. Jama. 2002;287:742–748. doi: 10.1001/jama.287.6.742. [DOI] [PubMed] [Google Scholar]
- 5.Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155:1081–1087. doi: 10.1093/aje/155.12.1081. [DOI] [PubMed] [Google Scholar]
- 6.Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21:65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 7.Rovio S, Kareholt I, Helkala EL, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer's disease. Lancet Neurol. 2005;4:705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 8.Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003;58:P249–255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- 9.Velkoff VA, He W, Sengupta M, DeBarros KA. 65+ in the United States: 2005. U.S. Census Bureau; Washington, D.C.: 2006. [Google Scholar]
- 10.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Blood ML, Sack RL, Percy DC, Pen JC. A comparison of sleep detection by wrist actigraphy, behavioral response, and polysomnography. Sleep. 1997;20:388–395. [PubMed] [Google Scholar]
- 12.Jean-Louis G, von Gizycki H, Zizi F, et al. Determination of sleep and wakefulness with the actigraph data analysis software (ADAS). Sleep. 1996;19:739–743. [PubMed] [Google Scholar]
- 13.Brown AC, Smolensky MH, D'Alonzo GE, Redman DP. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int. 1990;7:125–133. doi: 10.3109/07420529009056964. [DOI] [PubMed] [Google Scholar]
- 14.Muller U, Czymmek J, Thone-Otto A, Von Cramon DY. Reduced daytime activity in patients with acquired brain damage and apathy: a study with ambulatory actigraphy. Brain Inj. 2006;20:157–160. doi: 10.1080/02699050500443467. [DOI] [PubMed] [Google Scholar]
- 15.Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–410. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 18.Motionlogger® User's Guide . Act Millenium, Ambulatory Monitoring Inc.; Ardsley, NY: [Google Scholar]
- 19.Blackwell T, Ancoli-Israel S, Gehrman PR, Schneider JL, Pedula KL, Stone KL. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–1605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 20.Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–394. doi: 10.1037/h0044509. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Sheikh J, Yesavage J. Geriatric Depression Scale: recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 23.Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital & Health Statistics-series 1: Programs & collection procedures. 1987;21:1–115. [PubMed] [Google Scholar]
- 24.Pincus T, Summey JA, Soraci SA, Jr., Wallston KA, Hummon NP. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 25.Lohman TG, Roche AF, Mavtorell R. Anthropometric standardization reference manual. Human Kinetic Books; Champaigne, IL: 1988. [Google Scholar]
- 26.Cleveland WS, Devlin SJ. Locally weighted regression: An approach to regression analysis by local fitting. J Am Stat Assn. 1988;83:596–610. [Google Scholar]
- 27.Barnes DE, Yaffe K, Satariano WA, Tager IB. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults. J Am Geriatr Soc. 2003;51:459–465. doi: 10.1046/j.1532-5415.2003.51153.x. [DOI] [PubMed] [Google Scholar]
- 28.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 29.Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161:1703–1708. doi: 10.1001/archinte.161.14.1703. [DOI] [PubMed] [Google Scholar]
- 30.Abbott RD, White LR, Ross GW, Masaki KH, Curb JD, Petrovitch H. Walking and dementia in physically capable elderly men. Jama. 2004;292:1447–1453. doi: 10.1001/jama.292.12.1447. [DOI] [PubMed] [Google Scholar]
- 31.Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504. doi: 10.1001/archneur.58.3.498. [DOI] [PubMed] [Google Scholar]
- 32.Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. Jama. 2004;292:1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 33.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services . Physical activity and health: A report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta: 1996. [Google Scholar]
- 35.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 36.MacKnight C, Rockwood K, Awalt E, McDowell I. Diabetes mellitus and the risk of dementia, Alzheimer's disease and vascular cognitive impairment in the Canadian Study of Health and Aging. Dement Geriatr Cogn Disord. 2002;14:77–83. doi: 10.1159/000064928. [DOI] [PubMed] [Google Scholar]
- 37.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 38.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 39.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 40.Lazarov O, Robinson J, Tang YP, et al. Environmental enrichment reduces abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]