Abstract
Animal models are used to decipher the pathophysiology of IFN-α-induced psychiatric complications in humans. However, the behavioral effects of IFN-α in rodents remain highly controversial. In contrast to homologous IFN-α, our recent study uncovered that human IFN-α, which was used in many previous investigations, had no biological activity in mice. To evaluate the behavioral effects of homologous IFN-α in mice, adult C57BL/6J mice were treated with carrier-free murine IFN-α and tested on a number of behavioral paradigms. Surprisingly, contrary to previous reports, IFN-α treatment decreased the time spent immobile in the forced-swimming test after a single intraperitoneal injection at 2×106 IU/kg, whereas general locomotor activity was not altered. Elevated plus-maze test showed a trend toward an increased anxiety profile in IFN-α-treated mice. Tail-suspension or light dark exploration test revealed no difference between IFN-α-treated and control animals. Interestingly, neurochemical analysis revealed significantly increased concentrations of tryptophan and 5-hydroxyindoleacetic acid (5-HIAA)/serotonin (5-HT) ratios following IFN-α treatment in selected brain regions. Thus, systemic murine IFN-α treatment increases swimming time in mice. Increased cerebral serotonin turnover as well as increased tryptophan concentration, induced by IFN-α, implicates serotonergic neurotransmission in behavioral dysfunction caused by this innate immune mediator.
Keywords: Interferon-α, swim test, elevated plus-maze, tryptophan, serotonin, and behaviors
Discovered for its anti-viral action, the innate immune mediator interferon-alpha (IFN-α) is the first cytokine approved by the FDA for clinical applications, and has been prescribed for patients suffering from chronic viral infection or several types of malignancies because of its anti-viral, anti-proliferative and immunoregulatory effects [10]. In addition to its therapeutic effects, increasing evidence shows that administration of IFN-α results in various neuropsychiatric side effects ranging from irritability, anxiety and depression, to mania and psychosis in 11–47% of patients [25, 26]. Because of limited access to human brains, animals, especially rodents, have been used to study the neurobiology of IFN-α-induced behavioral deficits and relevant molecular mechanisms [26]. The reported results to date have been highly controversial in regards to whether IFN-α treatment has a similar impact on the analogous behaviors in rodents measured by the testing paradigms used for screening of potential anti-depressants and anxiolytics [8]. Because of the limited availability and cost of relevant homologous IFN-α, heterologous IFN-α, especially human IFN-α, has been used in most previous animal studies. Human IFN-α was initially shown to induce depression-related behavior in both the Porsolt swim test [18, 20] and the tail suspension test [35]. However, subsequent studies have not replicated the earlier observations [9, 17].
The molecular basis for cell signaling by IFN-α has been shown to involve Janus kinase (JAK)/signal transducer and activator of the transcription (STAT) signaling pathway [29]. Activation of the type I IFN receptor following ligand binding triggers JAK-dependent tyrosine phosphorylation followed by nuclear-translocation of its transcription factors STAT1 and STAT2 to form IFN-stimulated gene factor 3 (ISGF-3) upon association of STAT1/STAT2 heterodimers with IFN regulatory factor (IRF)-9 (also called p48 or ISGF-3γ). This biologically active complex interacts with the IFN-stimulated response element (ISRE) and regulates transcription of the genes with ISRE in their promoter to carry out the biological activities of IFN-α. To explore the potential factor(s) responsible for the inconsistent observations on the behavioral impacts of IFN-α, we analyzed the expression of the genes directly regulated by IFN-α to track the biological activity of IFN-α and IFN-α-responsive cell populations. We found that systemic administration of homologous IFN-α triggered a rapid expression of IFN-regulated genes in brain parenchymal cells in mice [33], indicating direct access of circulatory IFN-α to the CNS. More importantly, human IFN-α, in contrast to mouse IFN-α, had no such activity in either brain or peripheral tissues in mice following intraperitoneal injection even at doses 10- to 15-fold higher than the homologous IFN-α, demonstrating a species specificity of this cytokines as reported in vitro [32]. This novel observation prompted us to revisit the behavioral consequences of murine IFN-α in mice.
In the present study, six- to 8-week-old male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) were group-housed in a vivarium (22±1°C) under 12–12h light-dark cycle conditions (lights on 7:00 AM) with free access to food and water. After two weeks of adaptation at our housing facility, animals were assigned to experimental groups randomly. Considering the potential impact of carrier protein albumin [27], a preparation of mouse IFN-α with no bovine serum albumin (BSA) (PBL Biomedical Laboratories, New Brunswick, NJ) was used. Mice were treated with recombinant mouse IFN-α (specific activity: 7.7×107 units/mg protein) or pyrogen-free DPBS (Dulbecco’s phosphate buffered saline) (Sigma-Aldrich, St. Louis, MO) by intraperitoneal injection (100 μl/mouse) and tested for behaviors or killed for sample collection at different times following the treatment. The handling of mice and experimental procedures were conducted in accordance with the National Institutes of Health guidelines and approved by the University of Missouri-Kansas City Institutional Animal Care and Use Committee (IACUC).
Porsolt forced swimming [24], tail suspension [31], elevated plus maze (EPM) [16] and light dark exploration [2] tests were carried out as previously described by other investigators. Automatic testing apparatus for each of these four paradigms including forced swim station FS2000, tail suspension system TS100, elevated plus-maze apparatus EPM2000 and Light/Dark SF7×15 Cage Rack System (all acquired from Kinder Scientific; Poway, CA) were utilized. A standard 5 min test duration was adopted and the data were captured in real time by a Dell computer with the software provided by the vendor. The intensity of light was 300 lux (Porsolt and tail suspension), or 50 lux (EPM) and 150 lux (light dark exploration). The mice were used only once for behavioral tests in order to collect reliable data. All behavioral experiments were conducted between 12:30 PM and 4:30 PM to avoid circadian variation.
Poly (A+) RNAs were extracted from collected brain tissue and expression of IFN-stimulated genes was analyzed by RNase protection assay (RPA) as described previously [33]. For cerebral biogenic amine detection, brain regions were dissected and HPLC performed within 7–10 days of homogenization as previously described [11]. All results were presented as the mean ± S.E.M (standard error of mean). Unpaired student’s t-tests were utilized for statistical analysis for two-group experiments. For multiple-group comparisons, a two-way ANOVA was carried out followed by Fisher’s LSD test for post-hoc comparisons. Significance was set at a p value less than or equal to 0.05.
To determine behavioral impact of the homologous IFN-α treatment in mice, these animals were treated with 2×106 IU/kg (which was 5×104 IU and a total of 0.65 μg of protein for a 25-g mouse) of IFN-α by a single intraperitoneal injection according to previous studies in mice [7, 12, 33], and tested in the Porsolt swim test 2 hours later. Mice treated with IFN-α exhibited altered performance in the swim test compared with controls. The two measurements of general locomotor activity, total distance traveled and total number of beam breaks, did not differ between mice treated with IFN-α or vehicle. To our surprise, however, the total swim time in the 5-minute session was increased in IFN-α-treated mice. Figure 1 represents one of two such experiments. As illustrated in Figure 1, IFN-α treatment decreased resting time (immobility) by 26% (p = 0.017; n = 15) compared with the control group. In separate experiments, when the animals were injected with a lower dose of IFN-α (i.e., 2×105 IU/kg) on the same schedule, a smaller, but not statistically significant decrease of immobility was also observed (data not shown). Nevertheless, the same IFN-α treatment did not change the performance of mice in the tail suspension test, another commonly used paradigm for depression-like behavior (data not shown). To verify the activity of the IFN-α used, we found that cerebral expression of several prototypic IFN-stimulated genes, including dsRNA-dependent protein kinase R (PKR), ubiquitin-specific proteinase 18 (USP18), signal transducer and activator of transcription (STAT1), IFN-induced 15 kDa protein (ISG15) and guanylate-binding protein 3 (GBP3), were highly activated following the intraperitoneal injection of mouse IFN-α (Figure 2).
Figure 1.
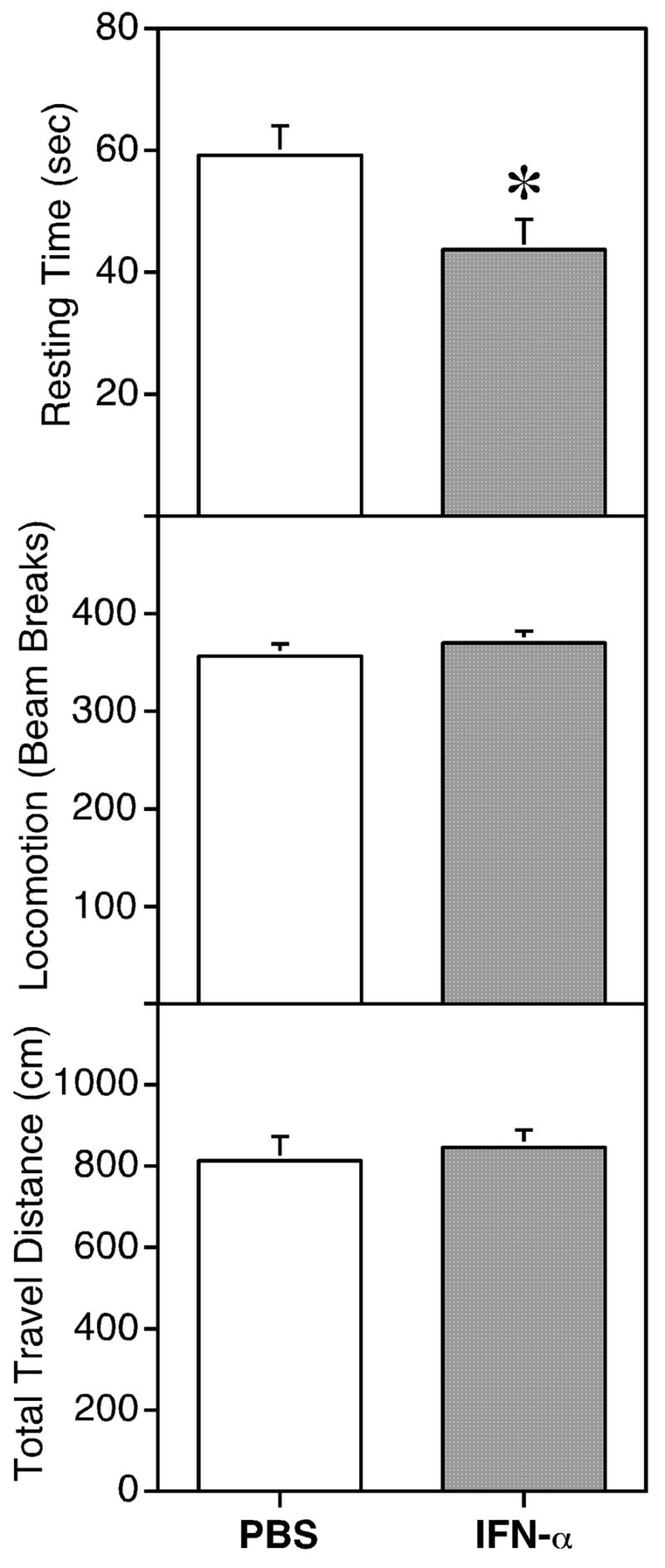
The effect of IFN-α on behavior of mice in the Porsolt swim test. Mice (N=15) were injected ip with vehicle or IFN-α (2×106 IU/kg) and tested in a 5-minute swim session at 2 hrs following the treatment. The time immobile (sec), total distance traveled (cm) and total number of beam breaks were recorded. Significantly different from vehicle-treated mice (* p ≤ 0.05).
Figure 2.
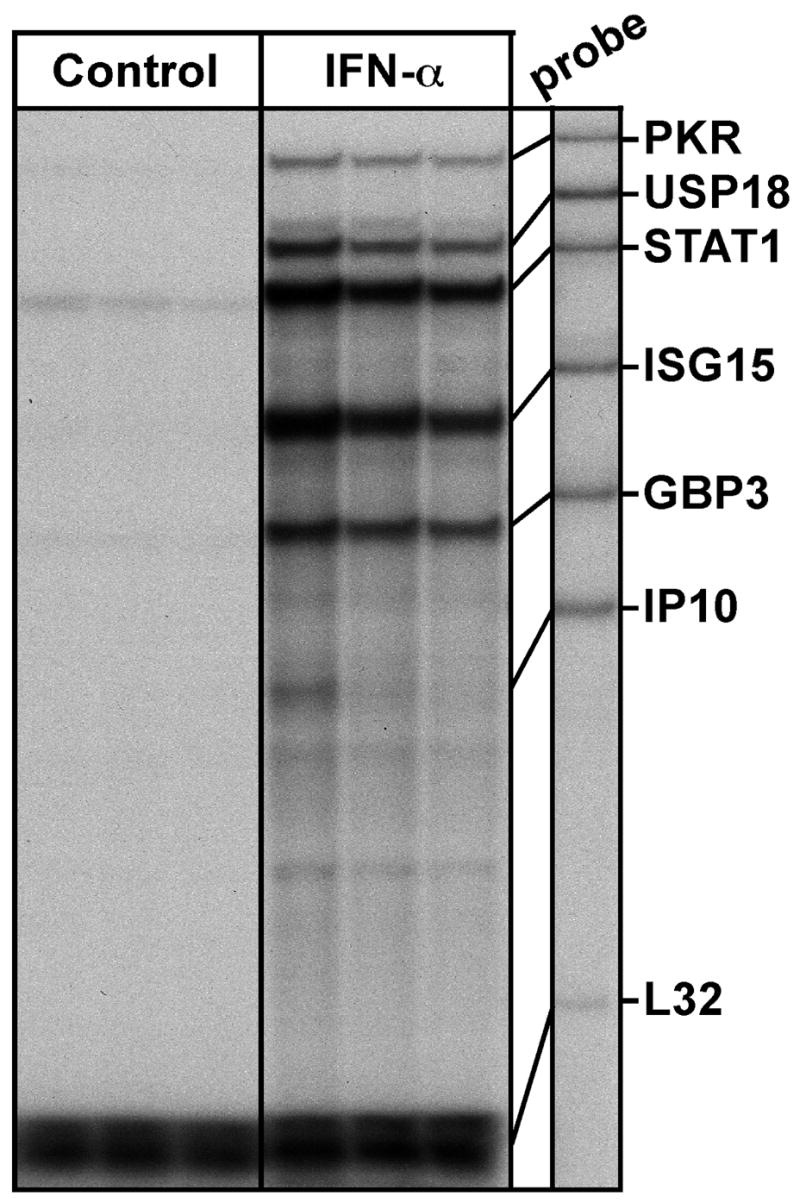
The effect of IFN-α on cerebral expression of the IFN-regulated genes. Mice were injected ip with vehicle (PBS) or mouse IFN-α (2×106 IU/kg) and forebrains were collected at 4 hours following the treatment for poly(A+) RNA preparation. For the analysis of RNA levels, 1.0 μg of poly(A+) RNA was analyzed by RPA. Each lane represents an individual animal.
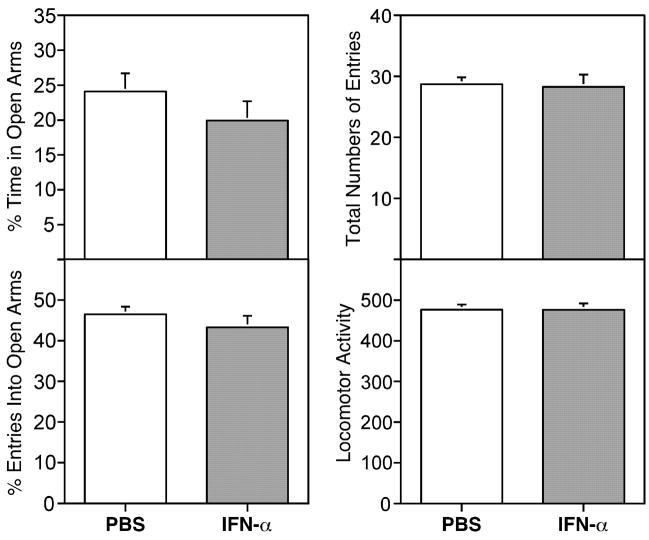
Based on clinical and animal studies [6, 7], we also evaluated whether similar IFN-α treatment had any influence on the performance of mice in two widely used paradigms, the EPM and the light dark box for anxiety-like behavior. Interestingly, in the EPM test, IFN-α-treated mice decreased the percentage of the time spent on the open arms by 17% and the percentage of the entries into open arms by 9% respectively, suggesting that IFN-α induced an increase in anxiety-like behavior (Figure 3). However, the decreases were not statistically significant compared to mice treated with vehicle. The locomotor activity of these mice measured by total arm entries and total number of beam breaks was not altered by IFN-α treatment and was consistent with the findings from the swim test. In addition, IFN-α treatment did not change the performance of mice in the light dark exploration test (data not shown).
Figure 3.
The effect of IFN-α on behavior of mice on the elevated plus-maze. Mice (N=16) were injected ip with IFN-α (2×106 IU/kg) or vehicle and tested on the elevated plus-maze at 2 hours following the injection. The percentage of time in open arms, percentage of entries into open arms, total number of entries and locomotion activity (total number of beam breaks) were calculated and presented.
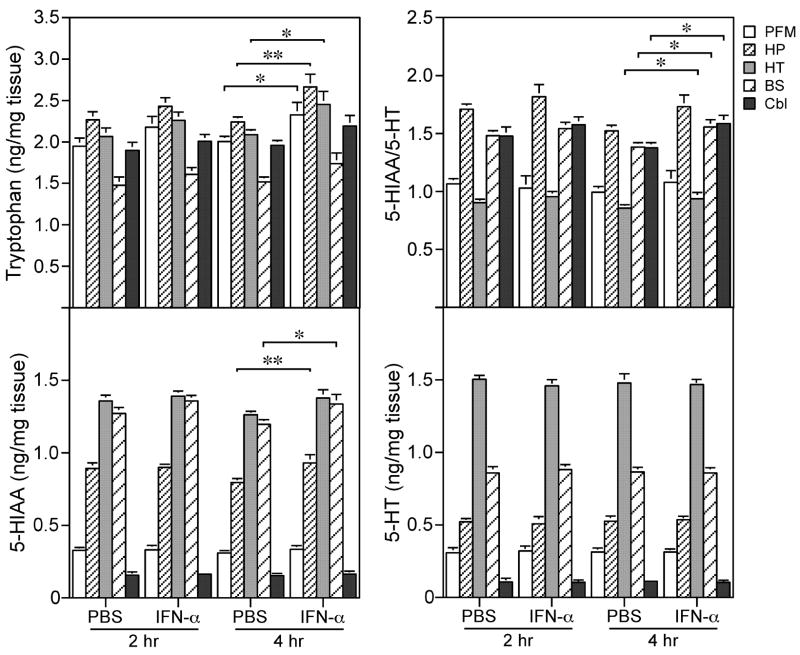
To evaluate the neurochemical impact of homologous IFN-α treatment on the brain, several brain regions such as the medial prefrontal cortex (PFM), hippocampus (HP), hypothalamus (HT), brain stem (BS) and cerebellum (Cbl) were dissected at 2 and 4 hours following intraperitoneal injection of IFN-α and analyzed for biogenic amine neurotransmitters and their metabolites by HPLC. Two-way ANOVA revealed significant effects of IFN-α treatment on the tryptophan level in PFM (F (1, 35) = 5.76, p = 0.022), HP (F (1, 35) = 7.32, p = 0.01) and HT (F (1, 35) = 6.50, p = 0.016) and the 5-HIAA/5-HT ratio in HT (F (1, 35) = 5.96, p = 0.02), BS (F (1, 35) = 5.31, p = 0.028) and Cbl (F (1, 35) = 5.26, p = 0.029) respectively. Post-hoc statistical analysis showed that IFN-α treatment significantly increased tryptophan concentrations or 5-HIAA/5-HT ratios at 4, but not 2 hours following injection in different brain regions (Figure 4). In addition, a single IFN-α administration also resulted in significantly increased 5-HIAA concentrations in hippocampus (p = 0.01) and brain stem (p = 0.04) at 4-hour time point. However, such IFN-α treatment had no effect on cerebral 5-HT concentration. The 5-HT levels in all brain regions analyzed were essentially unchanged at both 2 and 4 hours after single IFN-α injection (Figure 4).
Figure 4.
The effect of IFN-α treatment on tryptophan, 5-HT, 5-HIAA and 5-HIAA/5-HT ratios. Mice (N=8) were injected ip with IFN-α (2×106 IU/kg) or vehicle and decapitated at 2 and 4 hours after injection for HPLC sample collection. Significant differences from vehicle-treated mice were indicated (*p < 0.05; **p < 0.01).
The present study showed that homologous IFN-α treatment did not change general activity (an indicator for sickness behavior) in C57BL/6J mice. However, when treated with homologous IFN-α, mice decreased the time spent immobile in the Porsolt swim test. Such an observation is in contrast to increased immobility described in earlier reports from studies in which mice were treated with human IFN-α [18, 19]. The reason for this discrepancy is currently unknown. However, application of homologous IFN-α in the current study versus heterologous IFN-α used in previous investigations may be an important factor contributing to conflicting results, given the lack of detectable biological activity of human IFN-α in mice [33]. Other than activity of IFN-α itself, the carrier protein that is usually added to IFN-α preparation for maintaining the stability of recombinant proteins, such as BSA or human serum albumin has been shown to stimulate cytokine expression [27, 30].
An antidepressant effect of acute IFN-α was somewhat surprising as humans taking this compound often have problems with depressive-like symptoms. However, depression and other psychiatric complications are developed in patients who undergo chronic IFN-α therapy. An acute symptom experienced by human patients is confusion [26]; therefore, it is possible that the behavioral findings of the present study are revealing a correlate of confusion in mice. While extremely speculative, mice treated with IFN-α may continue to swim because, for example, they are not processing the contextual cues as well as vehicle-treated mice. This is interesting as it suggests a role of serotonin in even these early, acute effects of IFN-α. Clearly, behavioral characterization of mice following chronic IFN-α exposure including use of transgenic mice with chronic production of IFN-α will offer important insights into the relationship between IFN-α and behavioral dysfunction in mice.
Anxiety is among the most common psychiatric manifestation in IFN-α-treated patients [4, 6]. While the changes are not statistical significance, a trend of increased anxiety-like behavior indicated by the decreased percentage of time spent in open arms as well as the decreased number of entries into open arms were observed in our studies. In support of this, increased anxiety profiles have recently been reported in rats [23] and primates [13] after IFN-α treatment. In rhesus monkeys, treatment of IFN-α by a regimen extrapolated from human IFN-α therapy resulted in anxiety-like behavior, including self-scratching, body shaking and yawning [13].
The elevated tryptophan levels and the increased turnover of serotonin found in brain regions in the present study are in agreement with previous observations in rats [14] and humans [3, 5]. Here, a significant increase in the turnover of 5-HT (reflected by the increased ratio of 5-HIAA to 5-HT) was observed following IFN-α treatment. The present study also showed that acute IFN-α treatment resulted in an increased serotonin metabolite, 5-HIAA with no change in 5-HT itself in different brain regions. This increased 5-HT metabolism following acute IFN-α treatment suggests that serotonin depletion may be responsible for neuropsychiatric complications developed after IFN-α therapy in humans. However, compared to the behavioral changes, delayed neurochemical changes detected by HPLC of cerebral tissue homogenates is similar to the temporal profile of neurochemical alterations demonstrated in similar studies in which mice were treated with IL-1 or LPS [11]. Measurement of whole tissue homogenates other than extracellular fluids derived from in vivo microdialysis likely contributed to such delay [34]. Consistent with the notion that dysregulated serotonin neurotransmission is partially responsible for the behavioral changes induced by IFN-α, selective serotonin reuptake inhibitors (SSRIs) have been found effective in management of neuropsychiatric manifestations in human patients treated with IFN-α [22]. In addition, a recent in vitro study reported that IFN-α increases the expression of serotonin transporter (SERT), a key molecule which regulates reuptake of 5-HT [21], suggesting a molecular basis for IFN-α-induced changes in serotonin neurotransmission.
Despite the effectiveness of SSRIs in the management of psychiatric complications (both depression and anxiety) in patients chronically treated with IFN-α, animal studies have only focused on the depression-like behaviors caused by IFN-α. The study of the neurobehavioral impact of this anti-viral cytokine remains in its early stages. In addition to the challenges of recapitulating human depression or anxiety in rodents, broader behavioral screening remains necessary before efforts to understand the cellular and molecular mechanisms of the effects underlying the behavioral abnormalities. In this regard, recent investigations have shown that IFN-α can lead to additional behavioral dysfunctions in circadian rhythm [28] and cognitive impairment [15]. It is also important to note that clinical studies have been carried out in patient populations with chronic viral infection or malignancy [3–5]. Such precondition (leading to immune stimulation) could completely change the neurochemical and behavioral responses to IFN-α in patient cohorts compared with healthy subjects. Indeed, a recent animal study demonstrated that psychological distress dramatically enhances the IFN-α-induced neuroendocrine, neurochemical and behavioral effects in mice [1].
In conclusion, systemic homologous IFN-α treatment decreases immobility in forced swimming test in mice. Increased tryptophan levels and serotonin turnover were found in several brain regions of IFN-α-treated mice. These results suggest that dysregulated serotonin neurotransmission may be responsible for the behavioral dysfunction observed in our studies.
Acknowledgments
We thank Mr. Charles Dempsey for his help with the HPLC analysis of the tissue samples. We also thank Dr. Orisa J. Igwe for his comments on the manuscript. This work was supported by NIH grant MH 69524 to JW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anisman H, Poulter MO, Gandhi R, Merali Z, Hayley S. Interferon-α effects are exaggerated when administered on a psychosocial stressor backdrop: cytokine, corticosterone and brain monoamine variations. J Neuroimmunol. 2007;186:45–53. doi: 10.1016/j.jneuroim.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Belzung C, Misslin R, Vogel E, Dodd RH, Chapouthier G. Anxiogenic effects of methyl-beta-carboline-3-carboxylate in a light/dark choice situation. Pharmacol Biochem Behav. 1987;28:29–33. doi: 10.1016/0091-3057(87)90006-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 5.Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- 6.Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, Henry C. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 7.Crnic LS, Segall MA. Behavioral effects of mouse interferons-α and -γ and human interferon-α in mice. Brain Res. 1992;590:277–284. doi: 10.1016/0006-8993(92)91106-o. [DOI] [PubMed] [Google Scholar]
- 8.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 9.De La Garza R, 2nd, Asnis GM, Pedrosa E, Stearns C, Migdal AL, Reinus JF, Paladugu R, Vemulapalli S. Recombinant human interferon-α does not alter reward behavior, or neuroimmune and neuroendocrine activation in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:781–792. doi: 10.1016/j.pnpbp.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Dorr RT. Interferon-α in malignant and viral diseases. A review. Drugs. 1993;45:177–211. doi: 10.2165/00003495-199345020-00003. [DOI] [PubMed] [Google Scholar]
- 11.Dunn AJ. Endotoxin-induced activation of cerebral catecholamine and serotonin metabolism: comparison with interleukin-1. J Pharmacol Exp Ther. 1992;261:964–969. [PubMed] [Google Scholar]
- 12.Dunn AL, Crnic LS. Repeated injections of interferon-α A/D in Balb/c mice: behavioral effects. Brain Behav Immun. 1993;7:104–111. doi: 10.1006/brbi.1993.1011. [DOI] [PubMed] [Google Scholar]
- 13.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur Neuropsychopharmacol. 2000;10:129–132. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 15.Lieb K, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Janssen G, Schaefer M. Cognitive impairment in patients with chronic hepatitis treated with interferon alpha (IFNα): results from a prospective study. Eur Psychiatry. 2006;21:204–210. doi: 10.1016/j.eurpsy.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 16.Lister R. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 17.Loftis JM, Wall JM, Pagel RL, Hauser P. Administration of pegylated interferon-α-2a or -2b does not induce sickness behavior in Lewis rats. Psychoneuroendocrinology. 2006;31:1289–1294. doi: 10.1016/j.psyneuen.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Makino M, Kitano Y, Hirohashi M, Takasuna K. Enhancement of immobility in mouse forced swimming test by treatment with human interferon. Eur J Pharmacol. 1998;356:1–7. doi: 10.1016/s0014-2999(98)00474-9. [DOI] [PubMed] [Google Scholar]
- 19.Makino M, Kitano Y, Komiyama C, Hirohashi M, Kohno M, Moriyama M, Takasuna K. Human interferon-α induces immobility in the mouse forced swimming test: involvement of the opioid system. Brain Res. 2000;852:482–484. doi: 10.1016/s0006-8993(99)02235-0. [DOI] [PubMed] [Google Scholar]
- 20.Makino M, Kitano Y, Komiyama C, Takasuna K. Human interferon-α increases immobility in the forced swimming test in rats. Psychopharmacology. 2000;148:106–110. doi: 10.1007/s002130050031. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa O, Sakai N, Obara H, Saito N. Effects of interferon-α, interferon-γ and cAMP on the transcriptional regulation of the serotonin transporter. Eur J Pharmacol. 1998;349:317–324. doi: 10.1016/s0014-2999(98)00187-3. [DOI] [PubMed] [Google Scholar]
- 22.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 23.Myint AM, O’Mahony S, Kubera M, Kim YK, Kenny C, Kaim-Basta A, Steinbusch HW, Leonard BE. Role of paroxetine in interferon-α-induced immune and behavioural changes in male Wistar rats. J Psychopharmacol. 2007;21:843–850. doi: 10.1177/0269881107077165. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 25.Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-α: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaefer M, Engelbrecht MA, Gut O, Fiebich BL, Bauer J, Schmidt F, Grunze H, Lieb K. Interferon alpha (IFNα) and psychiatric syndromes: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:731–746. doi: 10.1016/s0278-5846(01)00324-4. [DOI] [PubMed] [Google Scholar]
- 27.Shacter E, Arzadon GK, Williams JA. Stimulation of interleukin-6 and prostaglandin E2 secretion from peritoneal macrophages by polymers of albumin. Blood. 1993;82:2853–2864. [PubMed] [Google Scholar]
- 28.Shinohara A, Koyanagi S, Hamdan AM, Matsunaga N, Aramaki H, Ohdo S. Dosing schedule-dependent change in the disruptive effects of interferon-alpha on the circadian clock function. Life Sci. 2008;83:574–580. doi: 10.1016/j.lfs.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Stark GR, Kerr IM, Williams BRG, Silverman RH, Schreiber RD. How cells respond to interferons. Ann Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 30.Steere AC, Rifaat MK, Seligmann EB, Jr, Hochstein HD, Friedland G, Dasse P, Wustrack KO, Axnick KJ, Barker LF. Pyrogen reactions associated with the infusion of normal serum albumin (human) Transfusion. 1978;18:102–107. doi: 10.1046/j.1537-2995.1978.18178118551.x. [DOI] [PubMed] [Google Scholar]
- 31.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 32.Trown PW, Wills RJ, Kamm JJ. The preclinical development of Roferon-A. Cancer. 1986;57:1648–1656. doi: 10.1002/1097-0142(19860415)57:8+<1648::aid-cncr2820571303>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Campbell IL, Zhang H. Systemic interferon-α regulates interferon-stimulated genes in the central nervous system. Mol Psychiatry. 2008;13:293–301. doi: 10.1038/sj.mp.4002013. [DOI] [PubMed] [Google Scholar]
- 34.Wieczorek M, Dunn AJ. Relationships among the behavioral, noradrenergic, and pituitary-adrenal responses to interleukin-1 and the effects of indomethacin. Brain Behav Immun. 2006;20:477–487. doi: 10.1016/j.bbi.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamano M, Yuki H, Yasuda S, Miyata K. Corticotropin-releasing hormone receptors mediate consensus interferon-α YM643-induced depression-like behavior in mice. J Pharmacol Exp Ther. 2000;292:181–187. [PubMed] [Google Scholar]