Abstract
Heme oxygenase (HO) catalyzes the conversion of heme to carbon monoxide, iron, and biliverdin, which is immediately reduced to bilirubin (BR). Two HO active isozymes exist: HO1, an inducible heat shock protein, and HO2, which is constitutive and highly concentrated in neurons. We demonstrate a neuroprotective role for BR formed from HO2. Neurotoxicity elicited by hydrogen peroxide in hippocampal and cortical neuronal cultures is prevented by the phorbol ester, phorbol 12-myristate 13-acetate (PMA) via stimulation of protein kinase C. We observe phosphorylation of HO2 through the protein kinase C pathway with enhancement of HO2 catalytic activity and accumulation of BR in neuronal cultures. The neuroprotective effects of PMA are prevented by the HO inhibitor tin protoporphyrin IX and in cultures from mice with deletion of HO2 gene. Moreover, BR, an antioxidant, is neuroprotective at nanomolar concentrations.
Heme oxygenase (HO) catalyzes the cleavage of the heme ring to form ferrous iron, carbon monoxide, and biliverdin. Biliverdin (BV) is rapidly reduced by biliverdin reductase to bilirubin (BR) so that in intact tissues, BV rarely accumulates and the physiologic product of HO is generally thought to be primarily BR. Two principal forms of HO have been distinguished and molecularly cloned (1). HO1 is an inducible enzyme, also designated as heat shock protein-32, with new HO1 protein synthesis elicited by multiple stimulants (especially those associated with red blood cell damage, such as heme and other porphyrins). HO1 is concentrated in tissues such as the spleen and liver, which degrade heme from aged red blood cells. By contrast, HO2 is constitutive and most concentrated in the brain and testes (2), accounting for the great majority of HO activity in the brain (1). Evidence suggests that HO2, localized to selective neuronal populations (1, 3), plays a major role in neuromodulatory activities, with CO participating as a putative neurotransmitter. Thus, HO2 mRNA (3) and protein (2) are selectively concentrated in discrete neuronal populations, although most, if not all, neurons do possess HO2. A role for CO as a neurotransmitter is best supported by studies of neurotransmission in the intestine. About 70% of neurons of the myenteric plexus stain for HO2, with the same population of neurons staining for neuronal nitric oxide synthase (nNOS) (4). Nonadrenergic, noncholinergic transmission elicited by electrical field stimulation of the myenteric plexus is substantially reduced in mice with targeted deletion of HO2 (HO2−/−) (4).
Although HO gives rise to three products, most research has focused on CO. BR elicits substantial antioxidant effects and is probably the most abundant endogenous antioxidant in mammalian tissues (5–7). We wondered whether generation of BR by HO is neuroprotective. In the present study, we demonstrate that phorbol esters are neuroprotective in neuronal cultures by activating protein kinase C (PKC). The neuroprotective effects involve HO2, as they are abolished in cultures from HO2−/− mice and by HO inhibitors. Moreover, low nanomolar concentrations of BR are neuroprotective. We also show that PKC phosphorylates HO2 and stimulates enzyme activity, providing a potential mechanism for neuroprotection.
MATERIALS AND METHODS
Rat Primary Hippocampal and Cortical Neuronal Cultures and Survival Assays.
Knowing that the HO2 expression is enriched in the pyramidal cell layer of the hippocampus formation (3), we prepared cultures of hippocampal and cortical neuronal cells isolated from 17-day-old embryos of timed pregnant Sprague–Dawley rats and wild-type and HO2−/− mice (8). Neurons were cultured in serum-free conditions with the B-27 supplement as described (9, 10), cultured at high density (1.75 × 106 cells per well, 24 wells per plate). Experiments were performed after 7 days in vitro.
H2O2-Induced Toxicity in Cultured Neurons.
H2O2 was freshly diluted in the culture medium and immediately added to the cells. All experiments were conducted under dim light to avoid heme pigment photodegradation. BR was freshly dissolved in NaOH (0.1 M:50 mM) at a concentration of 1 mM. Human serum albumin was mixed at a ratio of 1:5 following the protocol established by Neuzil and Stocker (11, 12).
Immunocytochemistry for Bilirubin.
BR antibody was obtained by coupling the BR to BSA by using the 1-(ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride) (EDC) cross-linker, similarly to Okamura et al. (13) and was injected to rabbits by Cocalico (Reamstown, PA). The antiserum was purified on a column containing the BR–EDC coupled to thyroglobulin (TGB; Sigma). The purified antibody showed strong immunoreactivity by dot blot analysis to both BR–BSA or and BR–TGB. The antibody did not react with BSA alone or BSA treated with EDC. Preabsorption with heme–BSA or BV–BSA did not change the signal obtained.
Cells were rinsed with ice-cold PBS and then fixed with 6% EDC (Pierce) at 4°C for 2 h. Cells were washed with PBS, incubated with 0.1% Triton X-100 for 15 min at room temperature, and washed with PBS. Cells were preincubated for 1 h with 4% NGS in PBS and incubated with the anti-BR in 2% NGS for 2 h at room temperature. Cells were then washed and incubated with the secondary antibody and developed.
Establishment of Human Embryonic Kidney (HEK) 293 Cell Lines Overexpressing Human Cytochrome P450 Reductase (CPR) with hHO1 or hHO2.
CPR, hHO1, and hHO2 were subcloned into cytomegalovirus-based expression vector PRK5 (15). By using pRSV neo as a marker, CPR construct was stably transfected into HEK 293 cells (16), and cells were maintained in DMEM-fetal calf serum with G418. Human HO1 or HO2 were stably expressed in 293-CPR cells by cotransfection with pZeoSV2 (Invitrogen), resulting in 293-HO1 and 293-HO2 cell lines maintained in medium containing G418 and Zeocin (Invitrogen). HO activity (see below) was increased by approximately 10- and 6-fold in the 293-HO1 and 293-HO2 cell lines, respectively. The basal level of HO1 and HO2 is barely detectable under normal conditions by using standard Western blot protocols.
HO Assay.
HO activity was measured by the release of free 55Fe from [55Fe]mesoporphyrin (NEN) as previously described (4), with the following modifications: the reaction buffer was Hepes 50 mM (pH 7.4)/1 mM EDTA; the reaction was stopped with the addition of cold tin protoporphyrin IX (SnPPIX) at a final concentration of 10 μM, and the washing buffer on column was 50 mM Hepes (pH 7.4)/1 mM EDTA/1 M NaCl.
In Vitro Phosphorylation of hHO2.
Gltuathione S-transferase (GST)–hHO2 was ligated to GST fusion vector pGEX-4T-2 (Amersham Pharmacia). The fusion GST–HO2 protein was then purified from Escherichia coli. After isolation on glutathione agarose, purity and immunoreactivity of GST–HO2 were confirmed by SDS/PAGE and Western Blot analysis. For the in vitro phosphorylation assays, 1 μg of GST–HO2 was incubated with 250 μM MgATP (1,000 cpm/pmol; [32P]ATP, NEN) and 0.05 μg of purified PKC (Calbiochem), 1 mM CaCl2, and 4:1 phosphatidylserine/diacylglycerol (Avanti Polar Lipids) as described (17). Phosphorylation was analyzed after SDS/PAGE, autoradiography, and radioactive counting.
Phosphorylation of HO2 in Htk-293 Cells.
HO1 and HO2 cell lines were incubated for 90 min with artificial cerebrospinal fluid modified from Quinlan and Halpain (18). Cells were solubilized in cold immunoprecipitation buffer (50 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5 mM EDTA/1% Triton X-100/0.1 mM phenylmethylsulfonyl fluoride/1 μM okadaic acid/25 mM NaF/1 mM orthovanadate) before immunoprecipitation.
RESULTS
Phorbol Esters are Neuroprotective for Primary Hippocampal and Cortical Neuronal Cultures.
In preliminary studies, we noted that phorbol ester treatment augments HO2 activity (19). To explore a possible association of HO2 and neurotoxicity, we examined the influence of PMA on H2O2-elicited neurotoxicity in primary neuronal cultures (Fig. 1). In both hippocampal and cortical cultures (Fig. 1 A and B), H2O2 kills neurons with a concentration-response dependency. At 0.1, 0.3, and 0.5 μM, PMA markedly protects against this toxicity. Hippocampal neurons are slightly more responsive than cortical neurons to PMA’s neuroprotective effect, which may reflect the high levels of HO2 in hippocampal neurons (Fig. 1 A and B). The neuroprotective effect of PMA is most evident at 75–100 μM H2O2, whereas PMA fails to protect against 300 μM H2O2 (Fig. 1C). At 1.0 μM, PMA is not neuroprotective (Fig. 1C), which may reflect down-regulation of PKC by high concentrations of phorbol esters (20). To investigate this possibility, we conducted a Western blot analysis for PKC in hippocampal cultures treated with different concentrations of PMA (Fig. 1D). PKC levels are reduced at 0.3 μM PMA and abolished at 1.0 μM. This fact could explain the inability of 1.0 μM PMA to provide neuroprotection and further substantiates stimulation of PKC as a mechanism for neuroprotection by PMA. A phorbol ester that does not activate PKC, 4α-PMA, provides no neuroprotective effect (Fig. 1E). Additionally, bisindolylmaleimide, a potent PKC inhibitor (21), reverses the protective effects of PMA (Fig. 1F). Effects are evident with nanomolar concentrations of bisindolylmaleimide, concentrations at which PKC is selectively inhibited.
Figure 1.
Neuroprotective effect of PMA against H2O2-induced toxicity on rat primary neurons. Rat primary neurons were exposed to oxidative stress injury induced by H2O2 (75 μM), and neuron survival was estimated 24 h after the beginning of the experiment (A and B). Concentration-dependent biological effects of PMA were observed against H2O2 toxicity when PMA was added 24 h before exposure of H2O2 on hippocampal (A) and cortical (B) neurons. In C, hippocampal neurons were pretreated with PMA (0.1 and 1.0 μM) before the addition of different concentrations of H2O2 (30, 100, and 300 μM). Western blot analysis showed the expression of PKC in hippocampal neuronal cultures after PMA treatment (D). (E) An inactive enantiomer of PMA, 4α-PMA, was added in the same conditions as for PMA. Hippocampal neurons were treated by using identical conditions as described above and 5.0 μM of the HO inhibitor (SnPPIX) was added at the same time as PMA. (F) The effect of a PKC inhibitor, bisindolylmaleimide, on the neuroprotective effect of PMA in hippocampal neuronal cultures.
Phorbol Ester Neuroprotection Is Mediated by HO2.
To investigate the possibility that PMA neuroprotection involves HO activity, we examined the influence of a variety of metalloporphyrin inhibitors of HO on PMA neuroprotection in fetal rat hippocampal cultures. At 5 μM, SnPPIX reverses the neuroprotective effects of PMA (Fig. 1E). MnPPIX and zinc deuteroPPIX similarly reverse the neuroprotective effects of PMA. SnPPIX, MnPPIX, and zinc deuteroPPIX are all known HO inhibitors (22–24). PPIX alknoe, which does not inhibit HO, fails to reverse the neuroprotective effects of PMA (data not shown). None of these agents elicit neurotoxicity by themselves in control cultures.
Western blot analysis revealed no changes in CPR and BV reductase levels after treatment with PMA (0, 0.1, and 1.0 μM) (data not shown). At high concentrations, metalloporphyrins can influence a variety of enzymes, such as NOS and guanylyl cyclase (24, 25). However, at the low concentrations employed in this study these compounds are selective for HO (23). nNOS probably does not participate in the PMA neuroprotective effect, because under our cultured conditions nNOS is barely detectable by Western blot analysis and the NOS inhibitor 7-nitroindazole fails to affect PMA’s neuroprotective action (data not shown).
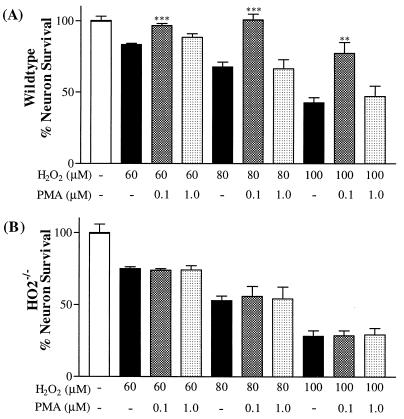
To discriminate between HO1 and HO2, we employed neuronal cultures from HO2−/− mice (Fig. 2). First, we ascertained that H2O2 toxicity to hippocampal cultures and protection by PMA are similar in mice and rats (Fig. 2A). In the mice (as in the rats), H2O2 toxicity is concentration-dependent, with a ≈60% decrease in neuronal survival at 100 μM H2O2. Also, in mice and in rats, PMA provides pronounced protection at 0.1 μM, with no protection at 1.0 μM. All concentrations of H2O2 examined (60, 80, and 100 μM) elicit substancially greater toxicity in HO2−/− than in control cultures, consistent with a neuroprotective action of endogenous HO2. In striking contrast to the wild-type cultures (Fig. 2B), 0.1 μM PMA fails to provide any protection in the HO2−/− cultures. The abolition of PMA neuroprotective effects in HO2−/− cultures establishes that HO2 is the primary, if not the sole, mediator of PMA’s neuroprotective actions.
Figure 2.
PMA neuroprotection is lost in hippocampal cultures of HO2−/− mice. Primary hippocampal neurons from wild-type (A) and HO2 knockout (B) mice were cultured similarly as rat neuronal cultures. Neurons were treated with various concentrations of H2O2 (60, 80, and 100 μM). PMA (0.1 and 1.0 μM) was added 24 h before the addition of H2O2. Neuronal survival was assessed 24 h later.
PMA Stimulates HO2 Activity by PKC Phosphorylation.
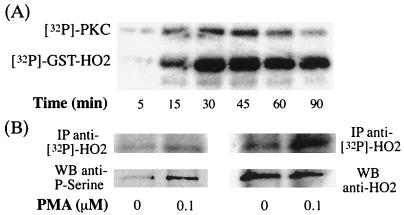
How might PMA elicit neuroprotection via HO2? Might PKC phosphorylate (and thus activate) HO2? To examine this possibility, we evaluated the effects of purified PKC on GST–HO2 in the presence of [32P]ATP and found a time-dependent phosphorylation of HO2 (Fig. 3A).
Figure 3.
In vitro phosphorylation of hHO2. (A) Time-dependent effect on phosphorylation of GST–hHO2 incubated with [32P]ATP and purified brain PKC. The upper band shows the phosphorylation of PKC itself and the lower band depicts the phosphorylation of GST–hHO2. (B) HO2 phosphorylation in HEK 293 cells stably overexpressing hHO2. The top row shows incorporation of [32P]ATP into HO2 after phosphorylation induced by treatment with PMA (0 and 0.1 μM). HO2 was immunoprecipitated with two different antibodies (Left, SPA297; Right, our antibody), then subjected to electrophoresis and transferred to nitrocellulose membrane. Quantification of the [32P]HO2 with the PhosphorImager reveals an increased of 1.63 (Left) and 1.72 (Right) times after PMA treatment over the control. The Lower row was obtained after Western blot analysis on the same nitrocellulose membrane with an anti-phosphoserine antibody, suggesting that immunoprecipitated HO2 was phosphorylated on serine residues. Right indicates that HO2 was loaded in similar amounts.
To explore whether HO2 is phosphorylated in response to PMA in intact cells, we utilized HEK 293 cells stably transfected with CPR, along with either HO1 or HO2 (Fig. 3B; Table 1). In one set of experiments, we incubated the cells with [32P]orthophosphate, immunoprecipitated HO2, and monitored radiolabeling in HO2 purified by gel electrophoresis in preparations from cells treated with 0.1 μM PMA or matched controls (Fig. 3B, Upper). PMA treatment produces a ≈1.7-fold increase in the incorporation of [32P] into HO2, estimated by PhosphorImager analysis. In similar experiments, we evaluated phosphorylation of HO2 with an anti-phosphoserine antibody (Fig. 3B, Lower). A pronounced augmentation of HO2 phosphorylation is elicited by 0.1 μM PMA treatment. By contrast, PMA treatment produces no change in HO2 protein levels (Fig. 3B, Lower). In the HEK 293 cells stably transfected with CPR along with HO1, we detected no phosphorylation of HO1 in response to PMA treatment (data not shown). Also, HO1 protein levels are unaffected by PMA treatment. PMA treatment elicits a 70% augmentation of HO2 activity in HO2–HEK 293 cells (Table 1). By contrast, PMA fails to alter HO1 activity. This suggests that HO2 phosphorylation by PKC stimulates HO2 catalytic activity.
Table 1.
Effect of PMA (1 h) on HO specific activity in HEK 293 cells stably transfected with human CPR and hHO1 (HO1) or hHO2 (HO2)
| HO | HO specific activity, cpm/mg·min
|
|
|---|---|---|
| Control | PMA (0.1 μM) | |
| HO1 | 16998 ± 152 | 16507 ± 81 |
| (100 ± 1%) | (97 ± 1%) | |
| HO2 | 11051 ± 290 | 19474 ± 438 |
| (100 ± 3%) | (176 ± 4%)** | |
, P < 0.001 compared with control fixed at 100%.
To ascertain whether HO activity is influenced by PMA in hippocampal cultures, we examined effects of 0.1 and 1.0 μM PMA on HO activity at various times (Table 2). At 1 h, PMA doubles HO activity, with similar effects at 3 h and a progressively smaller effect at later times. At 1 h, 1.0 μM PMA produces modest stimulation, which diminishes at later time points.
Table 2.
Time-dependent effect of PMA on HO specific activity in rat primary hippocampal neurons
| PMA, μM | Time, h | ||||
|---|---|---|---|---|---|
| 1 | 3 | 6 | 18 | 24 | |
| 0 | 2032 ± 6 | 2002 ± 9 | 1952 ± 121 | 1961 ± 4 | 2018 ± 70 |
| (100 ± 1%) | (99 ± 1%) | (96 ± 6%) | (96 ± 1%) | (99 ± 3%) | |
| 0.1 | 4287 ± 156 | 4195 ± 5 | 3508 ± 2 | 3043 ± 11 | 2575 ± 12 |
| (211 ± 8%)*** | (207 ± 1%)*** | (173 ± 1%)*** | (150 ± 1%)*** | (126 ± 1%)*** | |
| 1.0 | 2917 ± 18 | 2351 ± 1 | 2088 ± 52 | 2290 ± 31 | 2247 ± 189 |
| (142 ± 1%)*** | (125 ± 1%)*** | (103 ± 3%) | (113 ± 1%)** | (110 ± 9%) | |
Values are HO specific activity in cpm/mg·min.
, P < 0.001; ∗∗∗, P < 0.0001 compared with control (PMA; 0 μM) fixed at 100%.
We used several approaches to ascertain whether HO1 contributes to the PMA stimulated HO activity in hippocampal neuronal cultures. Whereas HO2 is a constitutive enzyme with a relatively long half-life, HO1 is stimulated by induction of new protein synthesis. Using Western blots analyzed by PhosphorImager with radiolabeled secondary antibody, PMA fails to alter levels of HO1 or HO2 protein at any of the time points examined (data not shown). This finding is consistent with observations that stress in mixed cultures stimulates HO1 synthesis in glia but not in neurons (26). Moreover, treatment with cycloheximide, an inhibitor of protein synthesis, does not attenuate the PMA-elicited neuroprotection under conditions in which cycloheximide depresses protein synthesis (data not shown). Knowing that HO1 is a PEST [a structural motif present in the majority of rapidly degraded enzymes (27)] protein (28), we also used antisense constructs to HO1. The antisense constructs reduce HO1 protein expression by more than 50%, whereas sense constructs and/or lipofectin vehicle preparations are inactive (data not shown). Antisense treatment has no effect on the ability of PMA to enhance neuronal survival in hippocampal cultures treated with H2O2. In these experiments, we first demonstrated a >50% reduction of HO1 expression by HO1 antisense but not by sense construct or lipofectin control-vehicle. Thus, HO1 does not contribute notably to the neuroprotective effects of PMA.
Bilirubin Is Neuroprotective.
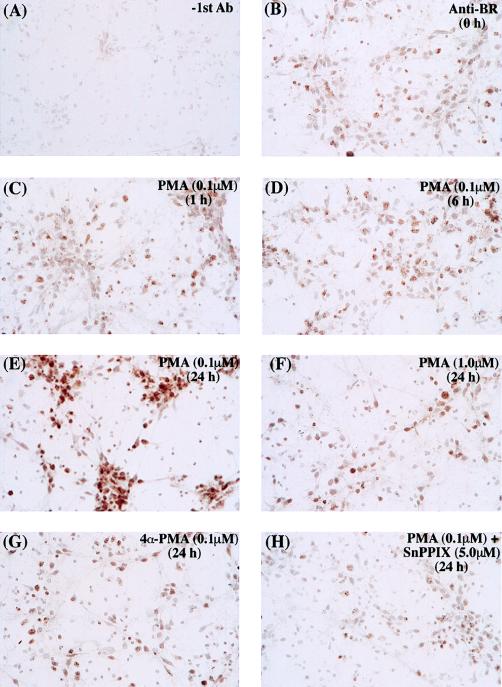
We wondered whether activation of HO2 is neuroprotective by stimulating the formation and accumulation of the antioxidant BR. We evaluated the formation of BR in response to PMA treatment by immunocytochemical staining of hippocampal cultures with a selective BR antibody (Fig. 4). Six hours after treatment with 0.1 μM PMA some accumulation of BR is shown (Fig. 4D), whereas at 24 h, BR staining is markedly augmented (Fig. 4E). By contrast, 1.0 μM PMA, which down-regulates PKC protein, fails to enhance BR staining (Fig. 4G). Also, 4α-PMA, which does not activate PKC, fails to stimulate staining (Fig. 4G). Evidence that BR accumulation derives from HO activity is provided by experiments in which the HO inhibitor SnPPIX (5.0 μM) prevents the PMA-elicited augmentation of BR staining (Fig. 4H). Thus, in hippocampal neurons, PKC activation stimulates HO2 activity, leading to BR accumulation.
Figure 4.
Photomicrograph of rat primary hippocampal neurons incubated with an antibody against BR showing BR accumulation after PMA treatment. (A) Control staining performed without the addition of the primary antibody. (B–H) Staining with the anti-BR under different stimuli. Cells were treated with 0.1 μM PMA for various time periods (0, 1, 6, or 24 h) (B–E), with 1.0 μM PMA for 24 h (F), with 0.1 μM inactive enantiomer 4α-PMA (G), or with 0.1 μM in combination with 5.0 μM HO inhibitor SnPPIX (H).
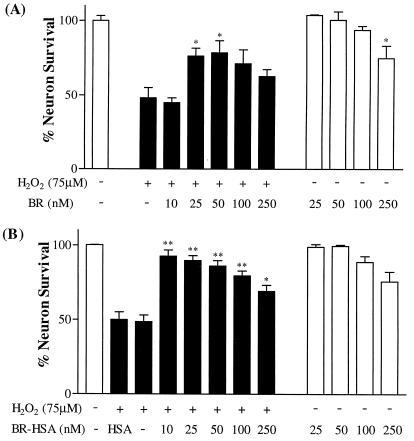
To directly ascertain whether BR is neuroprotective, we examined its effects on hippocampal neuronal cultures treated with H2O2 (Fig. 5). We used BR conjugated with human serum albumin (BR–HSA) to enhance its solubility (Fig. 5B). As little as 10 nM BR (BR–HSA) almost completely reverses the neurotoxic actions of H2O2. At 1 and 3 nM concentration, the neuroprotective effect is substantially diminished. BR conjugated to BSA is also neuroprotective, but to a lesser extent (data not shown). The neuroprotective effect decreases at higher concentrations of BR, presumably because higher levels of BR are themselves neurotoxic. This is suggested by the diminished neuronal survival of control cultures treated with 100 and 250 μM BR–HSA. BR also is neuroprotective when used without an albumin carrier, although it is less potent (Fig. 5A).
Figure 5.
Neuroprotective effect of bilirubin on H2O2-induced toxicity. (A) Increasing concentrations of free BR were added to rat primary hippocampal neurons before addition of 75 μM H2O2. (B) Increasing concentrations of BR complexed with human serum albumin (BR–HSA was added to neurons). An equivalent concentration HSA of albumin was added. Neuron survival was estimated 24 h after the beginning of the experiment.
DISCUSSION
In the present study, we evaluated neurotoxicity in hippocampal and cortical cultures by using hydrogen peroxide. H2O2 is thought to be one of the central components in the intracellular cascade of neurotoxic events involving reactive oxygen species (29, 30). H2O2 interacts with ferrous iron in the Fenton reaction to form the very toxic hydroxide free radical along with ferric iron. After neurotoxic stimulation, the hypoxic mitochondria generate substantial levels of superoxide. Superoxide interacts with protein-bound iron to form ferrous iron, which in turn further augments the Fenton reaction. Superoxide also combines with NO⨪ to form peroxynitrite, which decomposes to hydroxide free radical.
The phorbol ester PMA provides neuroprotection. Although activation of PKC by phorbol esters can influence numerous cellular events, our experiments using HO inhibitors and HO2−/− neuronal cultures imply that the neuroprotective actions of PMA involve HO2. A variety of HO inhibitors block the protective effects of PMA. The neuroprotective actions of PMA are abolished in HO2−/− cultures, and neurotoxicity is accentuated in HO2−/− cultures. These findings strongly suggest that HO2 is the isoform responsible for PMA’s neuroprotective actions, although we cannot fully rule out a role for HO1. We would have liked to employ HO1−/− cultures; however, HO1−/− animals typically die at approximately embryonic day 12 (31), and cultures are developed from embryonic day 18 animals.
The rapid activation of HO2 by PKC phosphorylation and other properties of HO2 parallel the disposition of nNOS. Both are constitutive enzymes localized to neurons. Although their neuronal localizations in the brain differ, in the myenteric plexus of the intestines, they occur in the same neurons and contribute similarly to nonadrenergic noncholinergic relaxation of the gut after physiologic nerve stimulation (24). nNOS is activated by calcium entry into cells binding to calmodulin on nNOS (32). PKC phosphorylation of HO2 would provide a way for relatively rapid stimulation of HO2 activity. Moreover, HO2, like nNOS, would be able to respond to calcium entry, because calcium is a major activator of PKC (33).
One of the attractive findings of the present study is the very potent neuroprotective effect of BR with complete neuroprotection evident at nanomolar concentrations. BR actions have been mostly characterized in the high micromolar range, where toxic effects also occur. How can nanomolar concentrations of BR protect against higher H2O2 concentrations? The most likely explanation is a cycle of oxidation–reduction between BR and BV, the major oxidation product of BR (34, 35). In mediating its antioxidant actions, BR would be transformed to BV. BV reductase, present in large functional excess in all tissues, would immediately regenerate BR.
BR is probably the most abundant endogenous antioxidant in mammalian tissues, accounting for the majority of the antioxidant activity of human serum (36). In an extensive series of antioxidants, BR displayed the most potent superoxide and peroxyl radical scavenger activity (37). Moreover, BR is an effective antioxidant of peroxynitrite-mediated protein oxidation (34). In the circulatory system, BR is largely complexed with albumin. We have observed more extensive neuroprotection with BR complexed with human serum albumin than with BSA, perhaps because human albumin has higher affinity for the primary and secondary binding sites for BR than its bovine homolog. Within cells, BR is stored in a complex with various isoforms of GST, also called ligandin and Y peptide (38). Thermodynamic parameters for BR dissociation from GST are similar to those for HSA (39). Binding of BR to these proteins keeps BR in solution and inhibits its efflux from the cell, thereby increasing the net accumulation. GST plays a role in cellular uptake and the intracellular transport of BR (40). Certain GST isoforms are selectively concentrated in neurons (41, 42). A GST isoenzyme-specific distribution also was found in various subcellular compartments, suggesting the possibility of scavenging free radicals in different cell compartments. GST isoforms are also highly concentrated in the testis (42), fitting with the uniquely high levels of HO2 expression in that tissue.
BR is best known as a potentially toxic agent that accumulates in the serum of neonates, causing jaundice. In high concentrations, BR deposits in selected brain regions to elicit the neurotoxicity associated with kernicterus (43). The “physiologic jaundice” of normal neonates, which occurs at BR levels fairly close to toxic levels, has been puzzling (44). Conceivably, physiologic jaundice has a protective effect. It could represent a transitional antioxidative mechanism in the neonatal circulation. Serum antioxidant activities are selectively associated with BR in neonatal Gunn rats (45) and in jaundiced newborn infants (6). In preterm infants, higher bilirubin levels are associated with a lower incidence of oxygen radical-mediated injury (46). BR administration protects against retinopathy in premature babies (47, 48). Moreover, beneficial effects of breastfeeding are often accompanied by high BR levels (49). BR may be particularly important as a cytoprotectant for tissues with relatively weak endogenous antioxidant defenses such as the myocardium (50, 51) and the nervous system (52). Interestingly, a decreased risk for coronary artery disease is associated with mildly elevated serum BR, with a protective effect comparable to that of HDL-cholesterol (7, 53). Our findings thus imply that HO2 generation of BR affords physiologic neuroprotection. This conclusion is supported by our recent observations that neuronal damage following middle cerebral artery occlusion is substantially worsened in HO2−/− mice (54).
Acknowledgments
We thank Drs. Kenneth D. Poss and Susumu Tonegawa for providing HO2−/− mice and matched controls. This work was supported by U.S. Public Health Service Grants DA-00266 and Research Scientist Award DA-00074 to S.H.S.; S.D. has a Centennial Fellowship from the Medical Research Council of Canada. C.D.F. has a Howard Hughes Fellowship for physicians.
ABBREVIATIONS
- BR
bilirubin
- BV
biliverdin
- CPR
cytochrome p450 reductase
- EDC
1-(ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride)
- GST
glutathione S-transferase
- HO
heme oxygenase
- nNOS
neuronal nitric oxide synthase
- PMA
phorbol 12-myristate 13-acetate
- PPIX
protoporphyrin IX
- PKC
protein kinase C
- HSA
human serum albumin
- HEK
human embryonic kidney
References
- 1.Maines M D. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Ewing J F, Maines M D. Brain Res Brain Res Protoc. 1997;1:165–174. doi: 10.1016/s1385-299x(96)00027-x. [DOI] [PubMed] [Google Scholar]
- 3.Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 4.Zakhary R, Poss K D, Jaffrey S R, Ferris C D, Tonegawa S, Snyder S H. Proc Natl Acad Sci USA. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stocker R, Yamamoto Y, McDonagh A F, Glazer A N, Ames B N. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 6.Bélanger S, Lavoie J C, Chessex P. Biol Neonate. 1997;71:233–238. doi: 10.1159/000244422. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins P N, Wu L L, Hunt S C, James B C, Vincent G M, Williams R R. Arterioscler Thromb Vasc Biol. 1996;16:250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- 8.Poss K D, Thomas M J, Ebralidze A K, O’Dell T J, Tonegawa S. Neuron. 1995;15:867–873. doi: 10.1016/0896-6273(95)90177-9. [DOI] [PubMed] [Google Scholar]
- 9.Doré S, Kar S, Quirion R. Proc Natl Acad Sci USA. 1997;94:4772–4777. doi: 10.1073/pnas.94.9.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer G J, Torricelli J R, Evege E K, Price P J. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 11.Neuzil J, Stocker R. J Biol Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 12.Brodersen R. J Biol Chem. 1979;254:2364–2369. [PubMed] [Google Scholar]
- 13.Okamura Y, Yamazaki M, Yamaguchi T, Komoda Y, Sugimoto A, Nakajima H. Biochim Biophys Acta. 1991;1073:538–542. doi: 10.1016/0304-4165(91)90227-8. [DOI] [PubMed] [Google Scholar]
- 14.Burnett A L, Johns D G, Kriegsfeld L J, Klein S L, Calvin D C, Demas G E, Schramm L P, Tonegawa S, Nelson R J, Snyder S H, Poss K D. Nat Med. 1998;4:84–87. doi: 10.1038/nm0198-084. [DOI] [PubMed] [Google Scholar]
- 15.Schall T J, Lewis M, Koller K J, Lee A, Rice G C, Wong G H, Gatanaga T, Granger G A, Lentz R, Raab H, et al. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- 16.Kingston R. In: Currents Protocols in Molecular Biology. Janssen K, editor. Vol. 1. New York: Wiley; 1995. pp. 9.0.1–9.9.6. [Google Scholar]
- 17.Ferris C D, Huganir R L, Bredt D S, Cameron A M, Snyder S H. Proc Natl Acad Sci USA. 1991;88:2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinlan E M, Halpain S. J Neurosci. 1996;16:7627–7637. doi: 10.1523/JNEUROSCI.16-23-07627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doré S, Zakhary R, Ferris C D, Hester L D, Snyder S H. Soc Neurosci Abstr. 1997;23:2214. [Google Scholar]
- 20.Ekinci F J, Shea T B. Int J Dev Neurosci. 1997;15:867–874. doi: 10.1016/s0736-5748(97)00037-3. [DOI] [PubMed] [Google Scholar]
- 21.Kuchera S, Barth H, Jacobson P, Metz A, Schaechtele C, Schrier D. Agents Actions. 1993;39:C169–C173. doi: 10.1007/BF01972756. [DOI] [PubMed] [Google Scholar]
- 22.Drummond G S, Kappas A. Proc Natl Acad Sci USA. 1981;78:6466–6470. doi: 10.1073/pnas.78.10.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshinaga T, Sassa S, Kappas A. J Biol Chem. 1982;257:7778–7785. [PubMed] [Google Scholar]
- 24.Zakhary R, Gaine S P, Dinerman J L, Ruat M, Flavahan N A, Snyder S H. Proc Natl Acad Sci USA. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grundemar L, Ny L. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- 26.Dwyer B E, Nishimura R N, Lu S Y. Brain Res Mol Brain Res. 1995;30:37–47. doi: 10.1016/0169-328x(94)00273-h. [DOI] [PubMed] [Google Scholar]
- 27.Rogers S, Wells R, Rechsteiner M. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 28.Dwyer B E, Nishimura R N, De Vellis J, Yoshida T. Glia. 1992;5:300–305. doi: 10.1002/glia.440050407. [DOI] [PubMed] [Google Scholar]
- 29.Henle E S, Linn S. J Biol Chem. 1997;272:19095–19098. doi: 10.1074/jbc.272.31.19095. [DOI] [PubMed] [Google Scholar]
- 30.Traystman R J, Kirsch J R, Koehler R C. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- 31.Poss K D, Tonegawa S. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Snyder S H. Annu Rev Pharmacol Toxicol. 1995;35:213–233. doi: 10.1146/annurev.pa.35.040195.001241. [DOI] [PubMed] [Google Scholar]
- 33.Sessoms J S, Chen S J, Chetkovich D M, Powell C M, Roberson E D, Sweatt J D, Klann E. Second Messengers Phosphoproteins. 1992;14:109–126. [PubMed] [Google Scholar]
- 34.Minetti M, Mallozzi C, Di Stasi A M, Pietraforte D. Arch Biochem Biophys. 1998;352:165–174. doi: 10.1006/abbi.1998.0584. [DOI] [PubMed] [Google Scholar]
- 35.De Matteis F, Dawson S J, Gibbs A H. Free Radic Biol Med. 1993;15:301–309. doi: 10.1016/0891-5849(93)90077-8. [DOI] [PubMed] [Google Scholar]
- 36.Gopinathan V, Miller N J, Milner A D, Rice-Evans C A. FEBS Lett. 1994;349:197–200. doi: 10.1016/0014-5793(94)00666-0. [DOI] [PubMed] [Google Scholar]
- 37.Farrera J A, Jauma A, Ribo J M, Peire M A, Parellada P P, Roques-Choua S, Bienvenue E, Seta P. Bioorg Med Chem. 1994;2:181–185. doi: 10.1016/s0968-0896(00)82013-1. [DOI] [PubMed] [Google Scholar]
- 38.Boyer T D. Hepatology. 1989;9:486–496. doi: 10.1002/hep.1840090324. [DOI] [PubMed] [Google Scholar]
- 39.Zucker S D, Goessling W, Gollan J L. J Biol Chem. 1995;270:1074–1081. doi: 10.1074/jbc.270.3.1074. [DOI] [PubMed] [Google Scholar]
- 40.Listowsky I, Abramovitz M, Homma H, Niitsu Y. Drug Metab Rev. 1988;19:305–318. doi: 10.3109/03602538808994138. [DOI] [PubMed] [Google Scholar]
- 41.Johnson J A, el Barbary A, Kornguth S E, Brugge J F, Siegel F L. J Neurosci. 1993;13:2013–2023. doi: 10.1523/JNEUROSCI.13-05-02013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otieno M A, Baggs R B, Hayes J D, Anders M W. Drug Metab Dispos. 1997;25:12–20. [PubMed] [Google Scholar]
- 43.Gourley G R. Adv Pediatr. 1997;44:173–229. [PubMed] [Google Scholar]
- 44.Newman T B, Maisels M J. Pediatrics. 1992;89:809–818. [PubMed] [Google Scholar]
- 45.Dennery P A, Rodgers P A. J Perinatol. 1996;16:S79–S83. [PubMed] [Google Scholar]
- 46.Hegyi T, Goldie E, Hiatt M. J Perinatol. 1994;14:296–300. [PubMed] [Google Scholar]
- 47.Gaton D D, Gold J, Axer-Siegel R, Wielunsky E, Naor N, Nissenkorn I. Br J Ophthalmol. 1991;75:532–534. doi: 10.1136/bjo.75.9.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heyman E, Ohlsson A, Girschek P. N Engl J Med. 1989;320:256. doi: 10.1056/NEJM198901263200420. [DOI] [PubMed] [Google Scholar]
- 49.Schneider A P d. J Am Med Assoc. 1986;255:3270–3274. [PubMed] [Google Scholar]
- 50.Wu T W, Carey D, Wu J, Sugiyama H. Biochem Cell Biol. 1991;69:828–834. doi: 10.1139/o91-123. [DOI] [PubMed] [Google Scholar]
- 51.Wu T W, Wu J, Li R K, Mickle D, Carey D. Biochem Cell Biol. 1991;69:683–688. doi: 10.1139/o91-102. [DOI] [PubMed] [Google Scholar]
- 52.Ewing J F, Haber S N, Maines M D. J Neurochem. 1992;58:1140–1149. doi: 10.1111/j.1471-4159.1992.tb09373.x. [DOI] [PubMed] [Google Scholar]
- 53.Schwertner H A, Jackson W G, Tolan G. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 54.Doré S, Sampei K, Koehler S, Blackshaw S, Takahashi M, Traysman R J, Snyder S H. Soc Neurosci Abstr. 1998;24:1233. [Google Scholar]