Abstract
Relationships between parent and child executive functioning were examined, controlling for the critical potential confound of IQ, in a family study involving 434 children (130 girls, 304 boys) and 376 parents from 204 community recruited families at high risk for the development of substance use disorder. Structural equation modeling found evidence of separate executive functioning and intelligence (IQ) latent variables. Mother’s and father’s executive functioning were associated with child’s executive functioning (beta = 0.34 for father-child, 0.51 for mother-child), independently of parental IQ, which as expected was associated with child’s IQ (beta = 0.52 for father-child, 0.54 for mother-child). Familial correlations also showed a significant relationship of executive functioning between parents and offspring. These findings clarify that key elements of the executive functioning construct are reliably differentiable from IQ, and are transmitted in families. This work supports the utility of the construct of executive function in further study of the mechanisms and etiology of externalizing psychopathologies.
Keywords: Executive functioning, Intelligence, Intergenerational transmission, Familial correlation, Neuropsychological tests
Introduction
Endophenotypes, intermediate component elements closer to genetic action than the indexed phenotype, are useful in psychopathology research because of the inherent difficulty of finding genes that have a one to one relationship with complex multifactorial behavioral disorders (Gottesman & Gould, 2003). A number of studies now suggest that executive functioning (EF) is one such variable, that is useful as an endophenotype for attention deficit-hyperactivity disorder (ADHD) (Doyle et al., 2005) and possibly also for other syndromes within the family of externalizing behavior disorders. Executive functioning is hypothesized to be closer to discrete avenues of gene expression than the more complex behavioral traits it indexes, as it refers to discrete cognitive skills that neuroimaging and lesion literatures have associated with specific brain circuits. In addition to attention-deficit hyperactivity disorder (Barkley, 1997), EF has been identified as an etiologically important intermediary component in literatures on conduct problems and antisocial behavior (Lynam & Henry, 2001), substance use disorders and alcoholism (Giancola & Moss, 1998), and personality disorder (Nigg, Silk, Stavro, & Miller, 2005).
A key property of an endophenotype is that it must run in families, and ultimately, be under some significant degree of heritable control. Although family transmission studies inevitably have genetic and family environment confounded, they are essential in the early stages of discovery when few large scale twin or adoption studies have been conducted due to prohibitive cost. Whereas several studies have looked at the familiality of components of EF, surprisingly few twin or family studies with adequate sample sizes have addressed this issue for EF per se (see review by Doyle et al., 2005). Kuntsi (2005) looked at variability of reaction times in approximately 4000 twin pairs, and found evidence for shared genetic effects between hyperactivity and response variability; however other executive measures were not assessed. In a study involving 52 sibling pairs with ADHD, significant correlations were found between many of the measures of response inhibition and attentional control (Slaats-Willemse, Swaab-Barneveld, De Sonneville, & Buitelaar, 2005). In still another study, involving 176 children (over half with ADHD) and their relatives, Nigg, et al (2004) found small (r ~ .20) but statistically significant familial correlations for individual EF measures. A recent study by Friedman et al. (2008) is a noteworthy exception both in sample size and in variety of EF measures assessed. Using a twin study design and a latent variable analysis, they observed a very large genetic component for the elements of EF that were shared within particular EF domains. They also were able to show that the EF latent factor involved components of variance that were independent of IQ. Additional studies are needed to extend and replicate their findings, both of the familial transmission of EF abilities and tasks, and of the differentiation of EF and IQ.
For simplicity, we use the term EF to refer to cognitive control processes that enable maintenance of behavior on a goal and adaptation to task context in light of goal directed action. EF is likely related neurally to a series of parallel prefrontal-thalamic-striatal neural loops (Fuster, 1997; Pennington & Ozonoff, 1996) in which various component operations may be instantiated. Prior studies have suggested that creating latent variables using EF measures which removes measure-specific variance also greatly enhances the external validity of EF as a core component in ADHD (Friedman & Miyake, 2004; Willcutt et al., 2001). The present study attempted to reached across multiple EF domains and therefore yielded lower factor loadings than when concentrating on a more homogenous conceptual domain. However, this heterogeneous conceptualization of EF may best detect family transmission of a domain-general EF ability.
To the extent that the EF construct cuts across multiple adaptation and goal direction tasks, a key issue is the extent to which the shared or latent variance in these tasks overlaps with IQ. As noted earlier, one possibility is that the shared variance overlaps with “g” or general intelligence. Most theories of intelligence suggest that it is influenced by a range of underlying abilities, and in several theories, executive functioning is one of these components (Borkowski & Burke, 1996). Although this does not reduce the importance of EF (particularly if EF helps to explain “g”), it is essential to consider the relation of a general EF factor with a general intelligence factor. Since IQ measures have well established familial transmission and substantial heritability (McGue, Bouchard, Iacono, & Lykken, 1993), it is essential that studies of familiality of EF consider IQ as well. To make this evaluation fully informative, it is ideal to have a latent variable assessment of IQ as well as of EF.
With these issues in mind, the present study examined four hypotheses: (a) that distinct latent variables for EF and IQ would be identified which would be moderately but not extremely correlated (i.e., a two –factor model of executive functioning and IQ model would fit better than a one –factor “g” model); (b) moderate familial transmission of IQ would be observed, consistent with prior research; (c) familial transmission of a latent EF factor would be observed; and (d) familial transmission of EF and IQ would be distinct when both domains were modeled simultaneously in a family design.
Method
Participants
Participants were 434 children (130 girls and 304 boys) and 376 of their parents (203 mothers and 173 fathers) from 204 families who were assessed with an executive function battery as part of an ongoing, prospective study. The Michigan Longitudinal Study (MLS) is following a community sample of families with high levels of alcohol and other substance use disorders (SUD), along with a community contrast sample of families drawn from the same neighborhoods but without the high substance abuse profile (Zucker et al., 2000). This sample is at elevated risk for a range of psychopathologies relevant to EF, including antisocial behavior, depression, and substance use disorders. Including a high risk as well as community control sample maximized the range of EF abilities measured, without moving into the range of frank neurological impairment.
Children completed IQ and EF measures at age 12-14 (MLS Wave 4, 64% of sample) and 15-17 (MLS Wave 5, 69% of sample). When both waves of data were available for a particular instrument (47% of sample), they were averaged. Parents were assessed at target child Wave 5 (mean age = 44.8, SD = 16.7) or at target child Wave 6 if missed at Wave 5.
Measures
Parents and children completed a four-subtest short form of the relevant Wechsler test for IQ, and five tests of EF, of which it was hoped that at least a subset would form a reasonable latent variable. The score for each cognitive measure was manipulated so that a higher score always reflects better performance.
IQ
Adults completed the four-subtest short form of the WAIS-R (Reynolds, Willson, & Clark, 1983) consisting of Block Design, Information, Picture Completion and Arithmetic. Children were administered the WISC-R (Wechsler, 1974) using the same four subtests as adults. This short form has excellent reliability and validity (Sattler, 2008).
Executive Functioning
EF was measured by five measures widely believed to tap either EF or an important component of it: Trailmaking, Tower of Hanoi, Stop Task, Paced Auditory Serial Additional Task (PASAT), and Wisconsin Card Sort.
The Trail Making Test (Trails) is a widely-used, timed paper-and-pencil test (Reitan, 1979) that activates prefrontal cortex (Stuss et al., 2001). Part A is a control condition tracing a line between numbers; part B is an executive condition alternating numbers and letters. To index set shifting, we created a Trails B Residual score variable by regressing Trails B on Trails A.
Tower of Hanoi (Tower) (Lezak, 1995) measures the ability to plan and to manipulate complex visual information in working memory. Poor performance is associated with frontal neural injury (Goel & Grafman, 1995) and we speculate that the task activates spatial working memory modules in right prefrontal cortex as described in the cognitive neuroscience literature (Courtney, Petit, Maisog, Ungerleider, & Haxby, 1998). Participants moved different-sized rings on a three- or four-peg board from one peg to another, following specific rules, including not placing a smaller ring on top of a larger one and moving only one ring at a time. Both time to completion and number of moves indicate performance effectiveness. Thus, these two scores were standardized and averaged; that score was divided by the minimum number of moves required for completion to correct for problem difficulty (three or four pegs).
The Wisconsin Card Sort (WCST) was computer-administered. It assesses working memory, concept formation, and set-shifting (Heaton, Chelune, Talley, Kay, & Curtiss, 1993). The test is associated with activation in dorsolateral prefrontal cortex (Weinberger, Berman, Gold, & Goldberg, 1994) perhaps due to its requirement to protect working memory (Botvinick, Braver, Barch, Carter, & Cohen, 2001). Up to 128 trials were administered in which the participant sorted cards based on criteria of color, shape, and number (the task ends when the participant consecutively sorts 10 cards correctly in each of six categories, or reaches the maximum of 128 cards). The participant was not told how to sort the cards, and instead deduced the current correct principle using only the computer’s feedback (“correct” or “incorrect”); the sorting principle changed periodically without the participant’s knowledge, and needed to be deduced again. Perseverative errors (reversed) and categories completed (r = -.70) were standardized and averaged to create a composite score in which higher scores indicate better performance.
The Stop Task (Stop) requires suppression of a prepared response. It entails activation of areas in prefrontal cortex, particularly the right inferior frontal gyrus and basal ganglia (A. R. Aron, Fletcher, Bullmore, Sahakian, & Robbins, 2003). Procedures were the same as those used by Logan, Schachar and Tannock (1997). Participants completed 8 blocks of 32 trials, after two practice blocks. They responded to an ‘X’ or ‘O’ as quickly as possible using their dominant hand; they withheld responding when they heard a tone (25% of trials). Timing of the warning signal (stop signal) was varied stochastically to identify the amount of warning that enabled a participant to interrupt a response 50% of the time, enabling efficient estimation of the stop signal reaction time (Stop RT) (Band, Van Der Molen, & Logan, 2003).
The Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977) measures verbal working memory and updating, as well as attention. The participant adds 60 pairs of randomized digits so that each is added to the immediately preceding one. Digits were presented at four rates of speed via an audio-taped presentation, yielding a score for mean number of correct responses. Neurological (Larrabee & Curtiss, 1995) and neuroimaging data (Deary et al., 1994) indicate that the task places demands on prefrontal cortex and anterior cingulate.
Alcoholism
Parent alcoholism, a control variable, was defined by either parent having any lifetime diagnosis of alcoholism (DSM-IV abuse or dependence) at the time of the neuropsychological assessments, as coded by a trained clinician using data from the Diagnostic Interview Schedule (Robins, Cottler, Bucholz, & Compton, 1995), the Drinking and Drug Use Questionnaire (Zucker, Fitzgerald, & Noll, 1990), and the Short Michigan Alcohol Screening Test (Selzer, Vinokur, & van Rooijen, 1975). The Lifetime Alcohol Problems Score (LAPS: Zucker, Davies, Kincaid, Fitzgerald, & Reider, 1997) collected concurrently with the neuropsychological testing provided a dimensional measure of the extent of alcohol problems over the life course. It is a three-component measure involving indicators of onset, course of disorder, and breadth of symptoms. It has been validated with regard to diagnosis, extent of alcohol-specific symptoms, and having been in treatment (Zucker et al., 1997). Two-group analysis of the structural equation modeling allowed comparisons of the models in families at higher and lower risk due to alcoholism.
Data Analysis
Each of the cognitive scores was evaluated for normality by examining normal probability plots, using SAS PROC UNIVARIATE. Some of the variables showed modest deviations from normality. However, since factor analysis is robust to departures from normality, no transformations were judged necessary to reduce these modest discrepancies. In the analyses which relied on linear relationships, nonlinearity and influential outliers were checked by examining a graph of the two variables (4 bivariate outliers on child Trails A and one parent-child Trails B residual outlier were excluded). For fathers’ data, which had more univariate outliers than either mothers or children, when values were more than 4 standard deviations from the mean, they were trimmed back to the next value closer to the mean. This affected one data point each for WCST, Stop, and Trails, and three for Tower.
Structural equation models were estimated using the Mplus program (Muthén & Muthén, 1998-2004). For the child and parent-child models, we used TYPE = COMPLEX which computes standard errors and chi-square tests of model fit taking into account non-independence of observations due to having multiple siblings from the same families. The missing data method used by this program estimates the parameters with a full information maximum likelihood estimator (FIML) using all observations in the data set (T. E. Duncan, Duncan, & Li, 1998; Schafer & Graham, 2002). Data were selected so that at most nine of the 18 variables used in the model were missing. In all, 13.9% of the data points were missing for the variables used in the structural equation model. The resulting covariance coverage matrix had more than half of the values greater than .8 and only one value less than .5. The recommended coverage is that all of the values be greater than .5 (Schafer, 1997). A combination of multiple fit indices are reported with acceptable values as follows: SRMR < .09, Tucker-Lewis Index (TLI) > .95, (Hu & Bentler, 1999), Comparative Fit Index (CFI) > .95, Root Mean Square Error of Approximation < .05. The chi-square is reported as well, although its significance is inflated due to large sample size. Family correlations were estimated using the FCOR program from S.A.G.E. (2004). It estimated main correlations (mother-father, parent-offspring, and sibling) as well as subtypes (e.g. mother-son, mother-daughter, father-son, father-daughter) and provided homogeneity tests for significant differences between the correlations.
Results
Descriptive statistics
Table 1 shows descriptive statistics for the sample organized with regard to high-risk (n=323 children; either parent having a lifetime alcoholism diagnosis) and the low-risk group (n=111 children; no diagnosis in parents). Each group was approximately 30% female and both groups were the same average age at the two waves of testing. The EF composite score (described subsequently) did not differ significantly between the two groups, but IQ was significantly lower in the high-risk group.
Table 1.
Descriptive statistics for high-risk and low-risk adolescents.
| Descriptor | High-risk | Low-risk | Cohen’s d | p |
|---|---|---|---|---|
| N | 323 | 111 | __ | __ |
| % Female | 30.0 | 29.7 | __ | __ |
| Age, Wave 4 | 13.7 | 13.7 | 0.01 | .9 |
| Age, Wave 5 | 16.5 | 16.6 | .08 | .5 |
| IQ | 104.6* | 109.1 | .36 | .003 |
| EF composite (standardized) | .05 | .02 | .04 | .8 |
p < .01
EF- Executive functioning: composite of Trailmaking, Wisconsin card sort and Tower of Hanoi
Preliminary Correlations
Table 2 shows the first-order correlations between child and parent performance on the neuropsychological tests; these ranged from.08 to .43. The child-parent correlations averaged .22 for individual EF variables (for both mothers and fathers), a benchmark we expected to improve with the latent variable approach. As expected, composite IQ correlations, at .44 to .47, exceeded those for individual neuropsychological tests, perhaps due to the higher reliability of the composite IQ score than the individual EF tasks.
Table 2.
Parent-adolescent correlations for individual neuropsychological tests and IQ
| Neuropsychological test | Mother-Adolescent Correlation | Father-Adolescent Correlation |
|---|---|---|
| Trailmaking B(residual) | .17** | .11* |
| Wisconsin card sort | .16** | .12* |
| PASAT | .11 | .43*** |
| Stopping task – Go RT | .12* | .16** |
| Stop RT | .29*** | .25*** |
| Variability | .34*** | .31*** |
| Tower of Hanoi | .17** | .17** |
| Average of EF tests | .22 | .22 |
| IQ | .44*** | .47*** |
p < .001;
p < .01;
p < .05;
PASAT - Paced Auditory Serial Addition Test; RT -Reaction time; EF - Executive function
Table 3 shows the inter-correlations among the EF and IQ individual subtest and composite scores. Although all of the EF tests were significantly correlated with the IQ subtests, the IQ subtests were for the most part more highly correlated with each other than with EF tasks.
Table 3.
Intercorrelations among Executive function tests and IQ subtests for children
| EF test | WCST | PASAT | Stop | Tower | Arithmetic | Block Design | Information | Picture Completion | Full IQ |
|---|---|---|---|---|---|---|---|---|---|
| Trailmaking B(residual) | .29*** | .09 | .23*** | .21*** | .28*** | .24*** | .25*** | .10 | .33*** |
| Wisconsin card sort | -- | .16** | .13** | .23*** | .25*** | .26*** | .32*** | .14* | .38*** |
| PASAT | -- | -- | .14* | .17** | .23*** | .24*** | .16** | .10 | .19** |
| Stop RT | -- | -- | -- | .09 | .13* | .11* | .11* | .10 | .17** |
| Tower of Hanoi | -- | -- | -- | -- | .29*** | .31*** | .16** | .17** | .34*** |
| Average | .26 | .23 | .20 | .12 | .28 | ||||
| IQ subtest | |||||||||
| Arithmetic | -- | .47*** | .52*** | .24*** | .66*** | ||||
| Block | .41*** | .41*** | .74*** | ||||||
| Information | -- | .29*** | .69*** | ||||||
| Picture completion | -- | .58*** |
p<.001;
p<.01;
EF - Executive function; WCST - Wisconsin Card Sort; PASAT - Paced Auditory Serial Addition Test; RT - reaction time
Development of latent variables for Executive functioning and IQ
The first objective of the modeling was to create latent variables for Executive functioning (EF) and intelligence (IQ) based on a theoretical model that would use the same indicators for parents and children. For the executive functioning latent variable, we started with a theoretical model for executive functioning that included all five of the measures described in the Method section. The latent variable for IQ included the four available subtests of IQ also as described in the Method section. The models were developed for the mothers, the fathers, and the children. To maximally differentiate the EF and IQ latent variables, we dropped PASAT and the Arithmetic subtest of the WAIS from the analysis, since modification indices indicated that allowing cross-loading of these two indicators onto both EF and IQ latent variables would provide a better model fit. The models were then examined for mothers, fathers, and children with the remaining four indicators for EF and three for IQ. The Stop RT did not load significantly (p > .05) on the EF latent variable for fathers, so it was also dropped from the model.
Figures 1-3 show the resulting structural equation models for each parent and for children, along with loadings for each indicator. All of the models have good fit and each indicator has significant loading on its factor. These models satisfied the goal of achieving the same model for EF and IQ, across all three groups (mothers, fathers, and children).
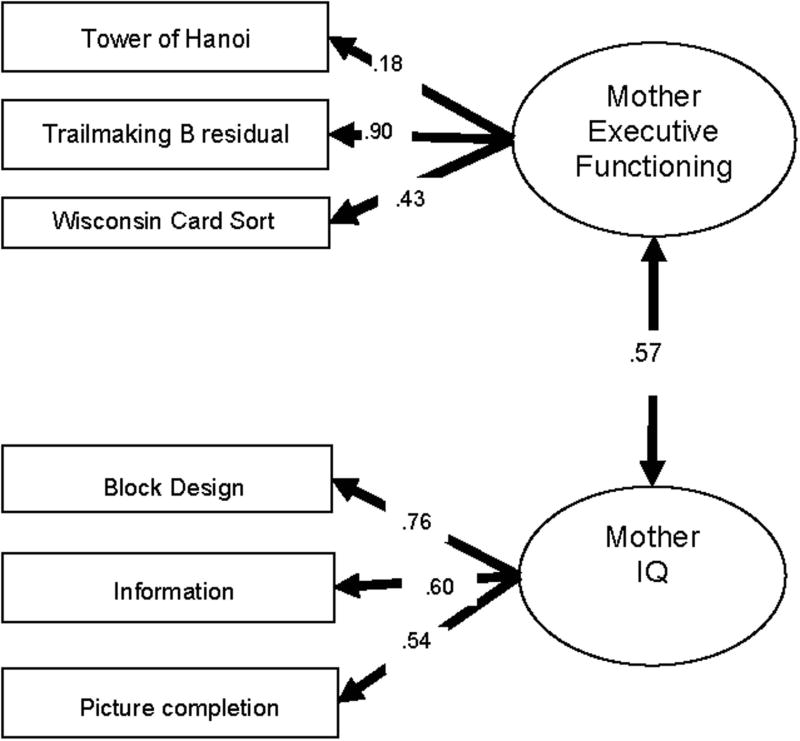
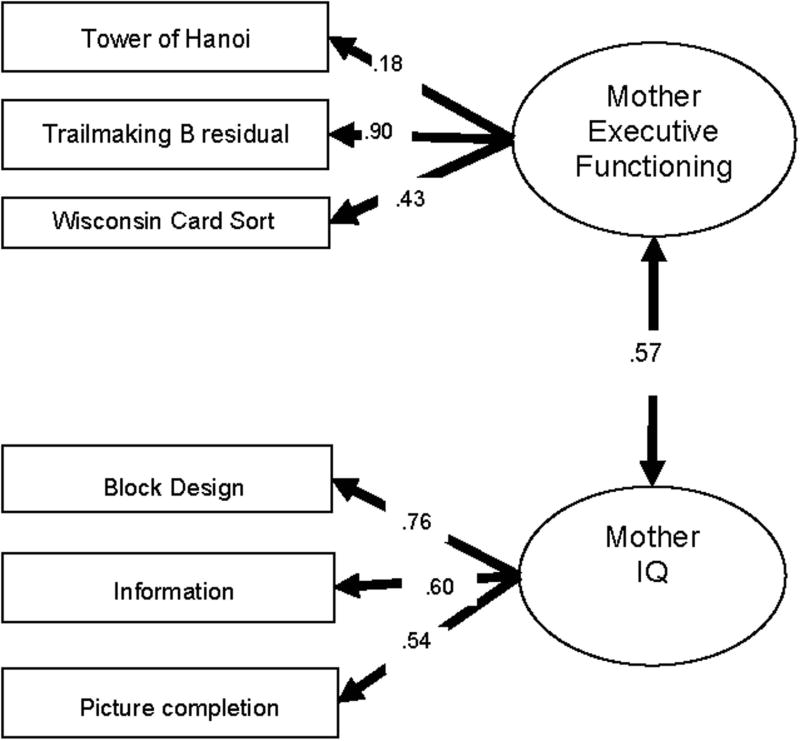
Fig. 1.
Structural equation model of executive function and IQ in mothers. CFI = 1.0, TLI = 1.0, RMSEA = .000, SRMR = .029.
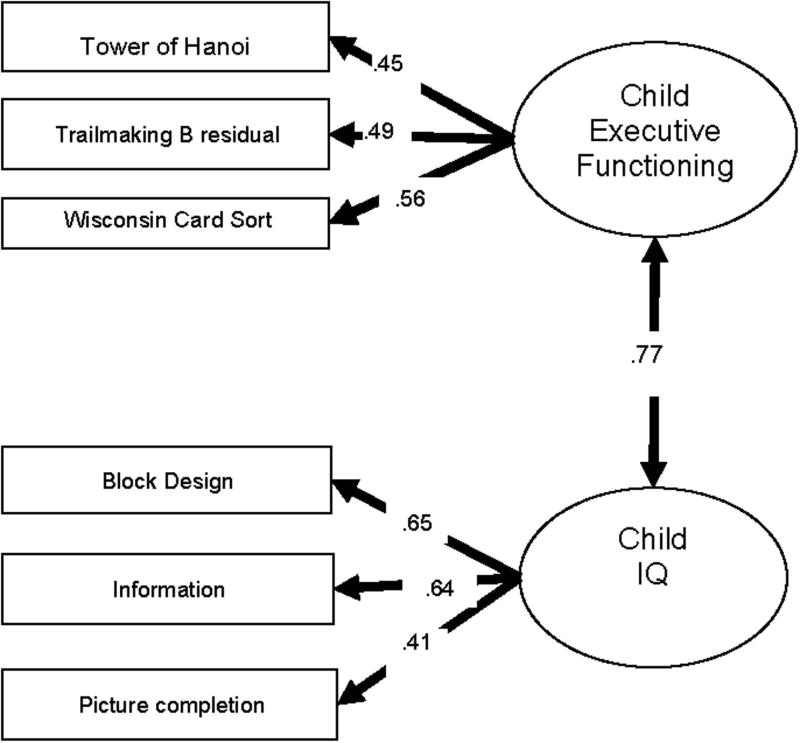
Fig. 3.
Structural equation model of executive functioning and IQ in adolescents. CFI = .99, TLI = .97, RMSEA = .036, SRMR = .027.
To evaluate the hypothesis that these were distinct latent factors, two-factor versus one-factor models were compared. For mothers, the model fit was significantly better for the two-factor solution (Δχ2 (1)= 15.1, p < .0001. For fathers, the two-factor fit was only marginally better fitting than the one-factor (Δχ2 (1)= 2.5, p = .11. For children, the model was estimated using a robust maximum likelihood estimator (MLR) that took into account the non-independence due to siblings. The scaled Satorra-Bentler chi-square difference (Muthén & Muthén, 1998-2004).was 5.3, 1 d.f., p < .05, showing that the two-factor model was a significantly better fit than the one-factor model. Overall, it was concluded that the two factor model (EF + IQ) provided a better solution and remaining analyses proceeded on this basis.
Parent-child models
Using the models developed above for the parents and children, structural equation modeling tested the strength of association between parents and children for the latent EF and IQ constructs. The modeling was performed separately for mothers and children, and then for fathers and children, in order to examine possible different effects of parent gender and to see if effects replicated across parents.
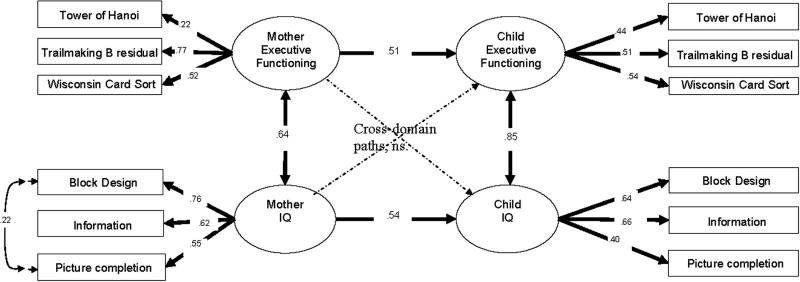
For the mother-child model, the structural model consisted of (1) a regression relationship with mother’s IQ and EF predicting children’s IQ and EF and (2) covariance allowed between (a) mother’s IQ and mother’s EF and (b) child’s IQ and child’s EF (pictured in Figure 4). The covariance cross-paths (between IQ and EF scores for each group) were not significant and small (less than 35% of the magnitude of the same-domain relation). These cross-domain paths were able to be omitted (fixed to 0) without loss of model fit (Δχ2 (2)=2.0, p > .3). The same-domain paths were significant and substantial, with the EF regression parameter from mother to children at .51 and the IQ regression from mothers to children at .54, as shown in Figure 4. The resulting model had acceptable fit (CFI = .97, RMSEA = .027, χ2 (49)=64.2, TLI =.95, SRMR = .04)
Fig. 4.
Structural equation models for the relationship of mothers’ and children’s executive functioning and IQ.
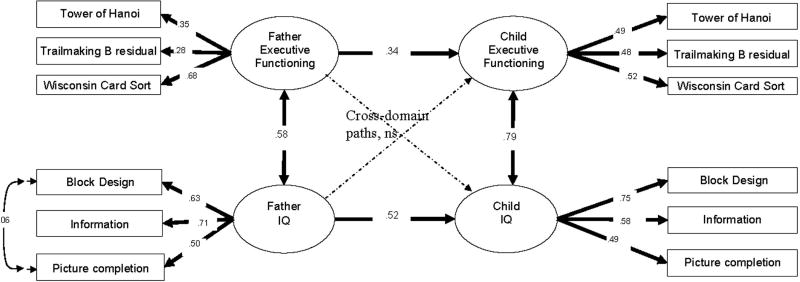
A similar structural equation model was tested for fathers and children. This model had poor fit (χ2 (47)=85.8, CFI=.90, TLI = .87, RMSEA= .044, SRMR = .047). Modification indices in this model suggested a better fit would be obtained if residual covariance were allowed between the Information subtest in fathers and in children and between the Block subtests in fathers and children. Since it is theoretically reasonable to expect covariance between these subtests measuring like constructs in fathers and children, these covariances were allowed. The resulting model had acceptable fit (χ2 (45)=68.7, CFI = .94, TLI = .91, RMSEA = .035, SRMR = .046) and all indicators loaded significantly on their factors. The regression pathway of child IQ on father IQ was significant (.72, p < .01). However, the regression pathway of child EF on father EF was not (.51, p = .12), when the cross-loading of child EF on father’s IQ was also included. Since the cross-loading of child EF on father IQ was substantially smaller than child EF on father EF (path coefficient=.20, p =.52), this pathway was removed without affecting the model fit (Δχ2 (1)=.238, p > .3, where the chi-square is adjusted for the COMPLEX type in Mplus). Figure 5 shows the final model, which fits well (χ2 (47)=69.3, CFI = .95 TLI = .92 SRMR = .047 RMSEA = .033) and has significant regression relationships of child EF on father EF (.34, p = .001) and child IQ on father IQ (.52, p < .001).
Fig. 5.
Structural equation models for the relationship of fathers’ and children’s executive functioning and IQ.
Father’s alcoholism
Two-group modeling examined the differences between the group with the most severe paternal alcoholism and the rest of the sample. Since about two-thirds of the men had a lifetime diagnosis of alcoholism, the Lifetime Alcohol Problem score (LAPS), a continuous measure of the severity of alcoholism, was used to create two groups of roughly equal size, using a median split. For 55 men, the LAPS score was missing. These men were assigned to the high risk group if they had a lifetime DSM-IV diagnosis of alcohol abuse or dependence, which was true for 50 of these 55 men. This resulted in 252 in the high risk group and 190 in the low risk group. The two structural equation models in Figures 4 and 5 were repeated using a two-group design. First all parameters were free to vary and then the two of most interest, the regression relationships between parent’s IQ and child’s IQ and between parent EF and child’s EF were fixed in the two groups. For both mother-child and father-child models, there were no significant differences in the model fit when these constraints were imposed (mother-child, Δχ2 (2)= 1.75, p > .1; father-child, Δχ2 (2)= 5.75, p > .05, using an adjusted chi-square difference test. This suggests that executive functioning transmission is similar in alcoholic and non-alcoholic families.
Familial correlations
To further confirm these relationships and to investigate them by subtype (parent and child gender), familial correlations were examined using composite scores created by averaging the subtests from the SEM models into single manifest variables. First, as seen in Table 4, for IQ, the parent:offspring correlation was .45, with no evidence for differences in correlations by sub-type (e.g. father-daughter vs. mother-son). This is in close agreement with a weighted average of correlations in a meta-analysis report of midparent-offspring correlation for IQ of .48 (Bouchard & McGue, 1981). Our finding of spousal correlation of .45 was somewhat higher than the same meta-analysis which showed an assortative mating effect of .33 (Bouchard & McGue, 1981), perhaps due to the nature of the current sample which has a high incidence of alcoholism, another basis for marital assortment. The overall sibling IQ correlation was .50, again very close to the weighted average (.47) from the Bouchard study. Subtype correlations ranged from .29 to .81 (the highest value based on only 17 sister pairs); thus there was evidence for heterogeneity of sub-types with sister-sister pairs showing the highest correlation, followed by sister-brother pairs and then brother-brother pairs. These data suggest that the present sample was comparable to other major samples with regard to familial transmission of IQ, lending some confidence to the EF data for which few comparable samples exist.
Table 4.
Familial correlations for IQ and EF residualized for IQ for all relationships in study, combined and separated by gender
| Relationship | IQ correlation | N | EF correlation (5 component average) | N | EF correlation (3 component average) | N | Residualized EF correlation (3 component average) | N |
|---|---|---|---|---|---|---|---|---|
| Parent:offspringa | .45*** | 1014 | .36*** | 698 | .24*** | 705 | .15*** | 806 |
| Father:son | .45*** | 344 | .26*** | 232 | .15 | 232 | .05 | 275 |
| Mother:son | .42*** | 353 | .45*** | 268 | .35*** | 257 | .31*** | 300 |
| Father:daughter | .49*** | 154 | .45** | 92 | .35*** | 103 | .24** | 108 |
| Mother:daughter | .49*** | 164 | .22** | 108 | .04 | 106 | -.06 | 123 |
| Sibling | .50b*** | 303 | .24*** | 267 | .15* | 267 | .04b | 202 |
| Brother:brother | .29*** | 88 | .40*** | 105 | .24** | 105 | .08 | 53 |
| Sister:brother | .55*** | 198 | .16** | 151 | .10 | 151 | .05 | 138 |
| Sister:sister | .81*** | 17 | -.04 | 9 | -.19 | 11 | -.29 | 11 |
| Spouse | .45*** | 266 | .31*** | 169 | .19** | 168 | .09 | 227 |
p<.05,
p<.01,
p< .001
These correlations are based on entire sample available and include some in the study who do not have EF data
Homogeneity tests show that there is significant variation in correlations among these groups (p < .05)
Table 4 then shows results for two kinds of composite EF scores: one based on 3 EF tests as validated in the Results section, and one based on 5 tests as initially theorized. Table 4 also shows results for a residualized EF score (from the 3-test composite) with IQ removed.
As the table illustrates, the zero order familial EF correlations for parent-offspring for the 5 subtest composite was .35 and for siblings was .24. These values were slightly lower, and in some instances non-significant, for the 3 subtest composite. Each group had substantial variation in subtype correlations (for the 3-test composite, parent-offspring (χ2(3) = 13.7, p=.003), siblings (χ2(2) = 7.1, p= .03). For parent-offspring relationships, the opposite sex parents had higher correlations of EF with their children than same sex parents; however for siblings, the highest correlation was of brothers with brothers.
Turning to the residual EF score with IQ partialled (final column of Table 4), the parent-offspring correlation for the residual EF measure was .15 (p<.001) and the sibling correlation was .04 (p>.05) (Table 4). Based on both the siblings and parent-offspring data, these results are consistent with a heritability estimate for EF of .08 to .30 (two times the correlation coefficients) (Falconer & TFC, 1996). Homogeneity tests indicated substantial variability in the subtype correlations for siblings (χ2(6) = 17.0, p< .01), as well as parent-offspring subtypes (χ2(12) = 24.5, p< .05).
Discussion
Executive functioning (EF) is widely studied as a potential genetically informative mechanism in clinical research, yet its familiality, and heritability have only recently begun to be examined. EF is a particularly interesting domain for examining family transmission because its neural instantiation is relatively well developed, related to regions of prefrontal cortex(Fuster, 1997), striatum, cerebellum (see Casey, Nigg, & Durston, 2007)and other regions. For that reason EF has been a crucial endophenotype for behavioral analysis of neural theories of psychopathology, among other uses. Showing that aspects of EF are familial, independent of familial transmission of IQ, is crucial to evaluating such theories. The present study provides further evidence that EF is familial thus providing more support for its potential utility as an endophenotype for genetic and other etiological research on psychopathology.
Our study helps to confirm in a high risk population-based sample the heritability of EF found in a recent twin study (Friedman et al., 2008). The utilization of a large sample, along with demonstration of a familiality effect even when IQ is controlled as a latent variable, lends credence to our findings. The family transmission effect we observed for the latent EF variable was moderate (beta .34 to .51) after correcting for measurement unreliability and controlling for IQ. This effect was also considerably larger than the effects seen with the raw family correlations, probably due to limited reliability of the individual measures (see Friedman et al., 2006, for a similar result), and thus provides evidence that aspects of EF are familial to a moderate degree. This suggests that measurement unreliability may depress familial correlations and heritability for individual EF measures. Further, the familial correlations also show evidence for transmission of EF from parent to offspring, while controlling for IQ. These findings amplify those of Friedman et al (2008) and further justify the investment in more expensive twin, adoption, and molecular studies to determine what proportion of this transmission is genetic and thus relevant to genetic models of psychopathology.
Of course, in a family study, transmission may be related to genetic or environmental factors, or their interplay. Environmental sources of familial transmission can easily be suggested. For example, families with low SES are likely to be exposed to poorer nutrition, additional toxic exposures, and chronic stresses that are correlated across family members and that interfere with attention, working memory and executive functioning and/or neural development. However, if EF proves to be related to these factors in families then it may usefully serve as an endophenotype for environmental transmission of risk as well. Recent data suggest, however, that at least the domain general aspect of EF is likely to be more influenced by genetic than environmental variation in families (Friedman et al., 2008).
The latent factor we identified held up across three distinct groups: adolescents, adult men, and adult women, which is consistent with the view that some aspect of EF ability is shared across a subset of these tasks. We chose to focus on this single factor for EF in order to isolate and differentiate this component from IQ. At the same time, it is important to recognize that these data do not provide a “best fit” description of the entire EF domain, which would require many additional measures in order to provide complete coverage. Indeed, once EF is broken into component cognitive operations, multiple neural circuits can be identified in which these components may be instantiated (Adam R. Aron, 2008) . The neural instantiation of the domain general EF ability that may be shared across tasks is an interesting question for further exploration, but is likely to include regions of prefrontal cortex.
Further, whereas a better fit for each group might have been attainable with the five available measures (particularly by including the stop task for children and for mothers), the advantage of having a consistent model for the three groups (mothers, fathers, children) outweighed searching for the best fit for each group. Our finding that measures of EF clustered into a domain-general latent variable is consistent with other factor analytic studies of executive functioning (Miyake, Friedman, Rettinger, Shah, & Hegarty, 2001; Zelazo & Müller, 2002).
Our findings also underscore that there are aspects of executive functioning which are reliably differentiated from IQ in relation to family transmission. One piece of support for this is that there were significant difference in EF but not IQ between low and high risk groups based on father’s alcoholism. Secondly, the 2-factor model provided a better fit to the data than a 1-factor model for women and for children, and was marginally better for men. The relation between general intelligence and executive functions is potentially complex, with theories positing that executive function is a component of general intelligence (Borkowski & Burke, 1996). Indeed, neuroimaging data suggest that tasks loaded for the general intelligence factor tend to recruit regions of prefrontal cortex similar to those associated with executive functioning (J. Duncan et al., 2000). Conversely, earlier theory (Luria, 1966) and recent data (Friedman et al., 2006; Friedman et al., 2008) suggest that at least some elements of the EF construct are distinct from IQ. The history of clinical lore on frontal lobe head injury indicates that intelligence (measured as full scale IQ) is often spared, whereas EF (the ability to modulate behavior to context) is impaired. Some refinement of this understanding has accrued as intelligence has been conceptualized as a set fluid abilities. Thus, recent data suggest that when IQ is partialled, EF still carries strong external validity to important outcomes such as externalizing psychopathology (Nigg, 1999; Nigg, Blaskey, Huang-Pollock, & Rappley, 2002; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005).
There has also been spirited debate about the relation of such executive abilities as working memory to general intelligence (Ackerman, Beier, & Boyle, 2005; Kane, Hambrick, & Conway, 2005; Oberauer, Schulze, Wilhelm, & Suss, 2005). Therefore, in the current study, it was viewed as essential to model a general intelligence factor as well as an EF factor. Our two-factor solution runs counter to a position argued in some of the recent literature (e.g. Salthouse, 2005), although that argument depended heavily upon working memory, an aspect of EF that was not emphasized here.
The question we are addressing is of particular relevance to high risk samples such as the current one, given the putative relationship between EF and risk for addictive disorder (Hester & Garavan, 2004). Thus the issue of how this factor is transmitted intergenerationally should be of special interest to the field of addiction.
One of the limitations of this study is that the sample used is a high-risk one and thus the generalizability of results to other populations is not known. Another issue is that the EF latent variable had relatively low loadings, indicating that task-unique variance is present and remains to be explained. This issue is not new to the present work; it has often been the case in other studies of EF (Miyake et al., 2001; Zelazo & Müller, 2002) making it even more important to understand the phenomenon. Identification of a common EF factor is challenging from a psychometric standpoint because individual measures vary in their reliability. Such limited reliability may exist for numerous reasons. Different capacities are tapped at different ages by similar-seeming tasks. Also, the tasks rely on being novel, therefore making test-retest statistics difficult to evaluate (Delis, Kramer, Kaplan, & Holdnack, 2004). This remains a concern in the present work; problems with reliability would cause underestimation of the inter-generational transmission of the shared abilities that are part of EF.
Despite these limitations, the finding of specificity of EF in family relationships, independent of IQ, is parallel to the findings of Friedman et al. (2008) albeit using a different methodology. These results clarify that key elements of EF as a latent construct run in families independently of IQ, thus supporting the utility of EF in further study of the mechanisms and etiology of externalizing psychopathology.
Fig. 2.
Structural equation model of executive function and IQ in fathers. CFI = 1.0, TLI = 1.0, RMSEA = .000, SRMR = .036.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grants R01 AA12217 (RAZ & JTN) and R37 AA07065 (RAZ & HEF), as well as by NIMH R01 MH063146 (JTN). The S.A.G.E program package is supported by a USPHS Resource Grant (RR03655) from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackerman PL, Beier ME, Boyle MO. Working memory and intelligence: the same or different constructs? Psychol Bull. 2005;131(1):30–60. doi: 10.1037/0033-2909.131.1.30. [DOI] [PubMed] [Google Scholar]
- Aron AR. Progress in executive-function research: From tasks to functions to regions to networks. Current Directions in Psychological Science. 2008;17(2):124–129. [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6(2):115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Band GPH, Van Der Molen MW, Logan GD. Acta Psychologica. Vol. 112. Elsevier Science; 2003. Horse-race model simulations of the stop-signal procedure; pp. 105–142. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Borkowski JG, Burke JE. Theories, models, and measurements of executive functioning: an information processing perspective. In: Lyon GR, Krasnegor NA, editors. Attention memory and executive function. Baltimore, MD: Paul H. Brookes Publishing Co; 1996. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bouchard TJ, Jr, McGue M. Familial studies of intelligence: a review. Science. 1981;212(4498):1055–1059. doi: 10.1126/science.7195071. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Nigg JT, Durston S. New potential leads in the biology and treatment of attention deficit-hyperactivity disorder. Curr Opin Neurol. 2007;20(2):119–124. doi: 10.1097/WCO.0b013e3280a02f78. [DOI] [PubMed] [Google Scholar]
- Courtney SM, Petit L, Maisog JM, Ungerleider LG, Haxby JV. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279(5355):1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Ebmeier KP, MacLeod KM, Dougall N, Hepburn DA, Frier BM, et al. PASAT performance and the pattern of uptake of 99mTc-exametazime in brain estimated with single photon emission tomography. Biol Psychol. 1994;38(1):1–18. doi: 10.1016/0301-0511(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10(2):301–303. doi: 10.1017/S1355617704102191. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Faraone SV, Seidman LJ, Willcutt EG, Nigg JT, Waldman ID, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Duncan J, Seitz RJ, Kolodny J, Bor D, Herzog H, Ahmed A, et al. A neural basis for general intelligence. Science. 2000;289(5478):457–460. doi: 10.1126/science.289.5478.457. [DOI] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Li F. A comparison of model- and multiple imputation-based approaches to longitudinal analyses with partial missingness. Structural Equation Modeling. 1998;5(1):1–21. [Google Scholar]
- Falconer DS, M TFC. Introduction to quantitative genetics. 4. Harlow, Essex, UK: Addison Wesley Longman; 1996. [Google Scholar]
- Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent-variable analysis. Journal of Experimental Psychology General. 2004;133(1):101–135. doi: 10.1037/0096-3445.133.1.101. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17(2):172–179. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology General. 2008;137(2):201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology and neuropsychology of the frontal lobe. 3. New York: Raven; 1997. [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J. Are the frontal lobes implicated in “planning” functions? Interpreting data from the Tower of Hanoi. Neuropsychologia. 1995;33(5):623–642. doi: 10.1016/0028-3932(95)90866-p. [DOI] [PubMed] [Google Scholar]
- Gottesman I, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: A measure of recovery from concussion. Perceptual & Motor Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual. Lutz, FL: Publishing Assessment Resources; 1993. [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24(49):11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Kane MJ, Hambrick DZ, Conway AR. Working memory capacity and fluid intelligence are strongly related constructs: comment on Ackerman, Beier, and Boyle (2005) Psychol Bull. 2005;131(1):66–71. doi: 10.1037/0033-2909.131.1.66. author reply 72-65. [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Rijsdijk F, Ronald A, Asherson P, Plomin R. Genetic influences on the stability of attention-deficit/hyperactivity disorder symptoms from early to middle childhood. Biol Psychiatry. 2005;57(6):647–654. doi: 10.1016/j.biopsych.2004.12.032. [DOI] [PubMed] [Google Scholar]
- Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J Clin Exp Neuropsychol. 1995;17(4):536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. Oxford: Oxford University Press; 1995. [Google Scholar]
- Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychological Science. 1997;8(1):60–64. [Google Scholar]
- Luria AR. Higher Cortical Functions in Man. New York, N.Y: Basic Books; 1966. [Google Scholar]
- Lynam DR, Henry B. The role of neuropsychological deficits in conduct disorders. In: Hill J, Maughan B, editors. Conduct disorders in childhood and adolescence. Cambridge: Cambridge University Press; 2001. pp. 235–263. [Google Scholar]
- McGue M, Bouchard TJJ, Iacono WG, Lykken DT. Behavioral genetics of cognitive ability: A life-span perspective. In: Plomin R, McClearn GE, editors. Nature nurture & psychology. Washington, DC: American Psychological Association; 1993. pp. 59–76. [Google Scholar]
- Miyake A, Friedman NP, Rettinger DA, Shah P, Hegarty M. How are visuospatial working memory, executive functioning, and spatial abilities related? A latent-variable analysis. Journal of Experimental Psychology General. 2001;130(4):621–640. doi: 10.1037//0096-3445.130.4.621. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Third. Los Angeles, CA: Muthén & Muthén; 19982004. [Google Scholar]
- Nigg JT. The ADHD response-inhibition deficit as measured by the stop task: replication with DSM-IV combined type, extension, and qualification. J Abnorm Child Psychol. 1999;27(5):393–402. doi: 10.1023/a:1021980002473. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollock CL, Rappley MD. Neuropsychological executive functions and DSM-IV ADHD subtypes. J Am Acad Child Adolesc Psychiatry. 2002;41(1):59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Stawicki JA, Sachek J. Evaluating the endophenotype model of ADHD neuropsychological deficit: results for parents and siblings of children with ADHD combined and inattentive subtypes. J Abnorm Psychol. 2004;113(4):614–625. doi: 10.1037/0021-843X.113.4.614. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Silk K, Stavro G, Miller T. An inhibition perspective on borderline personality disorder. Development and Psychopathology. 2005;17:1129–1150. doi: 10.1017/s0954579405050534. [DOI] [PubMed] [Google Scholar]
- Oberauer K, Schulze R, Wilhelm O, Suss HM. Working memory and intelligence--their correlation and their relation: comment on Ackerman, Beier, and Boyle (2005) Psychol Bull. 2005;131(1):61–65. doi: 10.1037/0033-2909.131.1.61. author reply 72-65. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology & Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Manual for administration of neuropsychological test batteries for adults and chidlren. Tucson, AZ: Reitan Neuropsychological Laboratory; 1979. [Google Scholar]
- Reynolds CR, Willson VL, Clark PL. A four-test short form of the WAIS-R for clinical screening. Clinical Neuropsychology. 1983;5(3):111–116. [Google Scholar]
- Robins L, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV. St Louis: Washington University School of Medicine; 1995. [Google Scholar]
- S.A.G.E. Statistical Analysis for Genetic Epidemiology Release 5.0 2004 [Google Scholar]
- Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19(4):532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- Sattler JM. Assessment of children Cognitive foundations. LaMesa, CA: Jerome M Sattler, Publisher, Inc; 2008. [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. London, New York: Chapman & Hall; 1997. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36(1):117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Slaats-Willemse D, Swaab-Barneveld H, De Sonneville L, Buitelaar J. Familial clustering of executive functioning in affected sibling pair families with ADHD. J Am Acad Child Adolesc Psychiatry. 2005;44(4):385–391. doi: 10.1097/01.chi.0000153227.34473.c7. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13(2):230–239. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Revised. San Antonio: The Psychological Corporation; 1974. [Google Scholar]
- Weinberger DR, Berman KF, Gold J, Goldberg T. Neural mechanisms of future-oriented processes: In vivo physiological studies of humans. In: Haith MM, Benson JB, editors. Development of future-oriented processes. Chicago: University of Chicago Press; 1994. pp. 221–242. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Boada R, Ogline JS, Tunick RA, Chhabildas NA, et al. A comparison of the cognitive deficits in reading disability and attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2001;110(1):157–172. doi: 10.1037//0021-843x.110.1.157. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Müller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of childhood cognitive development. Malden, MA: Blackwell Publishers; 2002. pp. 445–469. [Google Scholar]
- Zucker RA, Davies WH, Kincaid SB, Fitzgerald HE, Reider EE. Conceptualizing and scaling the developmental structure of behavior disorder: the Lifetime Alcohol Problems Score as an example. Dev Psychopathol. 1997;9(2):453–471. doi: 10.1017/s0954579497002125. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Fitzgerald HE, Noll RB. Drinking and drug history. East Lansing, Michigan: 1990. Unpublished manuscript. [Google Scholar]
- Zucker RA, Fitzgerald HE, Refior SK, Puttler LI, Pallas DM, Ellis DA. The clinical and social ecology of childhood for children of alcoholics: Description of a study and implications for a differentiated social policy. In: Fitzgerald HE, Lester BM, Zuckerman BS, editors. Children of addiction Research health and policy issues. New York: Routledge Falmer; 2000. pp. 109–141. [Google Scholar]