Abstract
While many laboratories have shown that dietary NaCl (salt) intake increases nitric oxide (NO) production in rodents and humans, the mechanism has not been uncovered. In the present study, pharmacological and dominant-negative strategies were used to show that feeding a formulated diet containing increased amounts of salt to young male Sprague-Dawley rats induced the formation of an endothelial cell-signaling complex that contained proline-rich tyrosine kinase 2 (Pyk2), c-Src (also known as pp60c-src) and phosphatidylinositol 3-kinase (PI3-kinase). In the setting of a high-salt diet, Pyk2 served as the scaffold for c-Src-mediated PI3-kinase activation. PI3-kinase was the upstream activator of protein kinase B (Akt), which was responsible for phosphorylation of the rat endothelial isoform of nitric oxide synthase (NOS3) at S1176 and thereby promoted the increase in NO production. The combined findings illustrated the crucial role for a Pyk2-signaling complex in the endothelial response to salt intake.
Keywords: nitric oxide, cell signaling, cell biology, Animal models of human disease
Introduction
Following the original description of the role of nitric oxide (NO)1 in the blood pressure responses to changes in dietary NaCl (termed salt in this paper) intake,1 subsequent studies confirmed that increased salt intake increased NO production in rodents2-5 and healthy humans.6 NO plays an important role in the hemodynamic response to changes in salt intake. Salt-induced NO release promotes vasorelaxation of the afferent arteriole,7 augments glomerular filtration rate8 and improves the pressure-natriuresis curve, facilitating salt excretion.9 Inhibition of NO results in salt retention and salt-sensitive hypertension10 and, if protracted, leads to renal injury particularly if the animals are maintained on a high-salt diet.11
The direct involvement of the endothelium in mediating NO production in response to a high-salt diet has been demonstrated.12 The mechanism by which salt intake increases endothelial NO production appears to be initiated through generation of shear forces.13-15 The endothelial isoform of nitric oxide synthase, termed NOS3 in this paper, is a highly regulated enzyme that is controlled by a variety of post-translational events that include phosphorylation of multiple serine and threonine residues of NOS3. While NOS3 enzyme activity is dependent upon binding of a calcium/calmodulin complex to NOS3, displacing an autoinhibitory loop and activating function, several laboratories have shown that shear stress also promotes a calcium-independent activation of NOS3.16, 17 The present view is that calcium/calmodulin activation of NOS3 is responsible only for transient increases in NO, while other post-translational events provide more prolonged NO release from NOS3.18, 19 In particular, NOS3 can serve as a substrate for protein kinase B (Akt), which promotes serine phosphorylation at residue 1176 in the carboxyl terminal portion of NOS3 and increases NOS3 sensitivity to calcium/calmodulin and enzyme activity.20
Recent studies show that dietary salt intake activates proline-rich tyrosine kinase 2 (Pyk2).21 Pyk2 (also designated FAK2, CAK-ß, CADTK, or RAFTK) is a member of the focal adhesion protein tyrosine kinase family.22 This non-receptor tyrosine kinase is typically activated by extracellular stress signals, such as shear stress,23 but also by G protein-coupled receptors, such as the angiotensin type I receptor.22, 24 Pyk2 has multiple binding partners that include c-Src, the 60-kDa protein of c-src (also known as pp60c-src), phosphatidylinositol 3-kinase (PI3-kinase) and Grb2.22, 25-27 Binding to Pyk2 activates c-Src and PI3-kinase and this signaling complex participates in a variety of intracellular processes.22, 28 Because PI3-kinase is an upstream activator of Akt, the present study has therefore been designed to determine if 1) an increase in the phosphorylation state of S1176 of NOS3 accounts for the augmented endothelial NO production that occurs in the setting of increased salt intake and 2) dietary salt intake induces a Pyk2/c-Src/PI3-kinase complex that in turn increases NOS3 activity through activation of Akt.
Methods
Animal and Tissue Preparation
The Institutional Animal Care and Use Committee at the University of Alabama at Birmingham approved the project. Studies were conducted using male Sprague-Dawley (SD) rats (Harlan Sprague Dawley, Indianapolis, IN) that were 28 days of age at the start of study. The protocol that was followed has been standardized in our laboratory.13, 14, 29 The rats were housed under standard conditions and given formulated diets (AIN-76A, Dyets, Inc., Bethlehem, PA) that contained 0.3% and 8.0% (wt/wt) NaCl. These nitrite- and nitrate-free diets were prepared specifically to be identical in protein composition and differed only in NaCl and sucrose content. On the fourth day of the study, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium injection (OVATION Pharmaceuticals, Inc., Deerfield, IL), 50 mg/kg body weight, and aorta and isolated glomeruli were obtained under sterile conditions for incubation studies and immunoblot analyses as performed previously.13-15, 29-31 The primary antibodies were diluted 1:1000 and recognized specifically the 20-30 amino acid sequence around the phosphorylated serine residue at position 1177 in human NOS3 (Cell Signaling Technology, Beverly, MA), p-Akt(S473) p-Akt(T308), total Akt (Cell signaling Technology), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam Inc., Cambridge, MA). Because analysis of the published cDNA sequence of rat NOS3 (accession # NM_021838) showed that the serine residue that corresponded to S1177 in the human NOS3 sequence and S1179 in the bovine sequence was amino acid residue 1176, phosphorylation of this serine residue was referred to as p-NOS3(S1176) in this paper.
In vitro Incubation Studies
After removal of adherent fat and connective tissue, that aorta was cut into 3-mm ring segments and placed in 48-well plates. Isolated glomeruli (5 × 103 glomeruli/ml), which were obtained by sieving renal cortical tissue, and aorta ring preparations were washed with cold PBS. Pelleted glomeruli and aortic ring segments were resuspended in serum-free medium (DMEM; Invitrogen Corporation, Carlsbad, CA) that contained vehicle alone, 5 μM tyrphostin A9 (EMD Biosciences, Inc., La Jolla, CA), or 10 μM PP2 (EMD Biosciences, Inc.). Tyrphostin A9 served as an inhibitor of Pyk232 and PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine), was a potent, cell-permeable, Src family-selective tyrosine kinase inhibitor.33
Studies in vivo
To characterize the interactions further, peptide fragments of Pyk2 were designed to interfere with specific interactions between Pyk2 and potential binding partners. Transduction of these peptides was accomplished through the generation of fusion proteins that consisted of the peptide segments of Pyk2 and the minimal transduction domain of HIV, termed Tat.34 The constructs in the pTatHA bacterial expression vector were generous gifts from Dr. Carl Nathan (Weill Medical College of Cornell University). Tat-AP was a fusion protein that consisted of the Tat peptide fused to a portion of the kinase domain of Pyk2 and contained the recognition domain for the SH2 group of c-Src. Tat-PBM consisted of the Tat peptide fused to amino acid residues 581-700 of the kinase domain of Pyk2 and included the purported binding site (YTLM) for the SH2 domain of PI3-kinase. Tat-GBM consisted of the Tat peptide fused to a peptide fragment that corresponded to a carboxyl terminal portion of Pyk2 and contained the Grb2-binding motif. These Tat fusion proteins, which served as cell-permeant, site-specific, dominant-negative inhibitors of Pyk2 interaction and activation, have been used by this laboratory to determine that dietary salt intake promoted interaction between Pyk2 and c-Src in vitro and in vivo.21
On the third day on either the 0.3% or 8.0% NaCl diet, rats received a single intravenous bolus of 0.5 ml phosphate-buffered saline (PBS) that contained vehicle alone, 50 nmol of LY294002, a PI3-kinase inhibitor that is efficacious in vivo,35, 36 or 1.25 nmol of Tat-AP, Tat-GBM, or Tat-PBM. The following day, the rats were anesthetized and aortic tissue and glomeruli were obtained to generate tissue lysates for western blotting and immunoprecipitation experiments or for in vitro incubation studies. At the time of tissue harvesting, urine was collected from the bladder to determine nitric oxide metabolites (NOx), which was assayed using a kit (QuantiChrom Nitric Oxide Assay kit, BioAssay Systems, Hayward, CA) and creatinine concentration, which was assayed using an autoanalyzer (Creatinine Analyzer 2, Beckman Coulter, Inc., Fullerton, CA). In these studies, assays were performed in triplicate and averaged; NOx values were normalized using the creatinine concentration obtained in each sample. Isolated glomeruli and aortic ring segments were incubated in serum-free medium (DMEM; Invitrogen Corporation) at 37°C for 24 h. The conditioned medium was harvested, centrifuged at 300 ×g for 10 min at 4°C to remove cell debris, and then stored at -80°C until assayed for NOx; the results were factored by wet weight (for aortic tissue) or total protein (for glomeruli).
Co-immunoprecipitation Assays
Co-immunoprecipitation studies were performed to characterize the effect of dietary salt intake on the interactions between Pyk2 and phosphatidylinositol 3-kinase (PI3-kinase). Tissue lysates containing 500 μg total protein were obtained from the rats used in the in vivo studies and were incubated with 2 μg of an anti-Pyk2 polyclonal antibody (Cell Signaling) at 4°C for 2 h, followed by addition of 30 μl of protein A-Sepharose and incubation. Immune pellets were washed three times with ice-cold RIPA buffer and then boiled in SDS sample buffer containing dithiothreitol. The proteins were resolved on 7.5% SDS-polyacrylamide gels and transferred to PVDF membranes. The membranes were probed with antibodies directed against four isoforms of the catalytic unit of PI3-kinase (p110α, p110β, p110γ, p110δ) and the regulatory subunit, p85 (Upstate Chemicon, Temecula, CA). Immunoreactive bands were visualized with the use of enhanced chemiluminescence.
Statistical Analysis
Data were expressed as mean ± standard error. Significant differences among data sets were determined by analysis of variance with post-hoc testing (Fisher’s Protected Least Significant Difference) (Statview, version 5.0, SAS Institute, Inc., Cary, NC). A P value < 0.05 assigned statistical significance.
Results
Dietary salt intake increased NOS3 phosphorylation through an Akt-dependent mechanism
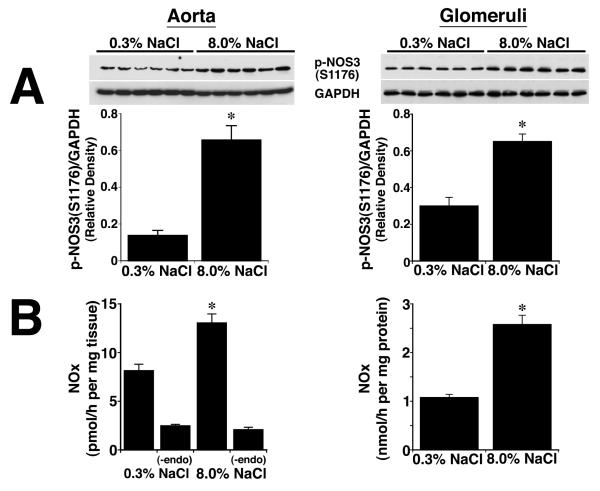
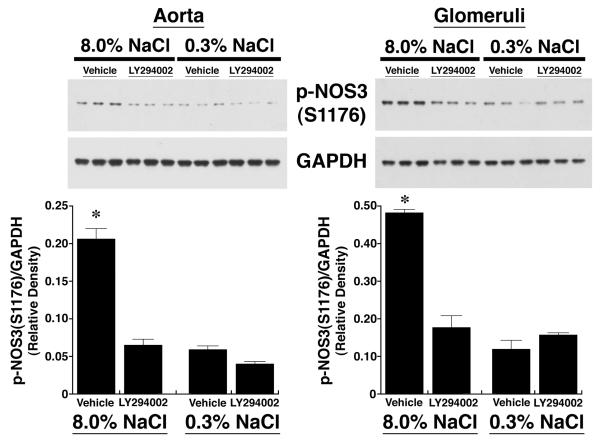
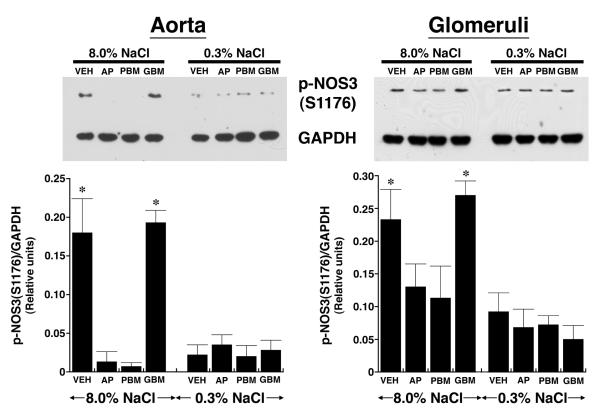
After four days on the two diets, lysates from aortic tissue and isolated glomeruli were obtained from the rats (n = 6 in each group) and phosphorylation of NOS3 at S1176 determined using an antibody that recognized specifically p-NOS3(S1176). Compared to samples from rats on the 0.3% NaCl diet, lysates from aorta and isolated glomeruli of rats on the 8.0% NaCl diet contained increased (P < 0.05) amounts of p-NOS3(S1176), which was expressed as the ratio of the density of phosphorylated NOS3 and GAPDH in each sample (Figure 1A). When incubated in medium, aortic rings and isolated glomeruli from rats on the 8.0% NaCl diet produced increased amounts of NOx, compared to samples from rats on the 0.3% NaCl diet. Mechanical removal of the endothelium reduced NOx production in both groups to levels that did not differ (Figure 1B). To determine if PI3-kinase was involved in salt-induced phosphorylation of NOS3, on the day prior to study rats on both diets received an intravenous bolus of LY294002 (Figure 2). Administration of LY294002 reduced p-NOS3(S1176) levels in both aorta and glomeruli to those seen in corresponding samples obtained from rats on the 0.3% NaCl diet.
Fig. 1.
Effect of dietary salt intake on NOS3 phosphorylation at S1176, expressed as a ratio of the density of the phosphorylated NOS3 to GAPDH, and NO production. (A) Western analyses using lysates from aortic and isolated glomerular preparations. In vascular tissues obtained from rats on the 8.0% NaCl diet, the amount of p-NOS3(S1176) was greater (P < 0.05) than levels of p-NOS3(S1176) in lysates from animals on the 0.3% NaCl diet. Each lane of the gels represented lysate obtained from a single rat (n = 6 rats in each group). (B) Aortic rings and isolated glomeruli from rats on the 8.0% NaCl diet released greater (P < 0.05) amounts of NOx into the medium, compared to tissues obtained from rats on the 0.3% NaCl diet. Mechanical removal of the endothelium reduced NOx production by aortic ring preparations to levels that did not differ between the two groups. *P < 0.05 compared to the 0.3% NaCl group
Fig. 2.
Effect of intravenous administration of LY294002 on p-NOS3(S1176), expressed as a ratio of the density of the phosphorylated NOS3 to GAPDH. LY294002 reduced the expression of p-NOS3(S1176) to levels that did not differ from levels of p-NOS3(S1176) in vascular tissues obtained from rats on the 0.3% NaCl diet. Each lane of the gels represented lysate obtained from a single rat (n = 3 rats in each group). *P < 0.05 compared to the other 3 groups
Activation of NOS3 by dietary salt occurred through a Pyk2/c-Src/PI3-kinase-dependent mechanism
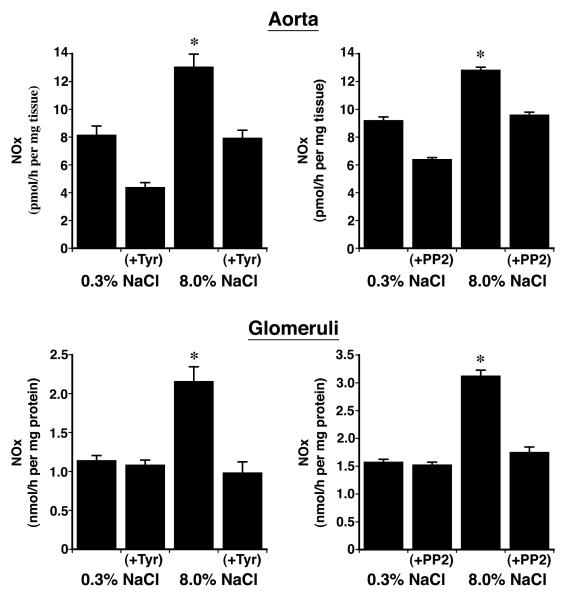
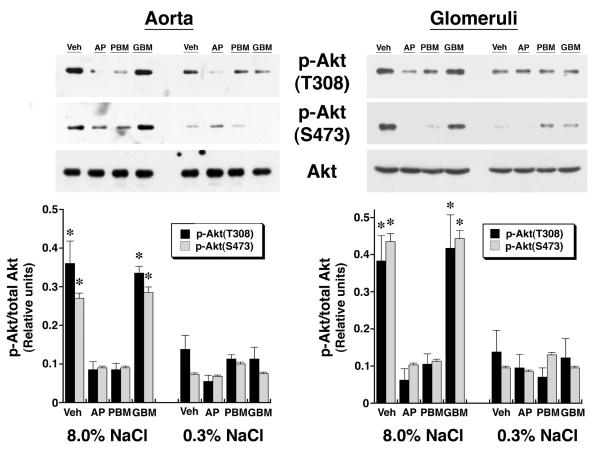
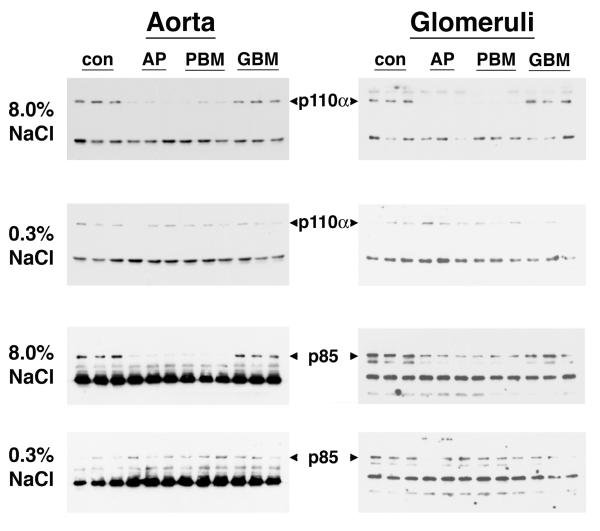
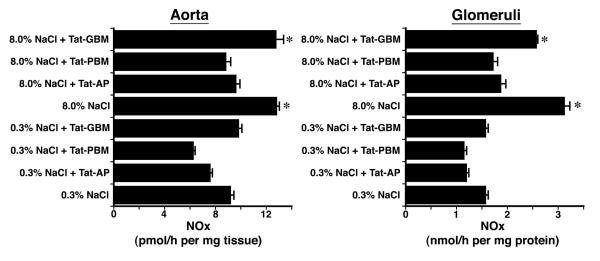
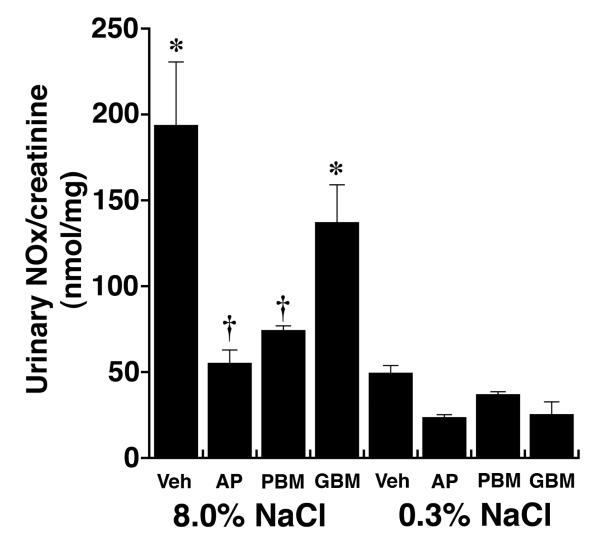
Previous studies showed that dietary salt induced the phosphorylation and activation of an endothelial Pyk2/c-Src complex.21 To test if these enzymes were also involved in NO production during increased salt ingestion, in initial experiments, tissue samples from rats on both diets were incubated in medium that contained tyrphostin A9, a Pyk2 inhibitor,32 and PP2, a c-Src inhibitor,33 and NOx release into the medium was quantified. Both inhibitors decreased NOx production by aortic segments and by isolated glomeruli (Figure 3). A dominant-negative approach was then employed to determine if Pyk2 was involved directly in Akt activation. On the day prior to study, 1.25 nmol of Tat-AP, Tat-PBM and Tat-GBM proteins were administered intravenously to groups of rats on both diets (Figure 4). While Tat-GBM did not alter the phosphorylation state of Akt at T308 and S473 in aortic and glomerular lysates from rats on the 8.0% NaCl diet, both Tat-AP and Tat-PBM decreased (P < 0.05) relative levels of p-Akt(T308) and p-Akt(S473), compared to corresponding samples from animals that received the 8.0% NaCl diet and intravenous bolus of vehicle alone. None of the three Tat fusion proteins produced a significant effect on Akt phosphorylation at either amino acid residue in samples obtained from rats on the 0.3% NaCl diet. Initial co-immunoprecipitation experiments determined that the p110α isoform and not p110ß, p110γ and p110δ isoforms served as the p85 partner in binding Pyk2 (data not shown). Intravenous administration of Tat-AP and Tat-PBM, but not Tat-GBM, resulted in diminished binding of p85 and p110α to Pyk2 in lysates from tissues obtained from rats on the 8.0% NaCl diet (Figure 5). Pre-administration of the Tat fusion proteins produced no demonstrable effect on binding of p85 and p110α to Pyk2 in lysates from tissues obtained from rats on the 0.3% NaCl diet. Together with prior studies,21 the combined experiments showed that Tat-AP and Tat-PBM disrupted a Pyk2/c-Src/PI3-kinase signaling complex that activated Akt. In other studies, both Tat-AP and Tat-PBM, but not Tat-GBM, also diminished relative levels of p-NOS3(S1176) in tissues from rats on the 8.0% NaCl diet (Figure 6). When aortic rings and isolated glomeruli from rats on the 8.0% NaCl diet were incubated in vitro, release of NOx into the medium was diminished by the pre-administration of Tat-AP and Tat-PBM, but not Tat-GBM, to levels observed in corresponding samples from rats on the 0.3% NaCl diet (Figure 7). Urinary NOx/creatinine ratios obtained the morning of the experiment showed the expected increase in urinary NOx in the groups of rats on the 8.0% NaCl diet, compared to the group of rats on the 0.3% NaCl diet (Figure 8). The parenteral administration of Tat-AP and Tat-PBM reduced urinary NOx/creatinine to levels that did not differ from that observed in the group of rats that were maintained on the 0.3% NaCl diet and received vehicle treatment alone.
Fig. 3.
Effect of addition of tyrphostin A (Tyr) and PP2 on release of NOx into the medium by aortic rings (upper panels) and isolated glomeruli (lower panels). The addition of Tyr and PP2 reduced (P < 0.05) NOx production by both vascular tissues to levels that did not differ from production by corresponding tissues obtained from rats on the 0.3% NaCl diet. There were 6 rats in each group. *P < 0.05 compared to the other 3 groups
Fig. 4.
Effect of intravenous administration of the Tat fusion proteins on expression of p-Akt(T308) and p-Akt(S473) relative to Akt in aortic and glomerular preparations. Total Akt levels did not differ among tissues obtained from the eight groups of rats (n = 4 rats in each group) on the two NaCl diets. Compared to rats that received vehicle (Veh), administration of Tat-AP and Tat-PBM, but not Tat-GBM, reduced (P < 0.05) levels of p-Akt(T308) and p-Akt(S473) in lysates from aortic tissue and isolated glomeruli from rats on the 8.0% NaCl diet to levels that did not differ from the groups of rats that received the 0.3% NaCl diet. Each lane in the gel represents a single animal; a total of 4 rats in each group were examined. *P < 0.05 compared to the other 6 groups
Fig. 5.
Effect of intravenous administration of the Tat fusion proteins on the association of Pyk2 with components of PI3-kinase. Following immunoprecipitation of Pyk2 from lysates of aortic tissue and isolated glomeruli of rats on both diets, the pelleted samples were separated using SDS-PAGE and probed for p110α and p85. Intravenous administration of Tat-AP and Tat-PBM, but not Tat-GBM, disrupted the association of Pyk2 with p110α and p85 in samples obtained from rats on the 8.0% NaCl diet, but did not alter binding to Pyk2 in lysates from rats on the 0.3% NaCl diet. Each lane represents data from a single rat (n = 3 rats in each group).
Fig. 6.
Effect of intravenous administration of the Tat fusion proteins on the expression of p-NOS3(S1176) relative to GAPDH in aortic tissue and isolated glomeruli. Intravenous administration of Tat-AP and Tat-PBM, but not Tat-GBM, decreased expression of p-NOS3(S1176) to levels that did not differ from the groups of rats on the 0.3% NaCl diet. Each lane in the gel represents a single animal; a total of 4 rats in each group were examined. *P < 0.05 compared to the other 6 groups
Fig. 7.
Effect of intravenous administration of the Tat fusion proteins on production of NOx by aortic rings and isolated glomeruli (n = 4 rats in each group). Administration of Tat-AP and Tat-PBM, but not Tat-GBM, reduced NOx production by both vascular tissues to levels that did not differ from production rates observed using vascular tissues from rats on the 0.3% NaCl diet. *P < 0.05 compared to the other 6 groups of rats
Fig. 8.
Effect of intravenous administration of the Tat fusion proteins on the urinary excretion of NOx in vivo (n = 4 rats in each group). Tat-AP and Tat-PBM decreased NOx/creatinine levels to that observed in the rats that received the 0.3% NaCl diet. *P < 0.05 compared to the remaining 6 groups; †P > 0.05 compared to the group that received the 0.3% NaCl diet and vehicle (Veh)
Discussion
The current series of experiments used two different vascular tissues to confirm that endothelial NO production increased as dietary salt intake increased; the mechanism of this increase has now been clarified. Recent observations from this laboratory demonstrated a dose-dependent effect of salt intake on activation of endothelial Pyk2, which recruited and activated c-Src.21 Novel findings of the present study include the observation that dietary salt intake induced an intracellular signaling complex that not only contained Pyk2 and c-Src but also p85 and p110α; this complex was directly involved in the activation of Akt that in turn produced the post-translational modification that increased NOS3 production of NO in the setting of a high-salt diet. By adding to the elegant studies of Matsui, et al.,37 who showed that Pyk2 is involved in modulating NOS3 activity in angiogenesis and ischemia, the data further supported an integral role for Pyk2 in signal transduction events that regulate NOS3 function in normal and disease states. The present study also is not at variance with the recent findings of Fisslthaler, et al.,38 who overexpressed Pyk2 to demonstrate that in the setting of shear stress, Pyk2 can associate directly with NOS3 and promote the phosphorylation of Y657, which serves as an inhibitor of enzyme function and mitigates, but does not prevent, shear-induced augmentation of NO production.
The variety of post-translational events that alter NOS3 activity has been reviewed.19, 39 Serine phosphorylation of amino acid residue 1176 in the carboxyl terminal portion of NOS3 is a particularly important regulator of enzyme activity and sensitivity to calcium/calmodulin activation.20 Besides Akt, AMP kinase, protein kinase A, protein kinase G, and calcium/calmodulin-dependent protein kinase II have been implicated in the regulation of the state of phosphorylation of NOS3 at 1176.19, 39 The evidence for the selective involvement of Akt in salt-mediated phosphorylation of NOS3 included the demonstration that 1) Akt activation and NOS3 phosphorylation were prevented by inhibition of recruitment and activation of PI3-kinase by Pyk2 following an increase in dietary salt intake and 2) LY294002 decreased p-NOS3(S1176) levels in animals receiving the 8.0% NaCl diet to that observed in rats on the 0.3% NaCl diet. Because the latter observation suggested that phosphorylation at 1176 was sufficient to explain the increase in NO production, other post-translational modifications of NOS3, such as tyrosine phosphorylation of NOS3 at T83, which also increases NOS3 activity and occurs through c-Src,40 were not explored.
The data demonstrating increased phosphorylation of NOS3 at S1176 in glomeruli conflicted with the findings of Mount, et al.,5 who also showed NOx production increased as dietary salt increased but did not demonstrate an increase in S1176 phosphorylation in kidney lysates. Their novel method for quantifying NOS3 initially employed precipitation from whole-kidney lysates using 2′,5′-ADP sepharose; aside from technical concerns related to this technique, their approach of using kidney lysates potentially obscured changes in regional or local expression of p-NOS3.
By permitting the intracellular delivery of our protein inhibitors without viral vectors,41, 42 the use of Tat fusion proteins enabled additional testing of the hypothesis in the in vivo condition.21 The Tat fusion proteins — Tat-AP, Tat-PBM, and Tat-GBM — were designed to interfere specifically with binding of Pyk2 to c-Src, p85, and Grb2, respectively. Several laboratories independently demonstrated the efficacy of these inhibitors.21, 43, 44 Addition of both Tat-AP and Tat-PBM inhibited binding of p85 and p110α to Pyk2 and subsequent activation of Akt and phosphorylation of NOS3. One interpretation of these data was that binding of c-Src to Pyk2, which occurs through the SH3 domain on c-Src,45, 46 was essential for binding to Pyk2 and activation of PI3-kinase. The data were consistent with the findings of Taniyama, et al.,28 who demonstrated that activated Pyk2 served as a scaffold to promote c-Src-dependent PI3-kinase activation. The data generated using Tat-GBM showed that there was no role for Grb2 in dietary salt-induced NO production and further permitted use of Tat-GBM as an additional control for the Tat fusion protein experiments.
Perspectives
In summary, the present series of experiments demonstrated that dietary salt intake directly promoted a complex endothelial cell-signaling cascade that induced Akt-mediated phosphorylation of NOS3 at S1176 and augmented NO production. It is relevant that salt-mediated vascular production of TGF-ß1 also occurs through a Pyk2-dependent process involving c-Src,21 and NO is an important compensatory response that mitigates the effects of TGF-ß1.31 Pyk2 therefore becomes a key signaling molecule in the vascular response to dietary salt intake. Although in the present study vascular reactivity was not examined, Atochin et al.,47 demonstrated the in vivo relevance of this post-translational modification by showing that transgenic mice expressing NOS3 possessing a S1179D (phosphomimetic) mutation had increased vascular reactivity when compared to transgenic mice expressing NOS3 possessing a S1179A mutation. While these findings suggest physiological benefit, in conditions that induce oxidative stress in the vessel wall, NOS3 can produce superoxide rather than NO and phosphorylation of human NOS3 at S1177 is also pivotal in regulation of superoxide production.48 While the present findings were limited to rats, elucidating the mechanism of dietary salt-induced alterations in NOS3 function permits improved understanding of the nature of the interaction between the endothelium and dietary salt intake in physiological and pathological states that alter NO production, such as diabetes mellitus, hypertension, and aging.
Acknowledgment and Sources of Funding
National Institutes of Health grant (R01 DK46199) and the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, supported this research.
Footnotes
- NO
- nitric oxide
- NOS3
- endothelial isoform of NO synthase
- Akt
- protein kinase B
- Pyk2
- proline-rich tyrosine kinase 2
- PI3-kinase
- phosphatidylinositol 3-kinase
- c-Src
- 60-kDa product of c-src also known as pp60c-src.
- (SD)
- Sprague-Dawley
- p-NOS3(S1176)
- NOS3 phosphorylated at S1176
- p-Akt(S473)
- Akt phosphorylated as S473
- p-Akt(T308)
- Akt phosphorylated at T308
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PP2
- 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine)
- Tat
- minimal transduction domain of HIV
- PBS
- phosphate-buffered saline
- NOx
- nitric oxide metabolites (nitrite and nitrate)
- p110
- catalytic subunit of PI3-kinase
- p85
- regulatory subunit of PI3-kinase
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Hypertension, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at Hypertension. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 3.Deng X, Welch WJ, Wilcox CS. Renal vasoconstriction during inhibition of NO synthase: Effects of dietary salt. Kidney Int. 1994;46:639–646. doi: 10.1038/ki.1994.316. [DOI] [PubMed] [Google Scholar]
- 4.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat: role of endogenous nitric oxide. J Clin Invest. 1993;91:642–650. doi: 10.1172/JCI116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mount PF, Fraser SA, Watanabe Y, Lane N, Katsis F, Chen ZP, Kemp BE, Power DA. Phosphorylation of Neuronal and Endothelial Nitric Oxide Synthase in the Kidney with High and Low Salt Diets. Nephron Physiol. 2005;102:36–50. doi: 10.1159/000089092. [DOI] [PubMed] [Google Scholar]
- 6.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB.Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans Am J Physiol 19982745 Pt 2F914–F923. [DOI] [PubMed] [Google Scholar]
- 7.Juncos LA, Garvin J, Carretero OA, Ito S. Flow modulates myogenic responses in isolated microperfused rabbit afferent arterioles via endothelium-derived nitric oxide. J Clin Invest. 1995;95:2741–2748. doi: 10.1172/JCI117977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng A, Baylis C.Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient Am J Physiol 19932642 Pt 2F212–F215. [DOI] [PubMed] [Google Scholar]
- 9.Patel A, Layne S, Watts D, Kirchner KA. L-Arginine administration normalizes pressure natriuresis in the hypertensive Dahl rats. Hypertension. 1993;22:863–869. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]
- 10.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 1994;46:230–236. doi: 10.1038/ki.1994.264. [DOI] [PubMed] [Google Scholar]
- 11.Fujihara CK, Michellazzo SM, De Nucci G, Zatz R.Sodium excess aggravates hypertension and renal parenchymal injury in rats with chronic NO inhibition Am J Physiol 19942665 Pt 2F697–F705. [DOI] [PubMed] [Google Scholar]
- 12.Ying W-Z, Sanders PW.Dietary salt increases endothelial nitric oxide synthase and TGF-ß1 in rat aortic endothelium Am J Physiol 19992774 Pt 2H1293–H1298. [DOI] [PubMed] [Google Scholar]
- 13.Ying W-Z, Sanders PW.Dietary salt modulates renal production of transforming growth factor-ß in rats Am J Physiol 19982744 Pt 2F635–F641. [DOI] [PubMed] [Google Scholar]
- 14.Ying W-Z, Sanders PW.Dietary salt increases endothelial nitric oxide synthase and TGF-ß1 in rat aortic endothelium Am J Physiol 19992774 Pt 2H1293–H1298. [DOI] [PubMed] [Google Scholar]
- 15.Ying W-Z, Sanders PW. Increased dietary salt activates rat aortic endothelium. Hypertension. 2002;39:239–244. doi: 10.1161/hy0202.104142. [DOI] [PubMed] [Google Scholar]
- 16.Corson MA, James NL, Latta SE, Nerem RM, Berk BC, Harrison DG. Phosphorylation of endothelial nitric oxide synthase in response to fluid shear stress. Circ Res. 1996;79:984–991. doi: 10.1161/01.res.79.5.984. [DOI] [PubMed] [Google Scholar]
- 17.Fleming I, Bauersachs J, Fisslthaler B, Busse R. Ca2+-independent activation of the endothelial nitric oxide synthase in response to tyrosine phosphatase inhibitors and fluid shear stress. Circ Res. 1998;82:686–695. doi: 10.1161/01.res.82.6.686. [DOI] [PubMed] [Google Scholar]
- 18.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton D, Gratton JP, Sessa WC. Post-translational control of endothelial nitric oxide synthase: why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–824. [PubMed] [Google Scholar]
- 20.Lane P, Gross SS. Disabling a C-terminal autoinhibitory control element in endothelial nitric-oxide synthase by phosphorylation provides a molecular explanation for activation of vascular NO synthesis by diverse physiological stimuli. J Biol Chem. 2002;277:19087–19094. doi: 10.1074/jbc.M200258200. [DOI] [PubMed] [Google Scholar]
- 21.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-ß1. Am J Physiol Renal Physiol. 2008;295:F406–414. doi: 10.1152/ajprenal.90294.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 23.Tai LK, Okuda M, Abe J, Yan C, Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol. 2002;22:1790–1796. doi: 10.1161/01.atv.0000034475.40227.40. [DOI] [PubMed] [Google Scholar]
- 24.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 25.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio JM, Plowman GD, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 26.Avraham S, London R, Fu Y, Ota S, Hiregowdara D, Li J, Jiang S, Pasztor LM, White RA, Groopman JE, Avraham H. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J Biol Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 27.Davies PF. Multiple signaling pathways in flow-mediated endothelial mechanotransduction: PYK-ing the right location. Arterioscler Thromb Vasc Biol. 2002;22:1755–1757. doi: 10.1161/01.atv.0000034391.00347.71. [DOI] [PubMed] [Google Scholar]
- 28.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol. 2003;23:8019–8029. doi: 10.1128/MCB.23.22.8019-8029.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ying W-Z, Sanders PW.Dietary salt enhances glomerular endothelial nitric oxide synthase through TGF-ß1 Am J Physiol 19982751 Pt 2F18–F24. [DOI] [PubMed] [Google Scholar]
- 30.Ying W-Z, Sanders PW. Dietary salt intake activates MAP kinases in the rat kidney. FASEB J. 2002;16:1683–1684. doi: 10.1096/fj.02-0982fje. [DOI] [PubMed] [Google Scholar]
- 31.Ying W-Z, Sanders PW. The interrelationship between TGF-ß1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol. 2003;285:F902–F908. doi: 10.1152/ajprenal.00177.2003. [DOI] [PubMed] [Google Scholar]
- 32.Fuortes M, Melchior M, Han H, Lyon GJ, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Invest. 1999;104:327–335. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 34.Becker-Hapak M, McAllister SS, Dowdy SF. TAT-mediated protein transduction into mammalian cells. Methods. 2001;24:247–256. doi: 10.1006/meth.2001.1186. [DOI] [PubMed] [Google Scholar]
- 35.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 36.Su JD, Mayo LD, Donner DB, Durden DL. PTEN and phosphatidylinositol 3′-kinase inhibitors up-regulate p53 and block tumor-induced angiogenesis: evidence for an effect on the tumor and endothelial compartment. Cancer Res. 2003;63:3585–3592. [PubMed] [Google Scholar]
- 37.Matsui A, Okigaki M, Amano K, Adachi Y, Jin D, Takai S, Yamashita T, Kawashima S, Kurihara T, Miyazaki M, Tateishi K, Matsunaga S, Katsume A, Honshou S, Takahashi T, Matoba S, Kusaba T, Tatsumi T, Matsubara H. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation. 2007;116:1041–1051. doi: 10.1161/CIRCULATIONAHA.106.645416. [DOI] [PubMed] [Google Scholar]
- 38.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 39.Mount PF, Kemp BE, Power DA. Regulation of endothelial and myocardial NO synthesis by multi-site eNOS phosphorylation. J Mol Cell Cardiol. 2007;42:271–279. doi: 10.1016/j.yjmcc.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, Jennings IG, Venema RC. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr-83. J Biol Chem. 2005;280:35943–35952. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 41.Gump JM, Dowdy SF. TAT transduction: the molecular mechanism and therapeutic prospects. Trends Mol Med. 2007;13:443–448. doi: 10.1016/j.molmed.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- 43.Han H, Fuortes M, Nathan C. Critical role of the carboxyl terminus of proline-rich tyrosine kinase (Pyk2) in the activation of human neutrophils by tumor necrosis factor: separation of signals for the respiratory burst and degranulation. J Exp Med. 2003;197:63–75. doi: 10.1084/jem.20021638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao T, Bokoch GM. Critical role of proline-rich tyrosine kinase 2 in reversion of the adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Immunol. 2005;174:8049–8055. doi: 10.4049/jimmunol.174.12.8049. [DOI] [PubMed] [Google Scholar]
- 45.Pleiman CM, Clark MR, Gauen LK, Winitz S, Coggeshall KM, Johnson GL, Shaw AS, Cambier JC. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-gamma 2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993;13:5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kapeller R, Prasad KV, Janssen O, Hou W, Schaffhausen BS, Rudd CE, Cantley LC. Identification of two SH3-binding motifs in the regulatory subunit of phosphatidylinositol 3-kinase. J Biol Chem. 1994;269:1927–1933. [PubMed] [Google Scholar]
- 47.Atochin DN, Wang A, Liu VW, Critchlow JD, Dantas AP, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric oxide synthase (eNOS) regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]