Summary
TRP channels are essential components of biological sensors that detect changes in the environment in response to a myriad of stimuli. A major difficulty in the study of TRP channels is the lack of pharmacological agents that modulate most members of the TRP superfamily. Notable exceptions are the thermoTRPs, which respond to either cold or hot temperatures and are modulated by a relatively large number of chemical agents. In the present study we demonstrate by patch clamp whole cell recordings from Schneider 2 and Drosophila photoreceptor cells that carvacrol, a known activator of the thermoTRPs, TRPV3 and TRPA1 is an inhibitor of the Drosophila TRPL channels, which belongs to the TRPC subfamily. We also show that additional activators of TRPV3, thymol, eugenol, cinnamaldehyde and menthol are all inhibitors of the TRPL channel. Furthermore, carvacrol also inhibits the mammalian TRPM7 heterologously expressed in HEK cells and ectopically expressed in a primary culture of CA3-CA1 hippocampal brain neurons. This study, thus, identifies a novel inhibitor of TRPC and TRPM channels. Our finding that the activity of the non thermoTRPs, TRPL and TRPM7 channels is modulated by the same compound as thermoTRPs, suggests that common mechanisms of channel modulation characterize TRP channels.
Introduction
Transient Receptor Potential (TRP) channels are essential components of biological sensors that detect changes in the environment in response to a myriad of stimuli including cold or hot temperatures, natural chemical compounds and mechanical stimuli. TRP channels are Ca2+ permeable and are crucially involved in: photoreception, pheromone sensing, taste perception, thermosensation, pain perception, mechanosensation, perception of pungent compounds, renal Ca2+/Mg2+ maintenance, smooth muscle tone and blood pressure regulation [1]. TRP channels form an evolutionary conserved cation channel family consisting of seven subfamilies which include nearly 30 human members [2;3]. The founding member of this family was discovered in Drosophila and was designated TRP [4]. TRP channels are classified into seven related subfamilies designated TRPC (Canonical or classical), TRPM (Melastatin), TRPN (NompC), TRPV (Vanilloid receptor), TRPA (ANKTM1), TRPP (Polycystin) and TRPML (Mucolipin, for reviews see [2;3;5–7]).
A major difficulty in the study of TRP channels is the lack of pharmacological agents that activate or inhibit most members of the various classes of the TRP subfamilies. This is especially true for channels in native tissues, which show characteristics similar to those of TRP channels but the genes encoding for these channels have not been identified. The lack of inhibitors and activators make it difficult to attribute the unknown native current to a specific channel.
A subgroup of TRP channels are activated by hot or cold temperatures and were therefore designated thermoTRPs. The thermoTRPs include TRPV1-4, TRPM8 and TRPA1 [8]. Since these channels are involved in thermal sensation, pungent natural compounds (e.g. carvacrol), which elicit hot or cold sensation, were tested for possible effect on thermoTRPs. Indeed, various studies have shown that thermoTRPs are regulated by pungent natural compounds [9;10]. Accordingly, thermoTRP channels have relatively large numbers of chemical modulators, in contrast to most members of the TRP superfamily that are non-thermoTRPs and belong to the TRPC and TRPM subfamilies. The question of whether pungent natural compounds affect non-thermoTRP channels has not been addressed.
The Drosophila photoreceptor cells constitute a native tissue in which two members of the TRPC subfamily, TRP and TRP-like (TRPL) are accessible to whole cell recordings and their physiological functions as the light activated channels is well defined in vivo [3;11–13]. Studying TRPC channel activation and inhibition by pharmacological agents in the Drosophila eye offers several advantages because of the power of Drosophila molecular genetics. The existence of null mutants in which either the TRP (trpP343) or TRPL (trpl302) are missing allow studying each of the two light activated channels in isolation. Unlike Drosophila TRP and TRPL channels, most studies on mammalian TRPC channels have been conducted solely on heterologously expressed channels in tissue culture cells. Often, channels expressed in heterologous systems differ in their properties from in vivo channels [14]. This, however, is not the case for the expressed TRPL channels in Drosophila Schneider 2 cells (S2), as they show similar biophysical properties of the native channel, except that the expressed channels are constitutively active, unlike the native channels that are closed in the dark [15;16]. Therefore, the S2 expression system allows studying the TRPL channel in isolation from the complex phototransduction cascade of Drosophila. In addition, the results obtained for the expressed TRPL channels can be verified in the native system during light exposure and provide physiological insight to the native system.
In the present study we show that carvacrol, which activates the thermoTRPs, TRPV3 and TRPA1 is an inhibitor of the non-thermoTRP, the Drosophila TRPL. We also show that carvacrol inhibits the mammalian TRPM7 channels, which belongs to the TRPM subfamily. This study, thus, identifies a novel inhibitor of TRPC and TRPM channels and shows that in addition to thermoTRPs, also non-thermoTRPs are targets of widespread natural compound. The fact that TRP channels of three different subfamilies are targets of a similar natural compound reveals commonality in the functional properties of various TRP channel subfamilies.
Results
Carvacrol is an inhibitor of TRPL channels expressed in tissue culture cells
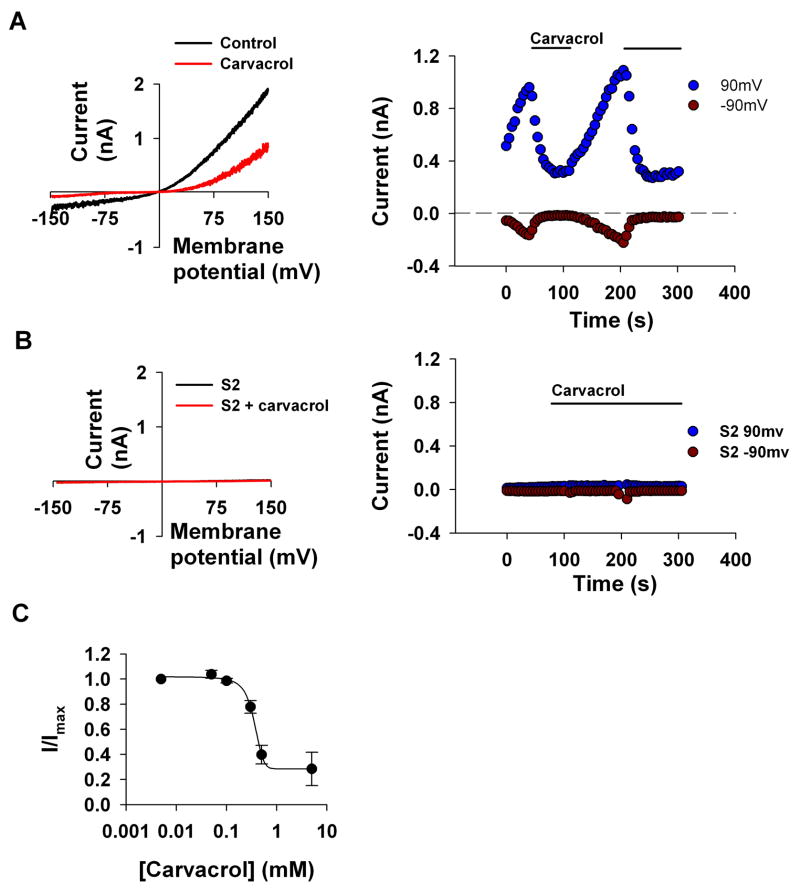
2-Aminoethoxydiphenyl borate (2-APB) is an efficient inhibitor of the TRPL channels [17] while this compound is known as an activator of TRPV3 [18]. Since carvacrol is also known as an activator of TRPV3 [10], we reasoned that carvacrol might inhibit TRPL channels. To test this possibility, we expressed TRPL channels in S2 cells and examined the effect of carvacrol on the TRPL-mediated currents [16]. TRPL channels expressed in S2 cells are constitutively active [15;16]. The level of this constitutive activity varies in individual cells and may show a slow increase of current with time [15]. Figures 1 and 2 demonstrate this variability in basal constitutive activity before application of the tested compounds (see also below). Since the time course of this phenomenon was slow relative to the effect of the tested compounds and we usually tested the effects of inhibitors (i.e in the opposite direction of current increase), this effect did not interfere with our measurements. To demonstrate the indifference of our results to the basal channels activity our results include cells that revealed variable constitutive activity. Figure 1A (left) shows the typical current-voltage relationships (I–V curves) of the active TRPL channels before and after application of carvacrol (for the chemical structure of carvacrol, see Table 1). Large suppression of the current is observed after application of carvacrol (500μM). The current values at −90mV and 90 mV obtained from a time series of I–V curves are plotted in Fig. 1A (right) as a function of time. Application of carvacrol reversibly blocked the channels as manifested by suppression of both inward and outward current (Fig. 1A). Under control condition when TRPL was not expressed carvacrol had no effect (Fig. 1B). Figure 1C shows that the IC50 of carvacrol inhibition is 357±14μM, which is similar to the effective concentration of carvacrol as an activator of TRPV3. Together, Fig. 1 indicates that carvacrol is an inhibitor of the TRPL channel.
Figure 1. Carvacrol inhibits TRPL channels expressed in S2 cells.
A. Left, Representative I–V curves measured from S2 cells by whole cell patch clamp recordings using voltage ramps from −150mV to 150mV within 1s. Carvacrol caused a large decrease in the TRPL-dependent current. right, Time course of the TRPL current measured from the I–V curves (left) at −90 mV and at 90 mV as indicated, before and after application of carvacrol (500μM) (n=15)
B. Control showing no effect of carvacrol when the TRPL channels were not expressed (n=7)
C. Measurements of the blocking efficiency of carvacrol as a function of its concentration (error bars are SEM, n≥5)
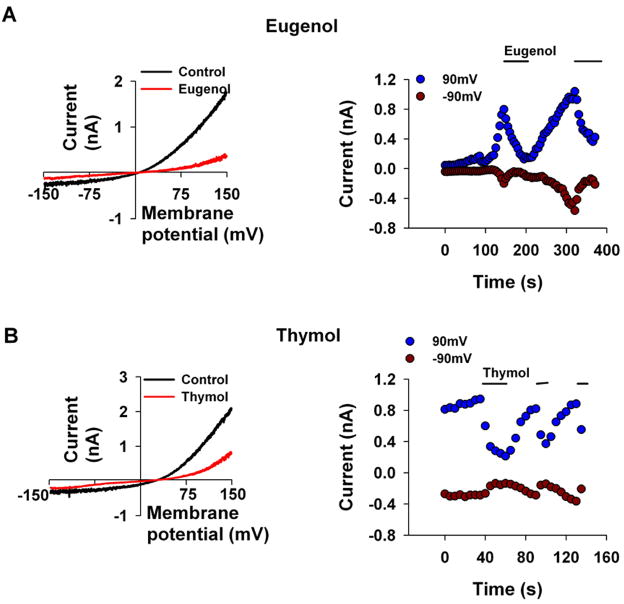
Figure 2. Eugenol and thymol inhibit TRPL channels expressed in S2 cells.
The paradigm of Fig. 1 was repeated. Eugenol (3mM, A, n=6) and thymol (1mM, B, n=7) caused a large decrease in the TRPL-dependent current. left, Representative I–V curves of the TRPL mediated current measured before and after application of the compounds. right, Time course of the TRPL mediated current measured from the I–V curves (left) at −90 mV and at 90 mV as indicated, before and after application of the compounds.
Table 1.
Inhibition/activation effect of different compounds on the TRPL channel
| Name | Formula | TRPL Activation/Inhibition |
|---|---|---|
| Carvacrol |
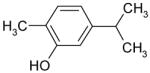
|
Inhibition |
| Thymol |
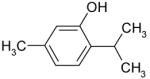
|
Inhibition |
| Eugenol |
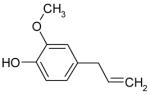
|
Inhibition |
| 2-APB |
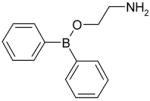
|
Inhibition |
| Cinnamaldehyde |
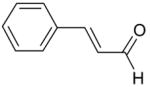
|
Inhibition |
| Caffeic acid |
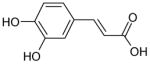
|
No effect |
| Menthol |
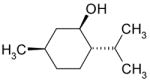
|
Inhibition |
| Carveol |
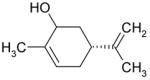
|
Inhibition |
| Borneol |
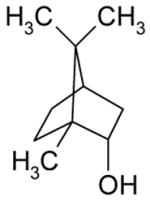
|
Activation |
| Camphor |
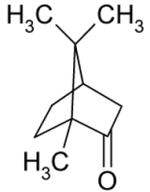
|
Activation |
Since both 2-APB and carvacrol inhibit TRPL but activate TRPV3, it was interesting to examine whether other activators of TRPV3 also inhibit the TRPL channel. Since all TRPV3 activators were shown to have an effect at a relatively high (< 10mM) concentrations, we used the same concentrations to test their inhibitory effect. Thymol and eugenol are alkyl phenols with closely related structure to carvacrol (see Table 1). These two compounds are also known activators of TRPV3 [10] and therefore are potential inhibitors of TRPL. To test this idea, we repeated the protocol of Fig. 1, except that eugenol (3mM Fig. 2A) and thymol (1mM Fig. 2B) were applied instead of carvacrol. Results similar to those of Fig. 1 were observed except that higher concentrations of the compounds had to be used in order to achieve an inhibitory effect. The I-V curves of Figs. 2A, B revealed a difference of ~15mV in their reversal potential. This difference is most likely due to the voltage ramp protocol, which is widely used and constitutes a reliable way to measure effects of chemicals on expressed channels. However, this protocol is not an optimal way to measure accurately the reversal potential, because of its inherent large variability [19].
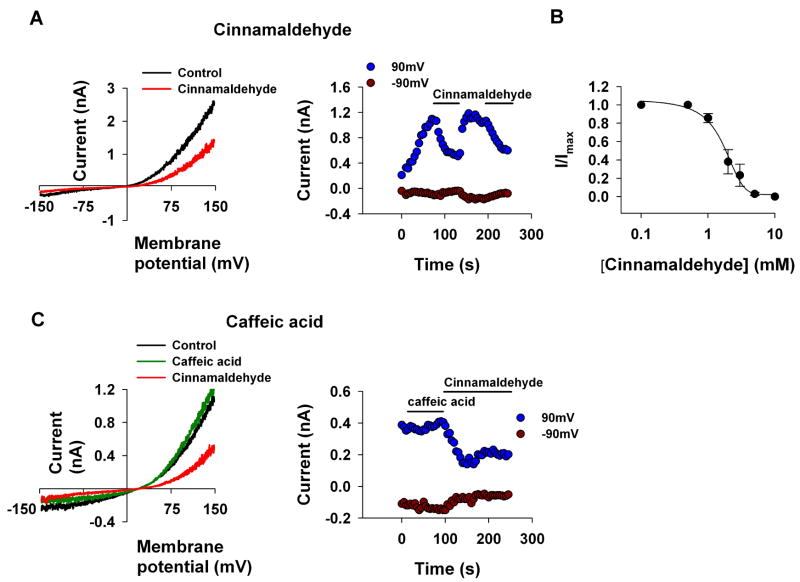
As the phenol moiety is a common structure of carvacrol, eugenol and thymol, the results of Figs. 1 and 2 raise the possibility that the phenol moiety of these compounds is important for their inhibition of the TRPL channel. In order to examine this possibility, we applied cinnamaldehyde, a known modulator of thermoTRPs which does not have a phenol moiety (for the chemical structure see Table 1). In addition, cinnamaldehyde activates the TRPA1 channel irreversibly by covalent modification [20]. We found that application of cinnamaldehyde inhibited the TRPL channel (Fig. 3A) with an IC50 of 1.6±0.3mM (Fig. 3B). However, in contrast to TRPA1 the inhibition of the TRPL channel by cinnamaldehyde was reversible, thus ruling out the possibility that covalent modifications are involved in the inhibition of the TRPL channel by cinnamaldehyde. We also applied caffeic acid, which is a diphenol carboxylic acid and is otherwise similar to cinnamaldehyde. In contrast to cinnamaldehyde, caffeic acid (up to 5mM) did not block the TRPL channel (Fig. 3C).
Figure 3. Cinnamaldehyde but not caffeic acid inhibits TRPL channels expressed in S2 cells.
A, C. The paradigm of Fig. 1 was repeated. Cinnamaldehyde (2mM, A, n=5) but not caffeic acid (5mM, C, n=7) caused a decrease in the TRPL mediated current. left, Representative I–V curves of the TRPL mediated current measured before and after application of the compounds. right, Time course of the TRPL mediated current measured from the I–V curves (left) at −90 mV and at 90 mV as indicated, before and after application of the compounds
B. Measurements of the blocking efficiency of cinnamaldehyde as a function of its concentration (error bars are SEM, n≥5)
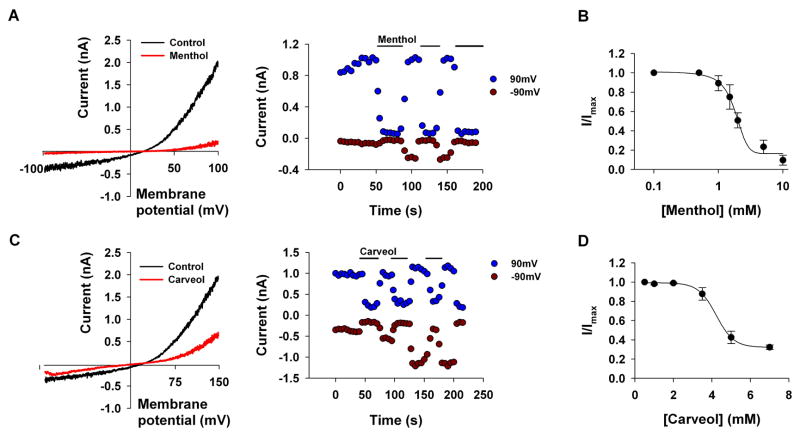
Together, the results of Figs. 1–3 rule out the possibility that the phenol moiety by itself is both necessary and sufficient for the inhibition of the TRPL channel by the alkyl phenol compounds. The fact that 2-APB, which does not have a phenol group, inhibits the TRPL channel (Table 1 and see [17]) further supports the above conclusion. We, therefore, hypothesized that the aromatic moiety of the alkyl phenols, cinnamaldehyde and 2-APB may be required for their inhibitory effect on the TRPL channel. To support this notion, we examined the effect of menthol and carveol, which have a 6-hydrocarbon ring, instead of aromatic ring (Table 1). Figures 4A, C show that both menthol and carveol inhibited the TRPL channel with an IC50 of 1.81±0.13mM and 4.22±0.4mM for menthol and carveol inhibition, respectively. Figure 4C shows an increase of the inward current relative to the outward current (i.e. a reduction of the outward rectification) with time. This phenomenon, which has appeared occasionally for the different tested compounds, is not due to the development of a leak current, since it was always blocked completely by the tested compound. Interestingly, a similar phenomenon has been demonstrated in several types of mammalian TRP channels without explanation and the underlying mechanism is still unknown [18;21;22].
Figure 4. Menthol and carveol inhibit TRPL channels expressed in S2 cells.
A, C. The paradigm of Fig. 1 was repeated. Menthol (5mM, A, n=5) and carveol (5mM, B, n=5) caused a large decrease in the TRPL-dependent current. left, Representative I–V curves of the TRPL mediated current measured before and after application of the compounds. right, Time course of the TRPL mediated current measured from the I–V curves (left) at −90 mV and at 90 mV as indicated, before and after application of the compounds.
B, D. Measurements of the blocking efficiency of menthol (B) and carveol (D) as a function of their concentration (error bars are SEM, n≥5)
The finding that menthol and carveol, which do not have an aromatic ring, still inhibit TRPL ruled out the possibility that the aromatic ring is important for the inhibitory effect of the above compounds (Figs. 1–3).
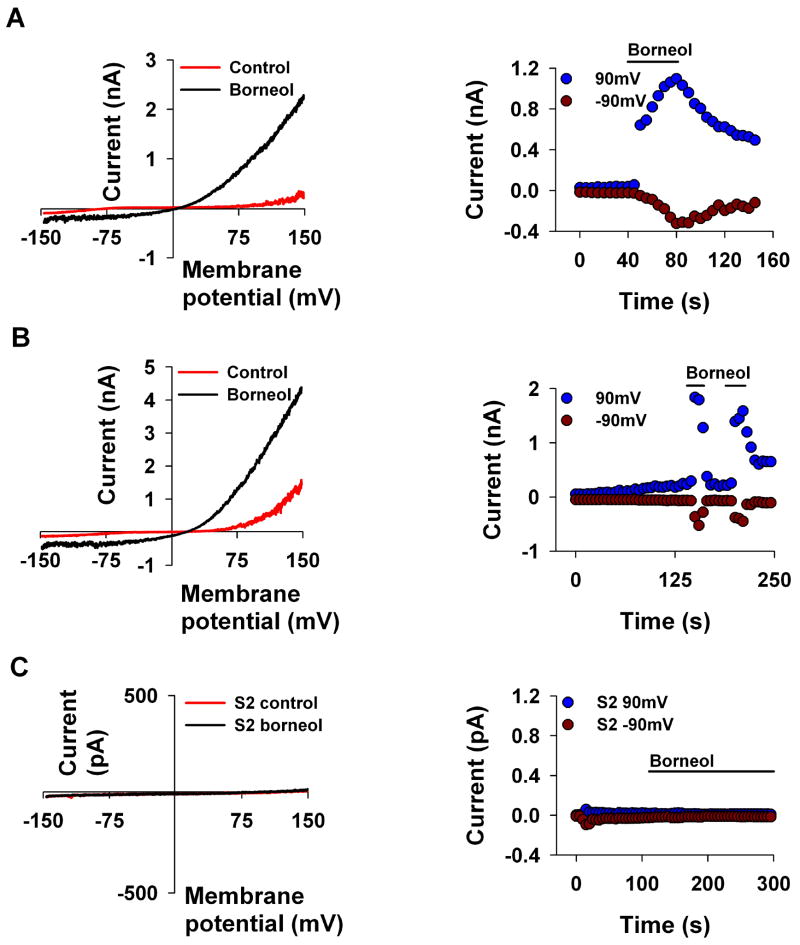
To examine whether other activators of TRPV3, which are not alkyl phenols, also inhibit the TRPL channel, we tested borneol and camphor, the known activators of TRPV3 [23]. Interestingly, borneol (5mM Fig. 5A) and camphor (10mM, Table 1) activated the TRPL channel. To make sure that the observed activation of the TRPL channel by borneol is not a manifestation of spontaneous increase in the basal activity of the channels (Fig. 1), we allowed in Fig. 5B the slow development of the basal constitutive current up to ~2 nA and only then applied the borneol. Figure 5B shows that after the slow development of the spontaneous activity a completely reversible rapid activation of the TRPL current was observed after the application of borneol. These results thus revealed, unexpectedly, novel activators of the TRPL channel.
Figure 5. Borneol activates TRPL channels expressed in S2 cells.
A, B. The paradigm of Fig. 1 was repeated. Borneol (5mM, n≥5) activated the TRPL mediated current when the channels were almost closed (A) and when the spontaneous activity of the TRPL channel was observed (B). left, Representative I–V curves of the TRPL mediated current measured before and after application of the compounds. right, Time course of the TRPL mediated current measured from the I–V curves (left) at −90 mV and at 90 mV as indicated, before and after application of the compound. Similar results were obtained when camphor was applied instead of borneol (Table 1).
C. Control experiment showing no effect of borneol when the TRPL channels were not expressed (n=5)
Together, Figs. 1–5 and Table 1 show that TRPL, a typical member of the TRPC subfamily is affected by natural compounds, which until now have been exclusively considered as activators of thermoTRPs because of their effects on thermal sensation [9].
Carvacrol inhibits the native TRPL channels in the Drosophila photoreceptor cells
Although the biophysical properties of the expressed and native TRPL channels are similar [15;16], it is important to establish the inhibitory effect of carvacrol on a typical TRPC channel in the native cells. To this end, we examined the effect of carvacrol on the native TRPL channel, which is one of the light activated channels of the Drosophila photoreceptor cell. This channel can be studied in isolation by measuring the light induced current (LIC) in the Drosophila mutant trpP343, which expresses only the TRPL channel (Fig. 6 [24]). Figure 6A shows the LIC in responses to a train of light pulses of constant intensity recorded from a photoreceptor cell of the trpP343 mutant (Fig. 6A left). Application of carvacrol (500μM) caused a dramatic reduction in the amplitude of the LIC (Fig. 6A, right, Fig. 6B). In contrast to the expressed TRPL in S2 cells, in the native photoreceptor cells the washout of lipophilic compounds is very slow (tens of min, [17]) and does not allow washout of the compound in a timescale in which the preparation is viable. It has been previously shown that inhibition of the TRPL and TRP channels by 2-APB is enhanced by light stimulation [17]. To examine if light activation is required for the inhibitory effect of carvacrol on the native TRPL channel, two light pulses were applied to the trpP343 mutant under control conditions, then carvacrol was applied in the dark and the constant test light was applied again. (see paradigm in Fig. 6C). We found that the inhibitory effect of carvacrol does not require light stimulation, since carvacrol induced inhibition was also effective upon application in the dark (data not shown and see Fig. 6C).
Figure 6. Carvacrol inhibits the native TRPL channels in photoreceptor cells.
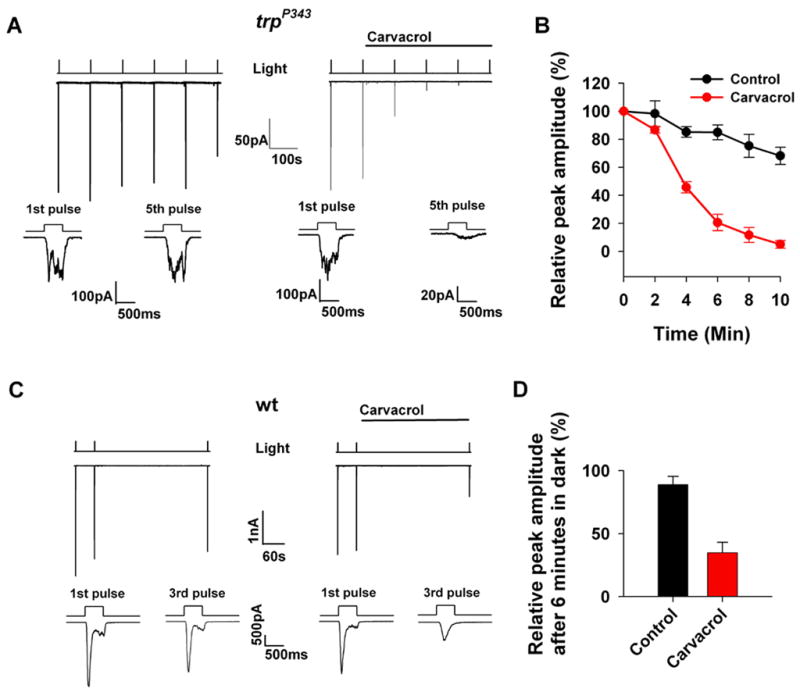
A. Representative whole-cell recordings of the light induced current (LIC) from isolated Drosophila ommatidia of the trpP343 mutant.
left, Control responses (middle trace) to a train of orange light pulses (top trace) of constant intensity (−logI=3.0, n=5). The bottom two traces show the waveform of the LICs in a faster time scale. right, The paradigm of the left panel was repeated, except that carvacrol (500μM) was applied as indicated (n=5).
B. The averaged peak amplitude of the light responses (such as in (A)) is plotted as a function of time (error bars are SEM, n=5).
C. The paradigm of (A) was repeated, except that wt flies were used and a larger dark interval was interposed after the second light pulse (n=5).
D. Histogram presenting the averaged and normalized peak amplitude of light responses after 6 min in the dark following application of carvacrol containing solutions (carvacrol, red) as compared to experiments in which carvacrol was not applied (control, black, error bars are SEM, n=5)
The use of the Drosophila photoreceptor cells allows testing the effect of carvacrol also on the major light activated channel TRP, which cannot be expressed in tissue culture cells [14]. The light response of wild-type (WT) flies in response to intense lights is mediated mainly via the TRP channels because genetic elimination of the TRPL channels has almost no phenotype under these conditions [24]. The use of WT flies therefore allows testing whether carvacrol also inhibits the TRP channel. Figure 6C and D show that carvacrol inhibits the LIC of WT flies implicating that both the TRPL and TRP channels are inhibited by carvacrol.
Together, Figures 1 and 6 show that carvacrol is an inhibitor of both native and expressed TRPL channels and native TRP channels.
Carvacrol inhibits the mammalian TRPM7 expressed in HEK cells
The TRPM7 channel, which belongs to the TRPM subfamily has features similar to the TRPL channel, such as activation by anoxia [25;26] and inhibition by divalent cations [16;27]. We, therefore, examined whether carvacrol also inhibits the TRPM7 channel.
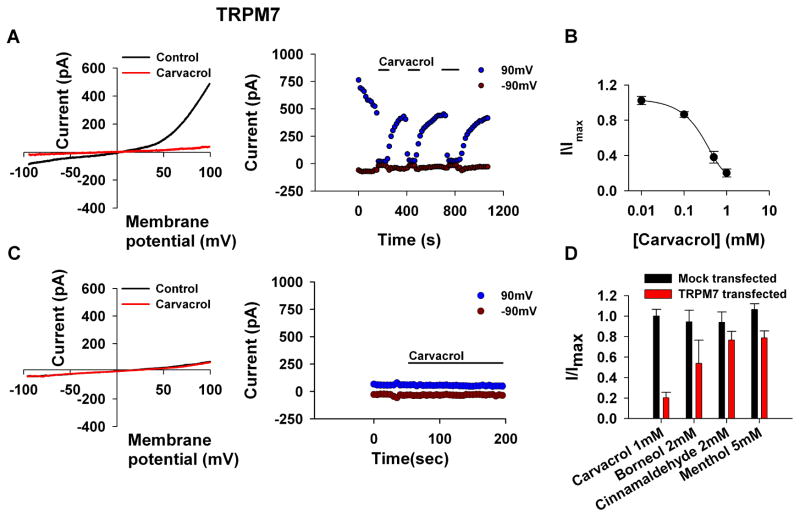
It has been previously shown that TRPM7 expressed in Human Embryonic Kidney (HEK) cells are constitutively active in the absence of Mg-ATP [27]. Accordingly, Figure 7A (left) shows the typical I–V curves of the spontaneously active TRPM7 channels expressed in HEK293 cells, before (control) and after application of carvacrol. Before application of carvacrol, the normal outward rectifying I–V curve of the expressed TRPM7 channel can be seen. Large suppression of the current was observed after application of carvacrol and the inhibitory effect was dependent on the concentration of carvacrol. The current values at −90mV and 90 mV obtained from a time series of I–V curves is plotted in Fig. 7A (right) as a function of time. Application of carvacrol to the active channels reversibly blocked the channels as manifested by suppression of both inward and outward current (Fig. 7A), with an IC50 of 306±65μM (Fig. 7B). In mock transfected HEK293 cells, carvacrol had no effect (Fig. 7C). We also examined, whether other pungent substances block the TRPM7 channel. Figure 7D presents a histogram of the maximal blocking effect of the TRM7 obtained for carvacrol, borneol, cinnamaldehyde and menthol showing that carvacrol is the most potent inhibitor of TRPM7 relative to the other compounds.
Figure 7. Carvacrol inhibits TRPM7 channel expressed in HEK cells.
A. The paradigm of Fig. 1 was repeated, except that HEK cells expressing TRPM7 were used and voltage ramps from −100mV to 100mV within 1s were applied every 5s. Carvacrol (500μM) caused a dramatic decrease in the TRPM7 mediated current. left, Representative I–V curves of the TRPM7 mediated current measured before and after application of carvacrol. right, Time course of the TRPL current measured from the I–V curves (right) at −90 mV and at 90 mV as indicated, before during and after application of carvacrol (500μM, n=8).
B. Measurements of the blocking efficiency of carvacrol as a function of its concentration (error bars are SEM, n≥5).
C. Control showing no effect of carvacrol when the TRPM7 channel was not expressed (n=5).
D. A histogram showing the maximal blocking effect obtained for the various compounds examined.
Carvacrol inhibits TRPM7 ectopically expressed in CA3-CA1 hippocampal cultures
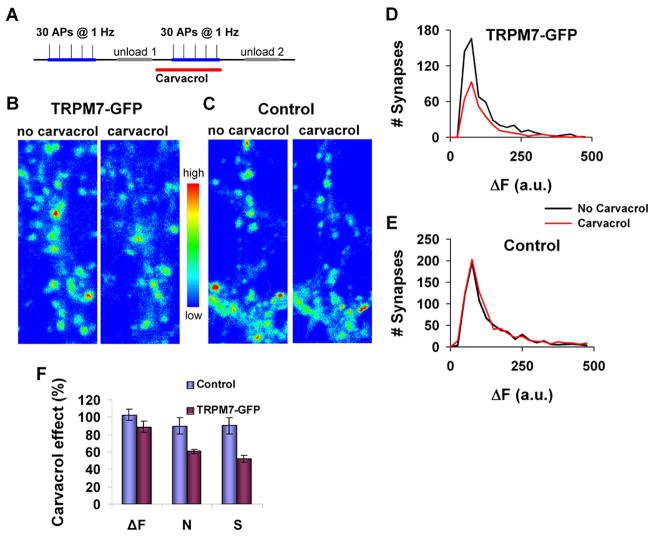
To further establish whether the inhibitory effect of carvacrol on TRPM7 is sufficient to block the functional effects of the TRPM7 channel, we examined the effect of carvacrol on TRPM7-dependent transmitter release. The activity of expressed TRPM7 channel was previously shown to enhance transmitter release in cholinergic sympathetic neurons [28]. A recent study on hippocampal brain slices of rat has shown that activation of TRPM7 channels takes place only under oxidative stress conditions [29]. Since over expression of TRPM7 results in constitutively active channels [27] we reasoned that over expression of TRPM7 in hippocampal brain neurons would mimic stressful conditions in which active TRPM7 channels are involved. We, therefore, examined the effect of carvacrol on the function of TRPM7 ectopically expressed in primary culture of hippocampal CA1-CA3 neurons. To test whether carvacrol affects synaptic vesicle release in hippocampal neurons, we used activity-dependent FM1–43 dye to directly monitor synaptic vesicle recycling [30;31]. Figure 8 compares vesicle release between control and TRPM7-GFP expressing synapses. The functional properties of synapses can be measured by estimating the probability of transmitter release and spatial distribution of functional presynaptic terminals. To this end, we measured the number of vesicles that have been released by a fixed number of simple spikes (30 action potentials (APs) at 1 Hz, Fig. 8A). The fluorescent intensity of individual punctum (ΔF) reflects the number of vesicles released, whereas the number of fluorescent boutons reflects the number of active terminals that released at least one vesicle (N, corresponding to synapses with release probability > 0.04). The total presynaptic strength (S) has been estimated by the product of these two parameters (S = ΔF × N).
Figure 8. Effect of carvacrol on synaptic vesicle release in CA3-CA1 primary hippocampal cultures.
A. Experimental protocol used to determine the number of presynaptic vesicles evoked by simple spikes (30 APs @ 1 Hz) using FM4–64 dye. Carvacrol was applied for 10 min before second dye loading.
B. Representative fluorescent images following stimulation with 30 APs @ 1 Hz in mature (15–28 days in-vitro (DIV)) CA3-CA1 hippocampal neurons that express TRPM7-GFP fusion protein before and 10 min after application of carvacrol (500μM). Fluorescence intensities (arbitrary units) were coded using a pseudocolor transformation shown on the right side of the images.
C. The same as in (B), but for control neurons, which did not express TRPM7-GFP.
D. Histograms of ΔF of boutons in TRPM7-GFP expressing neurons before (black line) and after (red line) carvacrol application (500μM) from the same experiment shown in (B). ΔF median changed from 73 to 75 arbitrary units (a.u) and the number of FM detectable boutons decreased from 578 to 318.
E. Histograms of ΔF of boutons in control neurons before (black line) and after (blue line) carvacrol application (500μM) from the same experiment shown in (C). ΔF median changed from 88 to 87 arbitrary units and the number of FM detectable boutons changed from 764 to 845.
F. Average magnitude of carvacrol effect on presynaptic strength for TRPM7-GFP and WT neurons (error bars are SEM, n = 4, P < 0.001).
An example for the effect of carvacrol on TRPM7-GFP expressing cells and on control cells, which did not express TRPM7-GFP is presented in Figs. 8B, C. We found that application of carvacrol (500μM) significantly decreased the vesicle release in TRPM7-GFP expressing neurons (Figs. 8D, F), but did not change vesicle release in control neurons (Figs. 8E, F). For the TRPM7-GFP expressing neurons, the reduction in vesicle release is due to a decreased number of active terminals. No change in the average number of vesicles released in each active terminal was observed (Fig. 8D). On average, carvacrol inhibited the total presynaptic strength (S) by 47 ± 3 %, while it did not affect presynaptic activity in control neurons (Fig. 8F).
These results demonstrate that carvacrol inhibits the function of mammalian TRPM7 expressed in synaptic terminals of hippocampal neurons. Together, the results show that carvacrol is a novel inhibitor of channel members of the TRPC and TRPM subfamilies.
Discussion
In the present study we identified carvacrol as a novel inhibitor of the TRPC and TRPM channel subfamilies. Since carvacrol is an activator of TRPV3 [10], we examined whether other activators of TRPV3 also inhibit the TRPL channel. An increase in the repertoire of TRPL inhibiting compounds should facilitate understanding the inhibitory effect of carvacrol. Indeed, we found that additional natural compounds that activate TRPV3, thymol, eugenol, cinnamaldehyde and menthol are all inhibitors of the TRPL channel. To investigate the structural features required for the blocking effect of these natural compounds, we examined several compounds with defined chemical properties. We could not identify a common structural feature, which is required for the blocking effect of all the natural compounds. Interestingly, all the TRPL inhibitors found in this study are known activators of TRPV3. Since carvacrol, thymol and eugenol are alkyl phenols with an inhibitory effect on the TRPL channel, it is surprising that caffeic acid, which is also an alkyl phenol, failed to block the TRPL channel. However, although caffeic acid is an alkyl phenol, it has a larger polarity than the other alkyl phenols that were examined in this study (see Table 1).
The blocking effect of carvacrol was further established by testing its inhibitory effect on the native TRP and TRPL channels of Drosophila photoreceptor cells. It was found that carvacrol is an inhibitor of both TRPL and TRP channels. In addition, carvacrol inhibits the TRPM7 channel expressed in both HEK cells and CA3-CA1 hippocampal primary cultured cells.
It was demonstrated that TRPM7 is an essential mediator of anoxic neuronal death [25]. Therefore, inhibition of TRPM7 is expected to reduce cell death following ischemia and brain damage. Indeed, suppression of TRPM7 expression in brain neurons protects the neurons from ischemic cell death [25]. To date, treatments for preventing ischemic cell death of brain neurons are only partially effective and antiexcitotoxic therapy with NMDA receptor antagonists have not proven clinically useful [32]. Accordingly, inhibitory compounds of a TRPM7-dependent current are expected to be important potential drugs for preventing neuronal cell death during brain damage. Thus, the blocking effect of carvacrol on TRPM7, which was demonstrated in this study, might serve as a potential candidate for further studies aiming at treatment of ischemic brain injury.
The natural compounds that we examined induce bitter, pungent or sharp taste and evoke warmth or cold sensation (possibly by activation of TRPA1 [33] but not TRPM8, on which carvacrol has only a minimal effect [34]). Although, it was found that these compounds are not selective for the different thermoTRPs, they were tested only for the thermoTRPs, due to their pungent nature. Our finding that TRPC and TRPM channels, which are not thermoTRP channels, are also modulated by the same compounds suggests that common mechanisms of modulation and activation characterize all TRP channels.
Methods
Fly stocks
Drosophila white-eyed flies of the following strains were used: wt and trpP343. Flies were raised at 24°C in a 12 h light/dark cycle.
Light Stimulation
A tungsten trans-illumination light (12 V, 100 W halogen lamp, in conjunction with a Schott OG 590 orange edge filter and a KG3 heat filter) provided illumination for stimulation of the photoreceptors in all the experiments. The stimulating light was applied via a condenser lens (Carl Zeiss MicroImaging, Inc.) and was attenuated by neutral density filters. The maximal luminous intensity of the unattenuated orange light at the level of the ommatidia was 3.2 mW/cm2.
Cell Culture
Schneider 2 cells were grown in 25 cm2 flasks at 25 °C in Schneider medium (Beit Haemek biological industries, Israel) supplemented with 10 % fetal bovine serum and 1 % pen-strep. TRPL-eGFP (TRPL accession number NM_165694) channels were stably expressed. Human embryonic kidney (HEK) cells were grown in 25 cm2 flasks at 37 °C in Dulbecco’s Modified Eagle’s Medium (DMEM) (Beit Haemek biological industries, Israel) supplemented with 10% fetal bovine serum (FBS), 1% pen-strep and 1% L-Glutamine. TRPM7 (accession number nm_021450) and pIRES-eGFP were transiently expressed using the transfection reagent Transit (Mirus).
Hippocampal cell culture
Primary cultures of hippocampal neurons were prepared as described [31]. The experiments were performed in mature (> days in vitro (DIV) 15) high density cultures (synaptic density > 1.5 synapses/μm2 of dendritic surface area). TRPM7-GFP was transiently expressed using Lipofectamin-2000 reagent (Invitrogen).
Electrophysiology
S2 and HEK cells
S2 and HEK cells were seeded on polylysine coated plates at a confluence of 25 %, 24–72 hours prior to the experiment. 24 hours prior to the experiment, 500μM CuSO4 was added to the medium of the S2 cells to induce expression of the channels. Whole-cell, patch-clamp recordings were performed as previously described [16]. Currents were recorded at room temperature using borosilicate patch pipettes of 5–8 MΩ and an Axopatch 1D (Molecular Devices, Inc.) voltage-clamp amplifier. Voltage-clamp pulses were generated and data captured using a Digidata 1322A interfaced to a computer running the pClamp 9.2 software (Axon Instruments, Inc.). Currents were filtered using the 8-pole low pass Bessel filter of the patch-clamp amplifier at 5 kHz and sampled at 20 kHz. To measure I–V curves with minimal distortions, only cells with low (<10 MΩ ) series resistance were used and the series resistance was compensated to ~80 %.
Drosophila ommatidia
Dissociated Drosophila ommatidia were prepared from newly emerged flies (<1 h after eclosion) that were kept in the dark 12–18 h before the experiment. Whole-cell, patch-clamp recordings were performed as previously described [35]. Currents were recorded at 21°C using borosilicate patch pipettes of 8–10 MΩ and an Axopatch 200B (Molecular Devices, Inc.) voltage-clamp amplifier. Voltage-clamp pulses were generated and data captured using a Digidata 1200 interfaced to a computer running the pClamp 8.0 software (Axon Instruments, Inc.). Currents were filtered using the 8-pole low pass Bessel filter of the patch-clamp amplifier at 5 kHz and sampled at 10 kHz. Series resistance was compensated to ~80 %.
Solutions
For S2 cells, the extracellular solution contained in mM: 150 NaCl, 5 KCl, 4 MgCl2, 10 TES, 25 proline, 5 alanine and 0.5 EGTA. The intracellular solution contained in mM: 130 K-gluconate, 10 TES, 2 MgCl2, 4 Mg-ATP and 0.4 Na-GTP. For Drosophila ommatidia, the extracellular solution contained in mM: 120 NaCl, 5 KCl, 4 MgSO4, 1.5 CaCl2, 10 TES, 25 proline and 5 alanine. The intracellular solution contained in mM: 140 K-gluconate, 10 TES, 2 MgSO4, 4 Mg-ATP, 0.4 Na-GTP and 1 β-NAD. All solutions were titrated to pH 7.15. For HEK cells, extracellular solution contained in mM: 140 NaCl, 2.8 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 Glucose. The intracellular solution contained in mM: 140 Cs-Aspartate, 8 NaCl, 10 EGTA and 10 HEPES. All solutions were titrated to pH 7.2. Cells were perfused via BPS-8 valve control system (Scientific Instruments) at a rate of ~30 chamber volumes per minute. Chemicals were applied via the perfusion system.
Estimation of synaptic vesicle release based on FM dye staining
Synaptic vesicle release at single synapses was determined by counting the number of presynaptic vesicles turned over by a fixed number of action potentials using activity-dependent FM dye uptake as a marker [30;31]. Briefly, during dye loading, action potentials in neurons were initiated by field stimulation and terminals, undergone vesicle exocytosis, were labelled by FM 4–64 (15 μM) presented in the extracellular solution during and 30 sec after electrical stimulation. Extracellular solution during FM loading and unloading procedures contained (in mM): NaCl, 145; KCl, 3; glucose, 15; HEPES, 10; MgCl2, 1.2; CaCl2, 1.2; kynurenic acid 0.5 (Sigma); pH adjusted to 7.4 with NaOH. Kynurenic acid has been added to prevent recurrent activity through block of excitatory postsynaptic responses during loading and unloading. Following the loading protocol (30 AP @ 1 Hz), external dye was washed away in Ca2+-free solution. To confirm that the fluorescent spots indeed corresponded to release sites, action potentials were evoked at frequency of 2 Hz for 4 min during unloading step in order to cause release of dye-filled vesicles. ΔF, the total amount of releasable fluorescence at each bouton (ΔF=Floading−Funloading), is proportional to release probability [31]. The total presynaptic strength (S) has been estimated by the product of these two parameters (S = ΔF × N), where N is the number of active terminals (defined as terminals that release at least 1 vesicle during loading protocol) per FM image.
Functional imaging and analysis
Images were taken using an Olympus (FV300) confocal laser inverted microscope. The 488 nm line of the argon laser was used for excitation, and the emitted light was filtered using a 510–530 nm band pass filter for GFP and 660 nm long pass filter for FM 4–64 and detected by photomultiplier. A 60×1.2 NA water-immersion objective was used for imaging. A confocal aperture was partially open and image resolution was 92 nm/pixel. The gain of the photomultiplier was adjusted to maximize the signal/noise ratio without causing the saturation by the strongest signals. The image after FM dye unloading was subtracted from the initial image; thus, only those terminals containing activity-dependent releasable FM dye (~90% of total staining) were analyzed. FM positive puncta were selected for further analysis using custom scripts written in ImagePro Plus (Media Cybernetics, Carlsbad, CA) and MATLAB (Mathworks, Natick, MA) programs.
Acknowledgments
We thank Dr. David Clapham for the TRPM7 and TRPM7-GFP plasmids and Ben Katz for critical reading of the manuscript. This research was supported by grants from the National Institute of Health (EY 03529), the Israel Science Foundation (ISF), the US-Israel Binational Science Foundation (BSF), the Moscona Foundation and the Minerva Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freichel M, Flockerzi V. Biological functions of TRPs unravelled by spontaneous mutations and transgenic animals. Biochem Soc Trans. 2007;35:120–123. doi: 10.1042/BST0350120. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 3.Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 4.Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- 5.Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39:585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- 6.Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channel. 2001:1–17. doi: 10.1126/stke.2001.90.re1. http://stkesciencemagorg/cgi/content/full/OC_sigtrans2001/90/rel. [DOI] [PubMed]
- 7.Patapoutian A, Peier AM, Story GM, Viswanath V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nat Rev Neurosci. 2003;4:529–539. doi: 10.1038/nrn1141. [DOI] [PubMed] [Google Scholar]
- 8.Bandell M, Macpherson LJ, Patapoutian A. From chills to chilis: mechanisms for thermosensation and chemesthesis via thermoTRPs. Curr Opin Neurobiol. 2007;17:490–497. doi: 10.1016/j.conb.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- 11.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 12.Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- 13.Scott K, Zuker C. TRP, TRPL and trouble in photoreceptor cells. Curr Opin Neurobiol. 1998;8:383–388. doi: 10.1016/s0959-4388(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 14.Minke B, Parnas M. Insights on TRP channels from in vivo studies in Drosophila. Annu Rev Physiol. 2006;68:649–684. doi: 10.1146/annurev.physiol.68.040204.100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie RC, Reuss H, Lansdell SJ, Millar NS. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21:431–440. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- 16.Parnas M, Katz B, Minke B. Open channel block by Ca2+ underlies the voltage dependence of Drosophila TRPL channel. J Gen Physiol. 2007;129:17–28. doi: 10.1085/jgp.200609659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chorna-Ornan I, Joel-Almagor T, Cohen-Ben AH, et al. A common mechanism underlies vertebrate calcium signaling and Drosophila phototransduction. J Neurosci. 2001;21:2622–2629. doi: 10.1523/JNEUROSCI.21-08-02622.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu HZ, Gu Q, Wang C, et al. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J Biol Chem. 2004;279:35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 19.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 20.Macpherson LJ, Dubin AE, Evans MJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 21.Hu HZ, Xiao R, Wang C, et al. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208:201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao R, Tang J, Wang C, Colton CK, Tian J, Zhu MX. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J Biol Chem. 2008;283:6162–6174. doi: 10.1074/jbc.M706535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moqrich A, Hwang SW, Earley TJ, et al. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- 24.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 25.Aarts M, Iihara K, Wei WL, et al. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 26.Agam K, von-Campenhausen M, Levy S, et al. Metabolic stress reversibly activates the Drosophila light-sensitive channels TRP and TRPL in vivo. J Neurosci. 2000;20:5748–5755. doi: 10.1523/JNEUROSCI.20-15-05748.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadler MJ, Hermosura MC, Inabe K, et al. LTRPC7 is a Mg. ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 28.Krapivinsky G, Mochida S, Krapivinsky L, Cibulsky SM, Clapham DE. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 29.Lipski J, Park TI, Li D, et al. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res. 2006;1077:187–199. doi: 10.1016/j.brainres.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Murthy VN, Sejnowski TJ, Stevens CF. Heterogeneous release properties of visualized individual hippocampal synapses. Neuron. 1997;18:599–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron. 2004;44:835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Aarts MM, Tymianski M. TRPM7 and ischemic CNS injury. Neuroscientist. 2005;11:116–123. doi: 10.1177/1073858404272966. [DOI] [PubMed] [Google Scholar]
- 33.Lee SP, Buber MT, Yang Q, et al. Thymol and related alkyl phenols activate the hTRPA1 channel. Br J Pharmacol. 2008;153:1739–1749. doi: 10.1038/bjp.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt-Eisele AK, Weber K, Sherkheli MA, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]