Abstract
Recognition that inflammation may represent a common mechanism of disease has been extended to include neuropsychiatric disorders including major depression. Patients with major depression have been found to exhibit increased peripheral blood inflammatory biomarkers, including inflammatory cytokines, which have been shown to access the brain and interact with virtually every pathophysiologic domain known to be involved in depression, including neurotransmitter metabolism, neuroendocrine function, and neural plasticity. Indeed, activation of inflammatory pathways within the brain is believed to contribute to a confluence of decreased neurotrophic support and altered glutamate release/reuptake, as well as oxidative stress, leading to excitotoxicity and loss of glial elements, consistent with neuropathologic findings that characterize depressive disorders. Further instantiating the link between inflammation and depression are data demonstrating that psychosocial stress, a well-known precipitant of mood disorders, is capable of stimulating inflammatory signaling molecules, including nuclear factor kappa B, in part, through activation of sympathetic nervous system outflow pathways. Interestingly, depressed patients with increased inflammatory biomarkers have been found to be more likely to exhibit treatment resistance, and in several studies, antidepressant therapy has been associated with decreased inflammatory responses. Finally, preliminary data from patients with inflammatory disorders, as well as medically healthy depressed patients, suggest that inhibiting proinflammatory cytokines or their signaling pathways may improve depressed mood and increase treatment response to conventional antidepressant medication. Translational implications of these findings include the unique opportunity to identify relevant patient populations, apply immune-targeted therapies, and monitor therapeutic efficacy at the level of the immune system in addition to behavior.
Keywords: Cytokines, depression, excitotoxicity, hypothalamic-pituitary-adrenal axis, inflammation, monoamines, stress
Major depression is a common and sometimes fatal disorder that is a leading cause of disability worldwide (1). Available antidepressant medications, which largely target monoamine pathways, are effective; however, more than 30% of depressed patients fail to achieve remission despite multiple treatment trials (2). Thus, there is a pressing need to identify novel pathophysiologic pathways relevant to depression that 1) reveal neurobiological targets for the development of new medications and 2) elucidate related biomarkers for the identification and monitoring of potentially responsive patients. One promising development in this regard is the emergence of inflammation as a common mechanism of disease. Indeed, numerous studies have demonstrated a clear relationship between inflammation and the development of cardiovascular disease, diabetes, and cancer (3,4). Mounting data indicate that inflammation may also play a role in neuropsychiatric diseases, including major depression. Given the accelerating development of biomarkers and treatments focused on the inflammatory response, there is tremendous promise that these advances, in addition to their relevance to general medicine, may have unique applications in psychiatry.
Evidence for a Cytokine Basis for Major Depression
When compared with nondepressed individuals, both medically ill and medically healthy patients with major depression have been found to exhibit all of the cardinal features of inflammation, including elevations in relevant inflammatory cytokines and their soluble receptors in peripheral blood and cerebrospinal fluid (CSF), as well as elevations in peripheral blood concentrations of acute phase proteins, chemokines, adhesion molecules, and inflammatory mediators such as prostaglandins (5,6). Associations between inflammatory markers and individual depressive symptoms such as fatigue, cognitive dysfunction, and impaired sleep have also been described (7-9). For example, both dysregulated sleep in depressed patients and sleep deprivation have been associated with increased interleukin (IL)-6, as well as activation of nuclear factor kappa B (NF-κB), a primary transcription factor in the initiation of the inflammatory response (8,10). Although much of the interest in inflammation and depression has been focused on cytokines, which mediate the innate immune response, including IL-1, tumor necrosis factor (TNF)-alpha, and IL-6, which appears to be one of the most reliable peripheral biomarkers in major depression (6,11), findings of increased markers of T cell activation (e.g., soluble IL-2 receptor) in depressed patients raises the specter that both acquired (e.g., T and B cell) and innate (e.g., macrophage) immune responses may be involved (11). Nevertheless, in contradistinction to the prominence of depression following administration of innate immune cytokines such as interferon (IFN)-alpha to humans (12,13), administration of the T cell cytokine, IL-2, is not uncommonly associated with profound changes in mental status including psychosis, delirium, and agitation (14).
In addition to correlative data linking inflammatory markers with depressive symptoms, several lines of evidence demonstrate that both acute and chronic administration of cytokines (or cytokine inducers such as lipopolysaccharide [LPS] or vaccination) can cause behavioral symptoms that overlap with those found in major depression. For example, normal volunteers injected with LPS exhibited acute increases in symptoms of depression and anxiety (15), and administration of a Salmonella typhi vaccine to healthy individuals produced depressed mood, fatigue, mental confusion, and psychomotor slowing (16). In both cases, symptom severity correlated with increases in peripheral blood cytokine concentrations. These data in humans are consistent with a large literature in laboratory animals demonstrating that cytokines and cytokine inducers can lead to a host of behavioral changes overlapping with those found in depression, including anhedonia, decreased activity, cognitive dysfunction, and altered sleep (17). Long-term exposure to cytokines also has been shown to lead to marked behavioral alterations in humans. For example, 20% to 50% of patients receiving chronic IFN-alpha therapy for the treatment of infectious diseases or cancer develop clinically significant depression (12,13). Of note, depressive syndromes induced by IFN-alpha exhibit considerable overlap with idiopathic major depression and like idiopathic major depression, respond to conventional antidepressant medication (12,13).
Finally, several studies in humans suggest that immune-targeted therapies may have clinical benefit. For example, acetylsalicylic acid (which blocks both cyclooxygenase-1 and 2 and the production of prostaglandins) when added to fluoxetine led to increased remission rates in an open-label study of depressed patients previously nonresponsive to fluoxetine alone (18). Similarly, medically healthy depressed patients who received the selective cyclooxygenase-2 inhibitor, celecoxib, in combination with reboxetine showed greater symptomatic improvement versus patients randomized to reboxetine plus placebo (19). Antidepressant activity of anti-inflammatory therapy has also been observed in patients with autoimmune and inflammatory disorders. For example, in a large double-blind, placebo-controlled trial of the TNF-alpha antagonist, etanercept, for the treatment of psoriasis, participants who received etanercept exhibited significant improvement in depressive symptoms compared with placebo-treated subjects, an effect that was independent of improvement in disease activity (20). These findings are consistent with a vast literature in laboratory animals indicating that cytokine antagonists or anti-inflammatory agents can block the development of behavioral changes following immune activation (17). Moreover, these data are consistent with findings that TNF-alpha receptor knockout (KO) mice exhibit an antidepressant phenotype (21).
Mechanisms and Mediators of Cytokine-Induced Behavioral Change
Studies have addressed fundamental pathways by which cytokines may contribute to depression. In general, cytokines have been shown to access the brain and interact with virtually every pathophysiologic domain relevant to depression, including neurotransmitter metabolism, neuroendocrine function, and neural plasticity (5,17). However, it remains unclear whether activation of inflammatory pathways in the central nervous system (CNS) during depression originate primarily in the periphery (e.g., as a function of overt or nascent medical illness or psychological stress-see below) and/or whether stress or other yet to be identified processes (e.g., vascular insults in late life depression) induce inflammatory responses directly within the brain. Such unresolved issues will have a major impact on whether relevant therapeutic targeting will require activity within the brain to be effective. Nevertheless, given that cytokines are relatively large polypeptides (~15-25 kD), experiments have been conducted in laboratory animals to determine how peripheral cytokine signals reach the brain. Pathways that have been elucidated include 1) cytokine passage through leaky regions in the blood-brain-barrier, 2) active transport via saturable transport molecules, 3) activation of endothelial cells and other cell types (including perivascular macrophages) lining the cerebral vasculature (which then produce cytokines and other inflammatory mediators), and 4) binding to cytokine receptors associated with peripheral afferent nerve fibers (e.g., the vagus nerve) that then relay cytokine signals to relevant brain regions including the nucleus of the solitary tract and hypothalamus (17,22). The inflammatory signaling molecule, NF-κB, has been found to be an essential mediator at the blood-brain interface that communicates peripheral inflammatory signals to the CNS. Central blockade of NF-κB in rodents inhibits c-fos activation in multiple brain regions following peripheral administration of IL-1 beta, while also inhibiting IL-1 beta and LPS-induced behavioral changes (23,24).
Data indicate that peripheral cytokine signals can also access the brain in humans and activate relevant cell types that serve to amplify central inflammatory responses. For example, peripheral administration of IFN-alpha to patients with hepatitis C led to increased CSF IFN-alpha, which correlated with increased CSF concentrations of IL-6 and the chemokine, monocyte chemoattractant protein (MCP)-1 (25). Monocyte chemoattractant protein-1, which is released by astrocytes and endothelial cells, has been found to prime microglia to produce IL-1 and TNF-alpha in response to LPS in rodents, an effect that is reduced in MCP-1 KO animals (26). Of note, microglia are a primary source of proinflammatory cytokines in the brain. Indeed, administration of the tetracycline agent, minocycline, which has been shown to inhibit LPS-induced cytokine production by microglia in vitro, attenuates both microglial activation and central cytokine induction, as well as behavioral changes following peripheral administration of LPS to mice (27,28). These effects may be mediated, in part, through minocycline's capacity to inhibit NF-κB(29).
Cytokine Effects on Neurotransmitter Metabolism
Once cytokine signals reach the brain, they have the capacity to influence the synthesis, release, and reuptake of mood-relevant neurotransmitters including the monoamines (30). There is a rich animal literature demonstrating that administration of cytokines or cytokine inducers can profoundly affect the metabolism of serotonin, norepinephrine, and dopamine (DA) (31,32). Moreover, drugs (serotonin and norepinephrine reuptake inhibitors) and gene polymorphisms (serotonin transporter gene) that affect monoamine metabolism have been shown to influence the development of cytokine-induced depressive-like behavior in laboratory animals and humans (12,33,34). Regarding the mechanisms involved, much attention has been focused on the enzyme, indoleamine 2,3 dioxygenase (IDO). Through stimulation of multiple inflammatory signaling pathways, including signal transducer and activator of transcription 1a (STAT1a), interferon regulatory factor (IRF)-1, NF-κB, and p38 mitogen-activated protein kinase (MAPK), cytokines can activate IDO (35). Indoleamine 2,3 dioxygenase, in turn, breaks down tryptophan (TRP), the primary amino acid precursor of serotonin, into kynurenine (KYN). The breakdown of TRP is believed to contribute to reduced serotonin availability (17,36)(Figure 1). Supportive of the role of IDO in cytokine-induced depression, decreased TRP and increased KYN in the peripheral blood have been associated with the development of depression in patients administered IFN-alpha (37). Moreover, blockade of IDO has been shown to inhibit the development of LPS-induced depressive-like behavior in mice (28). Of note, cytokine-induced IDO activation and the generation of KYN appear to have important effects on neurotransmitters and mood independent of effects on serotonin. For example, administration of KYN alone has been shown to induce depressive-like behavior in mice (28). In addition, based on the differential expression of relevant metabolic enzymes, KYN is preferentially converted to kynurenic acid (KA) in astrocytes and quinolinic acid (QUIN) in microglia (Figure 1)(36). Kynurenic acid has been shown to inhibit the release of glutatmate, which, by extension, may inhibit the release of dopamine, whose release is regulated in part by glutamatergic activity (38). Indeed, intrastriatal administration of KA has been shown to dramatically reduce extracellular DA in the rat striatum (39). In contrast, QUIN promotes glutamate release through activation of N-methyl-D-aspartate (NMDA) receptors (Figure 1). Quinolinic acid also induces oxidative stress, which in combination with glutamate release may contribute to CNS excitotoxicity (see below) (36,40-42). Thus, the relative induction of KA versus QUIN may determine the effects of cytokines on the CNS and remains an important area for future investigation, including the therapeutic targeting of IDO and KYN enzymatic pathways.
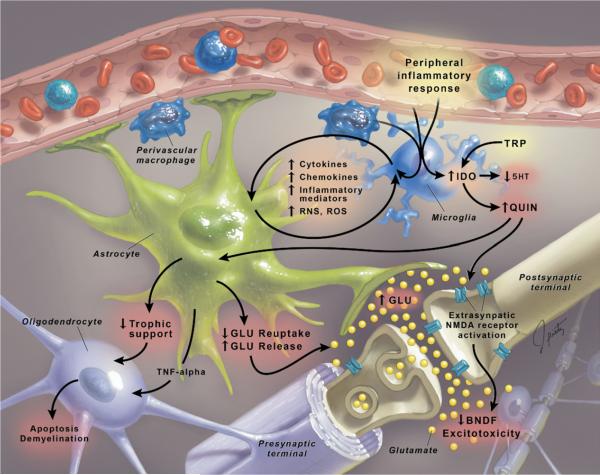
Figure 1.
Effects of the CNS inflammatory cascade on neural plasticity. Microglia are primary recipients of peripheral inflammatory signals that reach the brain. Activated microglia, in turn, initiate an inflammatory cascade whereby release of relevant cytokines, chemokines, inflammatory mediators, and reactive nitrogen and oxygen species (RNS and ROS, respectively) induces mutual activation of astroglia, thereby amplifying inflammatory signals within the CNS. Cytokines, including IL-1, IL-6, and TNF-alpha, as well as IFN-alpha and IFN-gamma (from T cells), induce the enzyme, IDO, which breaks down TRP, the primary precursor of 5-HT, into QUIN, a potent NMDA agonist and stimulator of GLU release. Multiple astrocytic functions are compromised due to excessive exposure to cytokines, QUIN, and RNS/ROS, ultimately leading to downregulation of glutamate transporters, impaired glutamate reuptake, and increased glutamate release, as well as decreased production of neurotrophic factors. Of note, oligodendroglia are especially sensitive to the CNS inflammatory cascade and suffer damage due to overexposure to cytokines such as TNF-alpha, which has a direct toxic effect on these cells, potentially contributing to apoptosis and demyelination. The confluence of excessive astrocytic glutamate release, its inadequate reuptake by astrocytes and oligodendroglia, activation of NMDA receptors by QUIN, increased glutamate binding and activation of extrasynaptic NMDA receptors (accessible to glutamate released from glial elements and associated with inhibition of BDNF expression), decline in neurotrophic support, and oxidative stress ultimately disrupt neural plasticity through excitotoxicity and apoptosis. 5-HT, serotonin; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; GLU, glutamate; IDO, indolamine 2,3 dioxygenase; IFN, interferon; IL, interleukin; NMDA, N-methyl-D-aspartate; QUIN, quinolinic acid; RNS, reactive nitrogen species; ROS, reactive oxygen species; TNF, tumor necrosis factor; TRP, tryptophan.
Cytokines also have been shown to influence the synthesis of DA. For example, intramuscular injection of recombinant, species-specific IFN-alpha to rats has been shown to decrease CNS concentrations of tetrahydrobiopterin (BH4) and DA in association with the stimulation of nitric oxide (NO) (43). Tetrahydrobiopterin is an important enzyme cofactor for tyrosine hydroxlylase, which converts tyrosine to L-3,4-dihydroxyphenylalanine (L-DOPA) and is the rate-limiting enzyme in DA synthesis. Tetrahydrobiopterin is also required for NO synthesis, and therefore increased NO generation is associated with increased BH4utilization (thus decreasing the availability of BH4 to support tyrosine hydroxylase activity). Treatment with an inhibitor of NO synthase was found to reverse the inhibitory effects of IFN-alpha on brain concentrations of both BH4 and DA (43). Activation of microglia is associated with increased NO production (44), suggesting that cytokine influences on BH4 via NO may be a common mechanism by which cytokines reduce DA availability in relevant brain regions.
Cytokines and their signaling pathways can also influence the reuptake of monoamines (30). Mitogen-activated protein kinase pathways, including p38 and extracellular signal-regulated kinases (ERK) 1/2, which mediate the effects of cytokines on cell proliferation/differentiation and apoptosis, as well as gene expression of inflammatory mediators, have been found to increase the activity of membrane transporters for serotonin and DA, as well as norepinephrine (45-47). For example, IL-1 and TNF-alpha have been shown to significantly increase serotonin reuptake in rat brain synaptosomes through activation of p38 MAPK (45). Of note, activated p38 in peripheral blood mononuclear cells has been associated with decreased CSF concentrations of the serotonin metabolite, 5-hydroxyindoleactectic acid (5-HIAA), in juvenile rhesus monkeys that were maternally abused as infants (48). Extending these findings, recent data in humans administered IFN-alpha have revealed that decreased CSF 5-HIAA was correlated not only with depressed mood but also increased CSF IL-6, which is capable of activating both MAPK and IDO pathways (25). Taken together with the influence of cytokines on monoamine synthesis, these data suggest that cytokines may exert a “double hit” on both monoamine synthesis and reuptake, thus contributing to reduced monoamine availability.
Cytokine Effects on Neuroendocrine Function
Some of the earliest observed effects of cytokines on mechanisms relevant to major depression involved their impact on the hypothalamic-pituitary-adrenal (HPA) axis (49). Cytokines, especially when administered acutely, have been shown to stimulate the expression and release of corticotropin-releasing hormone (CRH) and adrenocorticotropic hormone (ACTH), as well as cortisol, all of which have been found to be elevated in patients with depression (49,50). Although acute activation of the HPA axis in humans by IFN-alpha has been associated with the later development of depression (likely reflecting increased sensitivity of CRH pathways in the vulnerability to depression) (51), chronic administration of cytokines including IFN-alpha or chronic immune activation has not reliably been associated with persisting elevations in CRH or cortisol in humans or laboratory animals (25,32,52,53). For example, chronic administration of IFN-alpha to humans led to flattening of the diurnal cortisol slope and increased evening cortisol levels, which, in turn, was correlated with depression and fatigue (53). These findings are consistent with correlations between flattening of the cortisol curve and plasma IL-6 in patients with advanced cancer (54) and flattening of diurnal cortisol secretion and fatigue in breast cancer survivors (55), who have also been found to exhibit increased plasma cytokine soluble receptors (9).
One pathway by which cytokines may influence HPA axis function is through effects on negative feedback regulation. Impaired negative feedback regulation of HPA axis function is a hallmark of major depression and is reflected by decreased responsiveness to glucocorticoids (or glucocorticoid resistance) as manifested by increased cortisol concentrations following dexamethasone (DEX) administration in the DEX suppression test (DST) and the dexamethasone/corticotrophin-releasing hormone (DEX-CRH) test and decreased glucocorticoid-mediated inhibition of in vitro immune responses (50). Of note, flattening of the cortisol slope has been associated with nonsuppression on the DEX-CRH test in patients with breast cancer (56). Decreased feedback regulation of HPA axis function by glucocorticoids is believed to be mediated, in part, by alterations in the glucocorticoid receptor (GR) (50). Relevant to inflammation, cytokine activation of relevant inflammatory signaling molecules, including NF-κB, p38 MAPK, and signal transducer and activator of transcription 5 (STAT5), have been shown to inhibit GR through disruption of GR translocation from cytoplasm to nucleus, as well as through nuclear protein-protein interactions that inhibit GR-DNA binding (57). Cytokines can also influence GR expression, leading to decreased GR alpha, the active form of the receptor, and increased GR beta, a relatively inert GR isoform (57).
Given the potential effects of cytokines on GR signaling and the well-established inhibitory effect of glucocorticoids on inflammation (58), several studies have examined the relationship between inflammatory biomarkers and GR function in patients with major depression. Results from these largely cross-sectional studies have been mixed. One of the earliest studies in this regard showed a significant correlation between post-DEX concentrations of cortisol and the production of IL-1 by peripheral blood mononuclear cells (59). In addition, reduced skin sensitivity to topical glucocorticoid administration in depressed patients was found to significantly correlate with increased blood concentrations of TNF-alpha (60). Of note, in a study examining neuroendocrine and immune responses to LPS following DEX administration, subjects with evidence of glucocorticoid resistance (as manifested by increased ACTH and cortisol responses) exhibited increased cytokine (IL-6 and TNF-alpha) responses regardless of whether they were depressed or not, suggesting that the relationship between HPA axis sensitivity to glucocorticoids and responsiveness of the innate immune system may be unrelated to diagnosis (61).
Cytokine Effects on Neural Plasticity
Cytokines such as IL-1, IL-6, and TNF-alpha that subserve inflammation in the periphery have complex and Janus-faced functional roles in the CNS. Under physiological conditions, these cytokines are important for providing trophic support to neurons and enhancing neurogenesis, while contributing to normal cognitive functions such as memory in laboratory animals (62,63). However, significant data indicate that in the context of excessive and/or prolonged activation, cytokine networks in the CNS can promote an interconnected suite of abnormalities that are increasingly thought to be relevant to the pathophysiology of depression, including diminished neurotrophic support, decreased neurogenesis, increased glutamatergic activation, oxidative stress, induction of apoptosis in relevant cell types (e.g., astrocytes and oligodendrocytes), and dysregulation of glial/neuronal interactions and cognitive function (Figure 1)(63-77).
A rich animal literature demonstrates that activation of peripheral innate immune cytokine pathways-whether as a result of an immune challenge or acute or chronic stress-leads to increased proinflammatory cytokine production and decreased neurotrophic support and neurogenesis in brain areas important to behavior and cognition (17,64-67). For example, LPS administered peripherally produces cognitive impairment and increased hippocampal concentrations of TNF-alpha and IL-1, which are associated with decreased hippocampal expression of brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine kinase-B, as well as reduced hippocampal neurogenesis (67). Strongly supporting a causative role for inflammatory mediators in these behavioral and neurobiological changes are studies showing that the effects of acute and chronic stress on behavior, cognition, neurotrophic factors, and neurogenesis can be prevented by blockade of CNS cytokine activity through administration of IL-1 receptor antagonist (IL-1ra) or transplantation of IL-1ra secreting neural precursor cells into the hippocampus or the use of IL-1 receptor KO mice (64-66,78). Of note, in vitro studies have suggested that cytokine effects on neurogenesis are mediated in part by activation of NF-κB(64), while the release of glucocorticoids may be required for IL-1 effects on the brain during stress in vivo (78).
A related pathway to pathology in inflammation-induced effects on behavior includes the capacity of cytokines and inflammatory mediators to increase glutamate release and decrease the expression of glutamate transporters on relevant glial elements, thereby decreasing glutamate reuptake (68,71,75,79, 80). Of note, glutamate released by astrocytes has preferential access to extrasynaptic NMDA receptors, which mediate excitotoxicity and decreased production of trophic factors including BDNF (81,82). Cytokines, including TNF-alpha and IL-1, can also induce both astrocytes and microglia to release reactive oxygen and nitrogen species that in combination with QUIN (see above) can amplify oxidative stress and further endanger relevant cell types, including neurons and oligodendrocytes, which are especially vulnerable to oxidative damage (36,40,69,70,73,75-77,83). Astrocyte and microglial release of cytokines and inflammatory mediators also contributes to mutual amplification of inflammatory pathways within the brain (71,80). Consistent with the effect of cytokines and central inflammatory processes on glia, loss of glial elements, including oligodendrocytes and astrocytes, in multiple mood-relevant brain regions, including the subgenual prefrontal cortex and amygdala, has emerged as a fundamental morphologic abnormality in major depression (74,84,85). Of further relevance to the activation of inflammatory pathways within clinical populations are recent postmortem data suggesting that suicide is associated with increased microglia in the prefrontal cortex (86).
Cytokines, Stress, and Depression
The source of inflammation is clear when depression occurs in the context of medical illnesses in which there is an infectious, autoimmune, or inflammatory component or when there is tissue damage and/or destruction, all of which are associated with activation of peripheral, and in some cases, central inflammatory responses. However, in the case of presumably medically healthy depressed individuals, the source of inflammation is less apparent, albeit nascent inflammatory processes secondary to evolving medical pathologies remain a consideration. Nevertheless, a major breakthrough has been the increasing recognition that psychosocial stress can activate the inflammatory response both peripherally and in the brain (Figure 2). For example, peripheral blood mononuclear cells from healthy human volunteers exposed to a public speaking and mental arithmetic stressor were found to exhibit significant increases in NF-κB DNA binding (87). Interestingly, NF-κB and IL-6 responses to psychosocial stress have been shown to be exaggerated in patients with depression, consistent with findings that depressive symptoms are associated with amplified IL-6 responses to antigenic challenge (88,89). A rich database also indicates that chronic stress, including caregiving, marital discord, and perceived stress, is associated with increases in the acute phase protein, C-reactive protein (CRP), as well as IL-6 and other inflammatory mediators (90-92). In addition, increased inflammation appears to be a hall-mark of early life stress, in that childhood maltreatment has been associated with increased peripheral blood CRP (93). Of note, stress-induced activation of cytokine responses in the CNS appears to be largely dependent on activation of microglia (94).
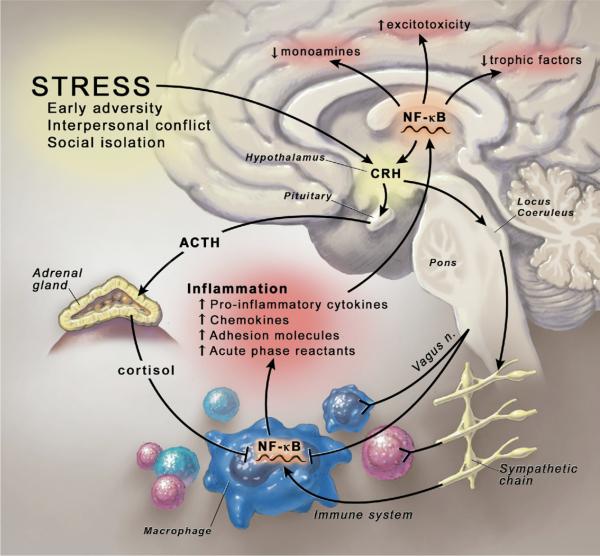
Figure 2.
Stress-induced activation of the inflammatory response. Psychosocial stressors activate central nervous system stress circuitry, including CRH and ultimately sympathetic nervous system outflow pathways via the locus coeruleus. Acting through alpha and beta adrenergic receptors, catecholamines released from sympathetic nerve endings can increase NF-κB DNA binding in relevant immune cell types, including macrophages, resulting in the release of inflammatory mediators that promote inflammation. Proinflammatory cytokines, in turn, can access the brain, induce inflammatory signaling pathways including NF-κB, and ultimately contribute to altered monoamine metabolism, increased excitotoxicity, and decreased production of relevant trophic factors. Cytokine-induced activation of CRH and the hypothalamic-pituitary-adrenal axis, in turn, leads to the release of cortisol, which along with efferent parasympathetic nervous system pathways (e.g., the vagus nerve) serve to inhibit NF-κB activation and decrease the inflammatory response. In the context of chronic stress and the influence of cytokines on glucocorticoid receptor function, activation of inflammatory pathways may become less sensitive to the inhibitory effects of cortisol, and the relative balance between the proinflammatory and anti-inflammatory actions of the sympathetic and parasympathetic nervous systems, respectively, may play an increasingly important role in the neural regulation of inflammation. CRH, corticotropin-releasing hormone; NF-κB, nuclear factor kappa B.
The mechanisms of stress-induced activation of immune responses involve both sympathetic nervous system (SNS) and HPA axis pathways (Figure 2). For example, catecholamines acting through alpha- and beta-adrenergic receptors have been shown to increase cytokine expression in the brain and periphery of rats (95), and alpha-adrenergic antagonists have been found to block increased peripheral blood IL-6 associated with altitude stress in humans (96). In addition, in vitro studies have demonstrated that stimulation of both alpha- and beta-adrenergic receptors can activate inflammatory signaling pathways, including NF-κB(87). Nevertheless, it should be noted that catecholamines have complex effects on multiple immune cell subtypes, and anti-inflammatory activities of catecholamines have been described (97). Moreover, the parasympathetic nervous system (PNS) may play a role in the autonomic nervous system regulation of inflammation. For example, studies have shown that stimulation of efferent vagus nerve fibers can inhibit cytokine responses to endotoxin in laboratory animals (98). These effects have been shown to be mediated, in part, by the release of acetylcholine, which, by binding to the α7 nicotinic acetylcholine receptor, is able to inhibit activation of NF-κB(98). The observation that increased inflammatory markers (e.g., CRP and IL-6) are associated with decreased parasympathetic activity, as reflected by decreased heart rate variability in young adults, supports the notion that the inhibitory effects of PNS activity on innate immune responses extends to humans (99). Taken together with the proinflammatory effects of catecholamines, these data suggest that there may be a yin-yang influence of the SNS and PNS on inflammation during stress (Figure 2).
Regarding the HPA axis, cortisol is one of the most potent anti-inflammatory hormones in the body (58); yet, in the context of chronic stress or depression, as noted above, the immune system can become glucocorticoid resistant. For example, in a recent study examining gene expression patterns in healthy control subjects versus individuals experiencing the chronic stress of caregiving for cancer patients, caregivers not only exhibited increases in plasma CRP and the expression of genes containing promoter response elements for NF-κB but also exhibited significant decreases in genes containing promoter elements for the GR, despite similar salivary concentrations of cortisol (92). Taken together, these data suggest that increased activity through SNS pathways coupled with reduced sensitivity to the inhibitory effects of glucocorticoids (e.g., cortisol) may conspire during chronic stress to contribute to chronic activation of inflammatory responses.
Neuroanatomical Substrates of Cytokine Effects on the Brain
An emerging literature is beginning to identify brain regions that may be targets of the effects of cytokines in humans. One important area in this regard is the basal ganglia. Both IFN-alpha and typhoid vaccination have been associated with psychomotor slowing and/or fatigue in association with changes in neuronal activity in the substantia nigra, putamen, and nucleus accumbens, as measured by functional magnetic resonance imaging (fMRI) and positron emission tomography (16,100). Given the role of basal ganglia in motivational states and locomotor activity (101), cytokine-induced effects on the basal ganglia and DA may represent an important mechanism whereby cytokines inhibit behavioral activation, thereby supporting evolutionarily derived pressures to reallocate energy resources from environmental exploration to fighting infection and wound healing (see below) (30).
Symptoms of anxiety, irritability, and hyperarousal are also apparent following cytokine administration to humans. Such symptoms have been observed after acute administration of endotoxin as well as chronic treatment with IFN-alpha (13,15,30,102). Indeed, a significant percentage of patients receiving IFN-alpha therapy have been shown to exhibit hypomanic and, in some cases, manic features, including marked irritability, inability to sleep, and hyperactivity (102). Similar findings have been reported in rhesus monkeys administered recombinant human IFN-alpha (32). Of potential relevance to these symptoms is that patients receiving IFN-alpha for hepatitis C exhibit significantly greater activation in the dorsal anterior cingulate cortex (dACC) (Brodmann's area [BA] 24), compared with non-IFN-alpha-treated control subjects (103). The dACC has been shown to play an important role in error detection and conflict monitoring (104), and increased activity in this brain region has been associated with high trait anxiety, neuroticism, obsessive-compulsive disorder, and bipolar disorder (105), all of which are associated with increased anxiety and arousal. Interestingly, activation of the dACC also has been found during an fMRI task of social rejection and consistent with the role of this brain region in the processing of social pain, was correlated with task-related emotional distress (105). Combined with its role in error detection and conflict monitoring, the processing of social pain by the dACC has been suggested to comprise a neural “alarm system,” which can both detect and respond to threatening environmental stimuli (105). Based on the neuroimaging data from IFN-alpha-treated patients, it appears that one mechanism by which cytokines may lead to increased arousal, anxiety, and alarm is through increased activation of neural circuits involving the dACC (103). Of note regarding the potential evolutionary significance of these data, an animal that has been infected or wounded is vulnerable to attack and therefore must maintain increased vigilance to respond to intrusions from a predator (30). Thus, taken together with the effects of cytokines on the basal ganglia, which serve to reduce exploratory behavior, the effects of cytokines on neurocircuits within the brain appear to subserve competing evolutionary survival priorities that promote reduced activity to allow healing, while fostering hypervigilance to protect against future attack (30).
Translational Implications
Relevant to the potential clinical applications of the association between inflammation and depression, data indicate that inflammatory biomarkers may identify depressed patients who are less likely to respond to conventional antidepressant treatment and may provide an indicator of treatment response. For example, patients with evidence of increased inflammatory activity prior to treatment have been reported to be less responsive to antidepressants, lithium, or sleep deprivation (a potent short-term mood elevator) (106-108). Moreover, patients with a history of nonresponse to antidepressants have been found to demonstrate increased plasma concentrations of IL-6 and acute phase reactants when compared with treatment-responsive patients (108,109). In addition, a nascent literature suggests that functional allelic variants of the genes for IL-1 beta and TNF-alpha, as well as genes critical for T cell function, may increase the risk for depression and may be associated with reduced responsiveness to antidepressant therapy (110-112). Of note, antidepressant treatment has been associated with decreases in inflammatory markers in 11 of 20 studies that examined immune responses as a function of antidepressant therapy (Supplement 1). Such data are consistent with in vitro findings that antidepressants can inhibit LPS-induced production of proinflammatory cytokines while promoting anti-inflammatory cytokines such as IL-10 (113). It should be mentioned that of studies that found increased markers of inflammation following antidepressant administration, two reported that the increases occurred in association with antidepressant-induced increases in body mass index (BMI) (Supplement 1). Of relevance in this regard is that BMI has been shown to correlate with increased peripheral markers of inflammation, in part related to the capacity of adipose tissue to produce IL-6 and other cytokines (114,115). Thus, the association between increased BMI and inflammation represents a complicating factor in the relationship among inflammation, depression, and antidepressant treatment.
More direct treatment implications of the inflammation-depression hypothesis are the development of treatments that target pathways by which the immune system impacts the brain. Obvious targets include the cytokines themselves, their signaling pathways, and downstream inflammatory mediators, as well as the activation of relevant CNS immune cell types (e.g., microglia) (Table 1). Anti-inflammatory cytokines such as IL-10, as well as insulin-like growth factor, which has been shown to block both LPS- and TNF-alpha-induced behavioral changes, also warrant consideration (116). In addition, treatments addressing immunologic effects on monoamine metabolism, CNS excitotoxicity (NMDA antagonists), and decreased trophic support are also indicated. Finally, given the influence of stress and stress-induced activation of the SNS, behavioral interventions that address psychological and autonomic reactivity to stress, including psychotherapy, exercise, and meditation, may have efficacy both regarding treatment and prevention. Indeed, in a recent study on compassion meditation, compared with an educational control group, individuals who engaged in meditation practice exhibited significantly reduced IL-6 responses to a laboratory psychosocial stressor (117).
Table 1.
Potential Translational Targets for Inflammation-Induced Depression
| Immune System |
| Cytokines (e.g., TNF-alpha, IL-1, IL-6) |
| Cytokine signaling pathways (e.g., NF-κB and MAPK) |
| Inflammatory mediators (e.g., COX-2, PG) |
| RNS/ROS (e.g., NO, H202) |
| Immune cells in the brain (e.g., microglia) |
| Central Nervous System |
| Monoamines (e.g., 5-HT, NE, DA) |
| IDO and its metabolites (e.g., KYN, QUIN, KA) |
| Extrasynaptic NMDA receptors |
| Excitotoxic neurotransmitters (e.g., glutamate) |
| Neurotrophic factors (e.g., BDNF) |
| Neuroendocrine System |
| HPA axis hormones (e.g., CRH, cortisol) and receptors (e.g., GR) |
| Autonomic Nervous System and Stress |
| Catecholamines and receptors (e.g., alpha and beta adrenergic receptors) |
| Parasympathetic outflow pathways (e.g., vagal nerve, alpha 7 nAChR) |
| Stress (e.g., interpersonal conflict, early adversity) |
5-H, serotonin; BDNF, brain-derived neurotrophic factor; COX-2, cyclooxy-genase-2; CRH, corticotropin-releasing hormone; DA, dopamine; GR, glucocor-ticoid receptor; H202, hydrogen peroxide; HPA, hypothalamic-pituitary-adrenal; IDO, indoleamine 2,3 dioxygenase; IL, interleukin; KA, kynurenic acid; KYN, kynurenine; MAPK, mitogen activated protein kinase; nAChR, nicotinic ace-tylcholine receptor; NE, norepinephrine; NF-κB, nuclear factor kappa B; NMDA, N-methyl-D-aspartate; NO, nitric oxide; PG, prostaglandin; RNS, reactive nitrogen species; ROS, reactive oxygen species; QUIN, quinolinic acid; TNF, tumor necrosis factor.
It should also be noted that an exciting opportunity regarding clinical trial design in studies targeting the immune system is the availability of pathophysiology-specific biomarkers that can be monitored early in treatment to determine whether a given therapy is effective in reducing immune activation. Such biomarkers can be used not only to monitor response but also, as noted above, can be used to identify patient populations that may be most likely to benefit from inflammation-targeted therapies. Of relevance in this regard is that published guidelines have already established categories of inflammation based on peripheral blood concentrations of CRP, with values >3 mg/L reflecting high inflammation (4). The availability of peripheral biomarkers that can both identify patients with specific pathophysiologic processes and serve to objectively monitor therapeutic responses within relevant pathways is truly unique and may represent a major advance in the personalization of the treatment of depression. Coupled with concerted efforts across medical disciplines to develop medications and biomarkers that target inflammatory responses, the notion that depression, like other medical disorders, may share an inflammatory component represents an exciting venue for transdisciplinary collaboration and the integration of resources from immunology, the neurosciences, and a variety of medical specialties to address a pressing need to develop novel approaches to treat patients with major depression.
Supplementary Material
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (NIH) (K05 MH069124, K23 MH064619, R01 MH070553, R01 HL073921, T32 MH020018), an NIH/National Center for Research Resources (NCRR) General Clinical Research Center Grant (M01 RR00039), and the Centers for Disease Control and Prevention.
Artwork was kindly provided by Jordan Pietz and Kim Hoggatt Krumwiede, Biomedical Communications Graduate Program, University of Texas, Southwestern Medical Center, Dallas, Texas.
Charles L. Raison is on the speakers' bureau for Lilly, Wyeth, and Schering-Plough and has served as a consultant or an advisory board member for Schering-Plough, Wyeth, Lilly, and Centocor; Vladimir Maletic is on the speakers' bureau for Lilly, Takeda, and Novartis and has served as a consultant for Lilly, Takeda, and Pfizer; Andrew H. Miller has served as a consultant for AstraZeneca, Schering-Plough, and Centocor and has received research support from Schering-Plough, Centocor, and GlaxoSmithKline.
Footnotes
Supplementary material cited in this article is available online.
References
- 1.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370:851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 2.Rush AJ. STAR*D: What have we learned. Am J Psychiatry. 2007;164:739–752. [Google Scholar]
- 3.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: How hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutr Rev. 2007;65:S253–259. doi: 10.1111/j.1753-4887.2007.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 5.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of major depression. Trend Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zorilla EP, Luborsky L, McKay JR, Roesnthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: A meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 7.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]
- 8.Motivala SJ, Sarfatti A, Olmos L, Irwin MR. Inflammatory markers and sleep disturbance in major depression. Psychosom Med. 2005;67:187–194. doi: 10.1097/01.psy.0000149259.72488.09. [DOI] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 2002;64:604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, et al. Consensus paper of the WFSBP Task Force on Biological Markers: Biological markers in depression. World J Biol Psychiatry. 2007;8:141–174. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- 12.Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 13.Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, et al. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 14.Kammula US, White DE, Rosenberg SA. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer. 1998;83:797–805. [PubMed] [Google Scholar]
- 15.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 16.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendlewicz J, Kriwin P, Oswald P, Souery D, Alboni S, Brunello N. Shortened onset of action of antidepressants in major depression using acetylsalicylic acid augmentation: A pilot open-label study. Int Clin Psychopharmacol. 2006;21:227–231. doi: 10.1097/00004850-200607000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: Results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 20.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: Double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 21.Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: Behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Nadjar A, Bluthe RM, May MJ, Dantzer R, Parnet P. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- 24.Godbout JP, Berg BM, Krzyszton C, Johnson RW. Alpha-tocopherol attenuates NFkappaB activation and pro-inflammatory cytokine production in brain and improves recovery from lipopolysaccharide-induced sickness behavior. J Neuroimmunol. 2005;169:97–105. doi: 10.1016/j.jneuroim.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: Relationship to monoamines and depression. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.010. [published online ahead of print September 16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rankine EL, Hughes PM, Botham MS, Perry VH, Felton LM. Brain cytokine synthesis induced by an intraparenchymal injection of LPS is reduced in MCP-1-deficient mice prior to leucocyte recruitment. Eur J Neurosci. 2006;24:77–86. doi: 10.1111/j.1460-9568.2006.04891.x. [DOI] [PubMed] [Google Scholar]
- 27.Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroin-flammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [published online ahead of print January 15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Si Q, Cosenza M, Kim MO, Zhao ML, Brownlee M, Goldstein H, et al. A novel action of minocycline: Inhibition of human immunodeficiency virus type 1 infection in microglia. J Neurovirol. 2004;10:284–292. doi: 10.1080/13550280490499533. [DOI] [PubMed] [Google Scholar]
- 30.Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.08.006. [published online ahead of print September 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anisman H, Merali Z, Hayley S. Neurotransmitter, peptide and cytokine processes in relation to depressive disorder: Comorbidity between depression and neurodegenerative disorders. Prog Neurobiol. 2008;85:1–74. doi: 10.1016/j.pneurobio.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, et al. Effects of interferon-alpha on rhesus monkeys: A non-human primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–1333. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, et al. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [published online ahead of print May 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, et al. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- 35.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1 alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: Involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–662. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 36.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: Glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 37.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism: Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 38.Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–229. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- 39.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: A new case of volume transmission in the brain? J Neural Transmission. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 40.Rios C, Santamaria A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- 41.Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 42.McNally L, Bhagwagar Z, Hannestad J. Inflammation, glutamate, and glia in depression: A literature review. CNS Spectr. 2008;13:501–510. doi: 10.1017/s1092852900016734. [DOI] [PubMed] [Google Scholar]
- 43.Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: Role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–114. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- 44.Zielasek J, Hartung HP. Molecular mechanisms of microglial activation. Adv Neuroimmunol. 1996;6:191–122. doi: 10.1016/0960-5428(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 45.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 46.Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, et al. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- 49.Besedovsky HO, del Rey A. Immune-neuro-endocrine interactions: Facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 50.Pariante CM, Miller AH. Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 51.Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 52.Harbuz MS, Chover-Gonzalez AJ, Jessop DS. Hypothalamo-pituitary-adrenal axis and chronic immune activation. Ann N Y Acad Sci. 2003;992:99–106. doi: 10.1111/j.1749-6632.2003.tb03141.x. [DOI] [PubMed] [Google Scholar]
- 53.Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: Relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.58. [published online ahead of print June 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rich T, Innominato PF, Boerner J, Mormont MC, Iacobelli S, Baron B, et al. Elevated serum cytokines correlated with altered behavior, serum cortisol rhythm, and dampened 24-hour rest-activity patterns in patients with metastatic colorectal cancer. Clin Cancer Res. 2005;11:1757–1764. doi: 10.1158/1078-0432.CCR-04-2000. [DOI] [PubMed] [Google Scholar]
- 55.Bower JE, Ganz PA, Dickerson SS, Petersen L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Spiegel D, Giese-Davis J, Taylor CB, Kraemer H. Stress sensitivity in metastatic breast cancer: Analysis of hypothalamic-pituitary-adrenal axis function. Psychoneuroendocrinology. 2006;31:1231–1244. doi: 10.1016/j.psyneuen.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids-new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 59.Maes M, Bosmans E, Meltzer HY, Scharpe S, Suy E. Interleukin-1 beta: A putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald P, O'Brien SM, Scully P, Rijkers K, Scott LV, Dinan TG. Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychol Med. 2006;36:37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- 61.Vedder H, Schreiber W, Schuld A, Kainz M, Lauer CJ, Krieg JC, et al. Immune-endocrine host response to endotoxin in major depression. J Psychiatr Res. 2007;41:280–289. doi: 10.1016/j.jpsychires.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- 63.Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad SciUSA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, et al. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- 66.Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, et al. Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience. 2003;121:847–853. doi: 10.1016/s0306-4522(03)00564-5. [DOI] [PubMed] [Google Scholar]
- 67.Wu CW, Chen YC, Yu L, Chen HI, Jen CJ, Huang AM, et al. Treadmill exercise counteracts the suppressive effects of peripheral lipopolysaccharide on hippocampal neurogenesis and learning and memory. J Neurochem. 2007;103:2471–2481. doi: 10.1111/j.1471-4159.2007.04987.x. [DOI] [PubMed] [Google Scholar]
- 68.Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85:2059–2070. doi: 10.1002/jnr.21325. [DOI] [PubMed] [Google Scholar]
- 69.Gavillet M, Allaman I, Magistretti PJ. Modulation of astrocytic metabolic phenotype by proinflammatory cytokines. Glia. 2008;56:975–989. doi: 10.1002/glia.20671. [DOI] [PubMed] [Google Scholar]
- 70.Matute C, Domercq M, Sanchez-Gomez MV. Glutamate-mediated glial injury: Mechanisms and clinical importance. Glia. 2006;53:212–224. doi: 10.1002/glia.20275. [DOI] [PubMed] [Google Scholar]
- 71.Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- 72.Pav M, Kovaru H, Fiserova A, Havrdova E, Lisa V. Neurobiological aspects of depressive disorder and antidepressant treatment: Role of glia. Physiol Res. 2008;57:151–164. doi: 10.33549/physiolres.930990. [DOI] [PubMed] [Google Scholar]
- 73.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 74.Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ida T, Hara M, Nakamura Y, Kozaki S, Tsunoda S, Ihara H. Cytokine-induced enhancement of calcium-dependent glutamate release from astrocytes mediated by nitric oxide. Neurosci Lett. 2008;432:232–236. doi: 10.1016/j.neulet.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 76.Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004;76:834–845. doi: 10.1002/jnr.20118. [DOI] [PubMed] [Google Scholar]
- 77.Li J, Ramenaden ER, Peng J, Koito H, Volpe JJ, Rosenberg PA. Tumor necrosis factor alpha mediates lipopolysaccharide-induced microglial toxicity to developing oligodendrocytes when astrocytes are present. J Neurosci. 2008;28:5321–5330. doi: 10.1523/JNEUROSCI.3995-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, et al. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- 79.Pitt D, Nagelmeier IE, Wilson HC, Raine CS. Glutamate uptake by oligodendrocytes: Implications for excitotoxicity in multiple sclerosis. Neurology. 2003;61:1113–1120. doi: 10.1212/01.wnl.0000090564.88719.37. [DOI] [PubMed] [Google Scholar]
- 80.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: Amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 81.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 82.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 83.Thornton P, Pinteaux E, Gibson RM, Allan SM, Rothwell NJ. Interleukin-1-induced neurotoxicity is mediated by glia and requires caspase activation and free radical release. J Neurochem. 2006;98:258–266. doi: 10.1111/j.1471-4159.2006.03872.x. [DOI] [PubMed] [Google Scholar]
- 84.Hamidi M, Drevets WC, Price JL. Glial reduction in amygdala in major depressive disorder is due to oligodendrocytes. Biol Psychiatry. 2004;55:563–569. doi: 10.1016/j.biopsych.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 85.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: Elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 87.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 89.Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- 90.McDade TW, Hawkley LC, Cacioppo JT. Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: The Chicago health, aging, and social relations study. Psychosom Med. 2006;68:376–381. doi: 10.1097/01.psy.0000221371.43607.64. [DOI] [PubMed] [Google Scholar]
- 91.Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, et al. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch Gen Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- 92.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 95.Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, et al. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 96.Mazzeo RS, Donovan D, Fleshner M, Butterfield GE, Zamudio S, Wolfel EE, et al. Interleukin-6 response to exercise and high-altitude exposure: Influence of alpha-adrenergic blockade. J Appl Physiol. 2001;91:2143–2149. doi: 10.1152/jappl.2001.91.5.2143. [DOI] [PubMed] [Google Scholar]
- 97.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain Behav Immun. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 99.Sloan RP, McCreath H, Tracey KJ, Sidney S, Liu K, Seeman T. RR interval variability is inversely related to inflammatory markers: The CARDIA study. Mol Med. 2007;13:178–184. doi: 10.2119/2006-00112.Sloan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine BJ, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 101.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 102.Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: Evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 103.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 105.Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trend Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 106.Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E. Interleukine-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Progr Neuropsychopharmacol Biol Psychiatry. 2002;26:1167–1170. doi: 10.1016/s0278-5846(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 107.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 108.Sluzewska A, Sobieska M, Rybakowski JK. Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology. 1997;35:123–127. doi: 10.1159/000119332. [DOI] [PubMed] [Google Scholar]
- 109.Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- 110.Yu YW, Chen TJ, Hong CJ, Chen HM, Tsai SJ. Association study of the interleukin-1 beta (C-511T) genetic polymorphism with major depressive disorder, associated symptomatology, and antidepressant response. Neuropsychopharmacology. 2003;28:1182–1185. doi: 10.1038/sj.npp.1300172. [DOI] [PubMed] [Google Scholar]
- 111.Jun TY, Pae CU, Hoon H, Chae JH, Bahk WM, Kim KS, et al. Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatr Genet. 2003;13:179–181. doi: 10.1097/00041444-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 112.Wong ML, Dong C, Maestre-Mesa J, Licinio J. Polymorphisms in inflammation-related genes are associated with susceptibility to major depression and antidepressant response. Mol Psychiatry. 2008;13:800–812. doi: 10.1038/mp.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 114.Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E745–751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- 115.Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, et al. Sleep apnea and daytime sleepiness and fatigue: Relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 116.Bluthe RM, Kelley KW, Dantzer R. Effects of insulin-like growth factor-I on cytokine-induced sickness behavior in mice. Brain Behav Immun. 2006;20:57–63. doi: 10.1016/j.bbi.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, et al. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.08.011. [published online ahead of print October 3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.