Abstract
Exercise promotes longevity and ameliorates type 2 diabetes mellitus and insulin resistance. However, exercise also increases mitochondrial formation of presumably harmful reactive oxygen species (ROS). Antioxidants are widely used as supplements but whether they affect the health-promoting effects of exercise is unknown. We evaluated the effects of a combination of vitamin C (1000 mg/day) and vitamin E (400 IU/day) on insulin sensitivity as measured by glucose infusion rates (GIR) during a hyperinsulinemic, euglycemic clamp in previously untrained (n = 19) and pretrained (n = 20) healthy young men. Before and after a 4 week intervention of physical exercise, GIR was determined, and muscle biopsies for gene expression analyses as well as plasma samples were obtained to compare changes over baseline and potential influences of vitamins on exercise effects. Exercise increased parameters of insulin sensitivity (GIR and plasma adiponectin) only in the absence of antioxidants in both previously untrained (P < 0.001) and pretrained (P < 0.001) individuals. This was paralleled by increased expression of ROS-sensitive transcriptional regulators of insulin sensitivity and ROS defense capacity, peroxisome-proliferator-activated receptor gamma (PPARγ), and PPARγ coactivators PGC1α and PGC1β only in the absence of antioxidants (P < 0.001 for all). Molecular mediators of endogenous ROS defense (superoxide dismutases 1 and 2; glutathione peroxidase) were also induced by exercise, and this effect too was blocked by antioxidant supplementation. Consistent with the concept of mitohormesis, exercise-induced oxidative stress ameliorates insulin resistance and causes an adaptive response promoting endogenous antioxidant defense capacity. Supplementation with antioxidants may preclude these health-promoting effects of exercise in humans.
Keywords: aging, hormesis, insulin resistance, oxidative stress, reactive oxygen species
Type 2 diabetes mellitus is increasing worldwide at epidemic rates and is associated with both microvascular and macrovascular complications (1). Type 2 diabetes mellitus is caused by a combination of insulin resistance involving a number of peripheral tissues, including skeletal muscle (2, 3), and an inadequate β-cell response despite normal or even increased amounts of circulating insulin.
Physical exercise exerts numerous favorable effects on general health (4) and specifically has been shown to improve glucose metabolism in the insulin-resistant state (5). This effect may be independent of exercise-related changes in body mass (6). Moreover, physical exercise has been shown to be effective in preventing type 2 diabetes in high risk individuals (7, 8) and may be even more effective than the most widely used anti-diabetic drug, metformin (9).
These beneficial effects of physical exercise on insulin resistance involve multiple mechanisms, including enhanced expression of glucose transporters and translocation of glucose transporters to the plasma membrane independent of insulin (10). Exercise, as well as weight loss, has been linked to activation of mitochondrial metabolism, and reduced mitochondrial metabolism has been functionally connected with type 2 diabetes (11). Mitochondria, however, are also the main source of reactive oxygen species (ROS), which are inevitable by-products of oxidative glucose metabolism. Muscle is also known to generate free radicals, especially during contraction and physical exercise (12). It has been suggested that ROS may mediate some health-promoting effects, at least in nonprimate model systems (13–17).
We here evaluated the possibility that ROS are required for the insulin-sensitizing capabilities of physical exercise in healthy humans and that commonly used antioxidants, such as vitamin C and vitamin E, may abrogate the health-promoting effects of both physical exercise and oxidative stress in humans.
Results
Baseline Characteristics.
Of the 40 individuals included in the present study, 20 were known to be previously trained, and 20 were previously untrained. Study subject characteristics in the preinterventional state are given in Table 1. No significant differences in age, height, body mass index, fat free mass, or VO2 maximum were observed within the groups (Table 1) and no significant differences in age, height and body mass index were observed between untrained and pretrained groups. Not surprisingly, pretrained individuals had a significantly higher fat free mass (P = 0.03) and VO2 maximum (P < 0.001).
Table 1.
Baseline characteristics of study subjects
| Previously untrained study group |
Pretrained study group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No supplements |
Vitamin C/Vitamin E |
P-Value | No supplements |
Vitamin C/Vitamin E |
P-Value | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Number/sex | 10/male | n.a. | 10/male | n.a. | n.a. | 10/male | n.a. | 10/male | n.a. | n.a. |
| Age, years | 26.70 | 4.34 | 27.44 | 3.02 | 0.69 | 25.40 | 2.15 | 26.00 | 1.95 | 0.54 |
| Height, cm | 179.20 | 4.98 | 182.32 | 8.83 | 0.38 | 182.96 | 3.87 | 177.91 | 7.16 | 0.08 |
| Body Mass Index, kg/m2 | 24.37 | 2.53 | 24.19 | 1.84 | 0.87 | 24.33 | 1.31 | 23.33 | 1.36 | 0.13 |
| Fat free mass, kg | 60.64 | 5.88 | 66.20 | 7.28 | 0.10 | 69.71 | 3.85 | 65.84 | 5.31 | 0.09 |
| VO2 max, ml/min·kg−1 | 45.85 | 5.46 | 45.21 | 7.03 | 0.84 | 54.42 | 4.90 | 54.31 | 5.10 | 0.96 |
n.a. = not applicable.
Half of the previously untrained and previously trained groups were randomly assigned to either antioxidant supplementation as described in Methods or to no supplementation (creating 4 groups of 10 each) (supporting information (SI) Fig. S1). All subjects underwent a 4 week exercise training program irrespective of antioxidant supplementation and previous training status. One untrained individual withdrew during the study for personal reasons unrelated to the experimental protocol.
Induction of Oxidative Stress by Short-Term Exercise.
It is well-established that physical exercise increases ROS formation in skeletal muscle (12); however, it is not known if the health-promoting effects of exercise are partly due to this effect. To replicate the ROS-inducing capacity of exercise in our specific experimental set-up, we subjected previously untrained individuals to 3 days of exercise with muscle biopsy before (Fig. S1, “pre”) and after this short-term intervention (Fig. S1, “early”). We measured concentrations of thiobarbituric acid-reactive substances (TBARS), a well-established marker of overall oxidative stress reflecting oxidized lipids and thus ROS formation in mammals, within skeletal muscle of these previously untrained individuals in the presence or absence of antioxidant treatment. As expected (12), we observed a more than 2-fold increase in oxidative stress, as reflected by TBARS levels, following physical exercise in the absence of antioxidants (Fig. S2, Left pair of bars, P = 0.008). By contrast, those individuals taking antioxidant supplements showed no significant increase in muscle TBARS levels after exercise (Fig. S2, Right pair of bars, P = 0.19) resulting in significantly reduced TBARS formation after 3 days of exercise in comparison to untreated individuals (Fig. S2, shaded bars, P = 0.03). Thus, consistent with previous findings (12), these observations suggest that short-term physical exercise induces skeletal muscle ROS formation and that antioxidant supplements reduce this formation, at least during the first 3 days.
Antioxidants Prevent Increase of Insulin Sensitivity Following Physical Exercise.
Glucose infusion rates.
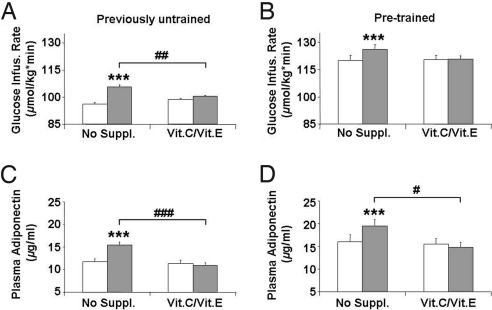
Physical training has been shown to ameliorate insulin resistance and to improve glucose metabolism (5). Thus, both previously untrained and previously trained individuals were subjected to training with twenty 85 min sessions of defined physical exercise on 5 days per week (with or without addition of antioxidant supplementation) with measurement of insulin sensitivity by glucose infusion rates (GIR) during a hyperinsulinemic euglycemic clamp (18). As expected (5), nonsupplemented individuals showed a significant increase in GIR, i.e., increased insulin sensitivity after 4 weeks of exercise irrespective of previous training status (previously untrained: Fig. 1A, Left pair of bars, P < 0.001; and previously trained: Fig. 1B, Left pair of bars, P < 0.001), confirming previous findings that physical exercise induces an increase in insulin sensitivity. By contrast, neither previously untrained individuals and pretrained individuals who received antioxidants exhibited significant changes in GIR following exercise (Fig. 1 A, Right pair of bars, P = 0.07, and B, Right pair of bars, P = 0.89). Thus physical exercise induced an increase in insulin sensitivity only in the absence of antioxidants. The same impaired effect of exercise on GIRs seen in the antioxidant-treated group was also apparent in the previously untrained individuals when the supplement-treated individuals were compared to nonsupplemented individuals (Fig. 1A, shaded bars, P = 0.003). Finally, analysis of all 39 individuals, irrespective of straining status, demonstrates a highly significant (Fig. S3A, P < 0.001 for ANOVA) effect of antioxidant supplementation on the blockage of exercise-induced improvement of GIR. Interestingly, inclusion of the baseline training status in the statistical analyses revealed that the effect of exercise on GIR was independent of the pretraining status of the study participants (P = 0.58 for interaction term of pretraining status by ANOVA), indicating that antioxidants can abolish the insulin sensitizing effects of exercise in both untrained and previously well-trained subjects. We additionally measured serum concentrations of TBARS. When comparing these to GIRs in the postinterventional state in all 39 individuals, we observed a significant correlation with TBARS serum levels (Pearson's r = 0.353, P < 0.05); this suggests that TBARS serum levels, at least to some extent, correlate with insulin sensitivity irrespective of antioxidant supplementation and previous training status, whereas no significant effect of antioxidant supplementation on postinterventional TBARS levels was observed (P = 0.73 for ANOVA, and P = 0.43 for interaction of vitamins by pretraining status by ANOVA).
Fig. 1.
Antioxidants prevent exercise-dependent induction of insulin sensitivity. (A) Glucose infusion rates (GIR) during euglycemic hyperinsulinemic clamps in previously untrained individuals before (white bars) and after (shaded bars) physical exercise over 4 weeks. (Left pair of bars) Individuals not taking any medication or placebo; (Right pair of bars) individuals taking both vitamin C (1000 mg/day) as well as vitamin E (400 IU/day). Bars depict means, error bars show standard error means (applies to all subsequent panels and figures). Significances (applies to all subsequent panels and Fig. 3): * indicates 0.01 < P < 0.05 comparing data before and after 4 weeks of exercise, # indicates 0.01 < P < 0.05 comparing “no suppl.” with “Vit.C/Vit.E” groups after intervention, ** indicates 0.001 ≤ P ≤ 0.01 comparing data before and after 4 weeks of exercise, ## indicates 0.001 ≤ P ≤ 0.01 comparing “no suppl.” with “Vit.C/Vit.E” groups after intervention, *** indicates P < 0.001 comparing data before and after 4 weeks of exercise, ### indicates P < 0.001 comparing “no suppl.” with “Vit.C/Vit.E” groups after intervention. (B) The same set of data derived from a physically pretrained group of individuals. (C) Plasma adiponectin levels in the previously untrained and previously trained (D) state.
Additional plasma markers of insulin sensitivity.
Plasma concentrations of the adipocyte-derived secretory protein adiponectin have been shown to be positively correlated with insulin sensitivity in humans and inversely correlated with type 2 diabetes risk (19). We observe an increase in circulating adiponectin levels following physical exercise in both previously untrained individuals (Fig. 1C, Left pair of bars, P < 0.001) and pretrained individuals (Fig. 1D, Left pair of bars, P < 0.001). In contrast, previously untrained individuals and pretrained individuals who received antioxidants did not exhibit any significant change in adiponectin levels following exercise when compared to the preintervention state (Fig. 1 C, Right pair of bars, P = 0.14, and D, Right pair of bars, P = 0.46), indicating that the exercise-induced increase in adiponectin levels is blocked by antioxidant supplementation. Moreover, comparing postintervention adiponectin levels there was a significantly impaired effect of exercise in the antioxidant-treated group compared to the placebo group (Fig. 1 C and D, shaded bars, P < 0.001 and P = 0.021, respectively). Again, as observed for GIR, there was a strong effect of antioxidant supplementation on postintervention adiponectin levels for the entire sample irrespective of training status (Fig. S3B, n = 39, P < 0.001 for ANOVA). As observed for GIR, additional analyses indicate that previous training status had no impact on the effects of exercise intervention and antioxidant supplementation on serum adiponectin levels (P = 0.94 for interaction of vitamins by pretraining-status by ANOVA).
Lastly, we compared fasting plasma insulin in individuals receiving antioxidants to those receiving no supplementation. We observed a significant decrease in fasting plasma insulin levels following the exercise intervention in previously untrained and pretrained individuals in the absence of antioxidants (P = 0.004 and P = 0.002), consistent with improved insulin sensitivity. Once again, antioxidant supplementation completely abrogated this effect of exercise (P = 0.74 and P = 0.94). As observed for GIR and adiponectin, this effect of antioxidant supplementation on postintervention fasting insulin levels was present for the entire sample (n = 39, P < 0.001 for ANOVA), and previous training status had no impact on the effects of exercise intervention and antioxidant supplementation on fasting insulin levels (P = 0.32 for interaction of vitamins by pretraining-status by ANOVA).
These results indicate that antioxidants severely impair the insulin-sensitizing effects of physical exercise as quantified by several measures, including GIR during hyperinsulinemic euglycemic clamps and plasma adiponectin and fasting plasma insulin concentrations, and that this effect occurs irrespective of previous training status.
Molecular Promotion of Insulin Sensitivity Following Physical Exercise Is Abrogated by Antioxidants.
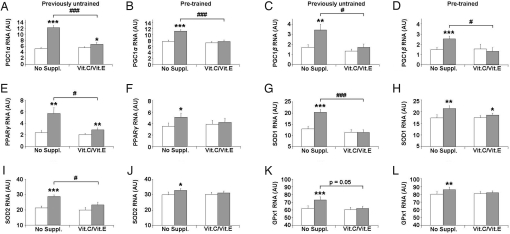
Several molecular regulators of insulin sensitivity have been proposed in the past, including peroxisome-proliferator-activated receptor gamma (PPARγ) and its 2 coactivators, PGC1α and PGC1β, all of which coordinate insulin-sensitizing gene expression within the nucleus of the cell. Using quantitative PCR (qPCR) to compare relative RNA expression levels for these regulators, we observed a strong induction of PGC1α, PGC1β, and PPARγ expression in skeletal muscle following 4 weeks of exercise training in previously untrained, antioxidant naïve individuals (Fig. 2A, C, and E, Left pair of bars, all P < 0.01 to P < 0.001). By contrast, individuals treated with antioxidants showed a markedly reduced exercise-related induction (Fig. 2 A, C, and E, Right pair of bars). Similarly, we observed induction of PGC1α, PGC1β, and PPARγ expression by physical exercise in pretrained individuals in the absence of antioxidants (Fig. 2 B, D, and F, Left pair of bars, P < 0.05 to P < 0.001), and antioxidant treatment prevented this induction (Fig. 2 B, D, and F, Right pair of bars). Likewise, when comparing the relative expression of PGC1α, PGC1β, and PPARγ in the postinterventional state in the presence and absence of antioxidants, exercise increased expression to a much lesser extent in antioxidant-supplemented individuals in all cases (Fig. 2 A–E, shaded bars, P < 0.05 to P < 0.001). As with parameters of insulin sensitivity, this effect of antioxidant supplementation on postintervention gene expression was observed for the entire sample (Figs. S4 A–C, n = 39, P < 0.001 for ANOVA), and previous training status had no impact on the effects of exercise intervention and antioxidant supplementation on expression of these genes (P = 0.21 to 0.89 for interaction of vitamins by pretraining-status by ANOVA).
Fig. 2.
Antioxidants prevent induction of molecular mediators of insulin sensitivity and antioxidant defense in exercised skeletal muscle. (A) depicts expression levels of PGC1α RNA transcripts in skeletal muscle biopsies derived from previously untrained individuals before (white bars) and after (shaded bars) physical exercise over 4 weeks as described in the Methods section. (Left pair of bars) Individuals not taking any medication or placebo; (Right pair of bars) individuals taking both vitamin C (1000 mg/day) as well as vitamin E (400 IU/day). Bars depict means, error bars show standard error means, “AU” abbreviates normalized arbitrary units. (B) depicts expression levels of PGC1α RNA transcripts in skeletal muscle biopsies derived from pretrained individuals before (white bars) and after (shaded bars) physical exercise over 4 weeks. (C and D) expression levels of PGC1β RNA transcripts in a similar fashion; (E and F) expression levels of PPARγ RNA; (G and H) levels of superoxide dismutase 1 (SOD1) RNA expression; (I and J) RNA levels of superoxide dismutase 2 (SOD2); (K and L) glutathione peroxidase 1 (GPx1) RNA expression levels.
Taken together, these findings indicate that physical exercise induces several molecular regulators of insulin sensitivity irrespective of previous training status and that this induction is widely inhibited by antioxidant supplementation.
Molecular Promotion of Muscle Antioxidant Defense Following Physical Exercise Is Abrogated by Antioxidants.
The transcriptional coactivators PGC1α and PGC1β have not only been linked to increased insulin sensitivity but have also been shown to induce expression of several enzymes known to be involved into detoxification of reactive oxygen species (ROS), including superoxide dismutase 2 (SOD2), glutathione peroxidase 1 (GPx1) and possibly other enzymes of similar biochemical function (20). Accordingly, in the present study, physical exercise resulted in a strongly increased expression of SOD1 (Fig. 2 G and H, Left pair of bars, P < 0.05 to P < 0.001), SOD2 (Fig. 2 I and J, Left pair of bars, P < 0.05 to P < 0.001), and GPx1 (Fig. 2 K and L, Left pair of bars, P < 0.001) in previously untrained and previously trained, antioxidant naïve individuals, whereas pretreatment with antioxidants prevented this induction (Fig. 2 G, I, and K, Right pair of bars, P = 0.92, P = 0.06, and P = 0.10, respectively). Similar while less pronounced effects were observed for catalase (CAT) (P = 0.045 and P = 0.13, not depicted). When comparing the relative expression of these enzyme-encoding mRNAs in the postinterventional state in the presence and absence of antioxidants, exercise increased expression to a much lesser, if any, extent in antioxidant-supplemented individuals whether previously untrained or previously trained (Fig. 2 G-L, shaded bars, P < 0.05 to P < 0.001). As observed for PPARγ, PGC1α, and PGC1β, we found a strong effect of antioxidant supplementation to block exercise training-induced expression of antioxidant enzyme mRNAs for the entire sample of previously trained and untrained individuals (n = 39) (Fig. S4 D–F) (SOD1: P < 0.001; SOD2: P = 0.01; GPx1: P < 0.001; and CAT: P = 0.81, all for ANOVA). In some of these cases, previous training status had an impact on the effects of exercise intervention and antioxidant supplementation on 2 of the expression levels (SOD1: P = 0.003; SOD2: P = 0.32; GPx1: P = 0.046; and CAT: P = 0.83, all for interaction of vitamins by pretraining-status by ANOVA). However, this effect was restricted to SOD1 and GPx1, while, notably, the mitochondrially active SOD2 appeared to be unaffected by previous training status.
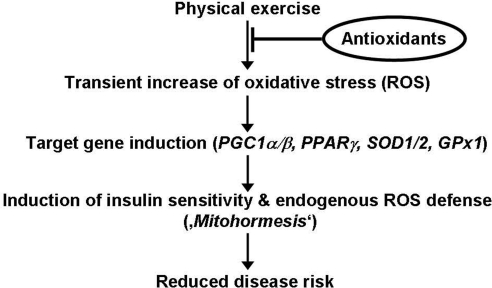
Taken together, physical exercise induces numerous molecular regulators of insulin sensitivity and antioxidant defense, most of which are almost completely inhibited by antioxidant pretreatment in healthy young men (Fig. 3).
Fig. 3.
Mitohormesis links physical exercise and subsequent formation of reactive oxygen species to insulin sensitivity and antioxidant defense. Physical exercise exerts ameliorating effects on insulin resistance by increasing mitochondrial formation of reactive oxygen species in skeletal muscle to induce expression of PGC1α, PGC1β, and PPARγ as inducers of insulin sensitivity, as well as superoxide dismutases 1 and 2 and glutathione peroxidase 1, key enzymes of ROS defense. Notably, by blocking exercise-dependent formation of reactive oxygen species due to ingestion of antioxidant supplements, health promoting effects of physical exercise are abolished, and physical exercise fails to promote insulin sensitivity and antioxidant defense in the presence of vitamin C and vitamin E.
Discussion
Based on the evidence derived from the current study, we here propose an essential role for exercise-induced ROS formation in promoting insulin sensitivity in humans. This induction appears to involve the ROS-dependent transcriptional coactivators PGC1α and PGC1β, and the transcription factor PPARγ and their targets SOD1, SOD2, GPx1, and, to a reduced extent, CAT. Most importantly, these changes in gene expression and the increase in insulin sensitivity following physical exercise are almost completely abrogated by daily ingestion of the commonly used antioxidants vitamin C and vitamin E. Thus, antioxidant supplementation blocks many of the beneficial effects of exercise on metabolism.
This direct molecular link between exercise-dependent formation of ROS, activation of PGC1α, PGC1β and PPARγ on the one hand and increased insulin sensitivity on the other hand, strongly suggest that oxidative stress can be instrumental in preventing type 2-diabetes. The transcriptional coactivator PGC1α has been previously linked to type 2 diabetes in humans (21, 22). This protein has also been shown to be inducible by various oxidative stressors (20), as well as physical exercise in rodents, notably in a vitamin C sensitive manner (17). Given its synergistic potency in coactivating the transcription factor PPARγ to promote insulin sensitivity, the previously established role of PGC1α as a ROS sensor in neurons that in turn induces ROS defense (20), as well as in rodent muscle (17), suggests that activation of PGC1α and possibly PGC1β may be important factors in promoting insulin sensitivity by both exercise and ROS in skeletal muscle. Moreover, in addition to the increase in insulin sensitivity following exercise-induced ROS formation, we also observe an induction of all relevant ROS defense enzyme expression levels, namely SOD1 and SOD2, GPx1, and, to a reduced extent, CAT. Notably, some of these enzymes have been previously linked to transcriptional promotion by PGC1α (20), and, at least for SOD2, exercise has been shown to induce its expression in rodents (23).
Nevertheless, the published evidence is ambiguous with a number of studies suggesting that exposure to ROS may promote insulin resistance (24, 25) whereas others find the opposite (14). In the present study, we find that increased ROS formation efficiently counteracts insulin resistance. Previously published findings in nonprimate models also support this interpretation (13–17). One possible explanation for the apparent conflict between the different studies may be that those studies suggesting an inverse relation between ROS and insulin sensitivity were obtained in models of continuous exposure to increased levels of ROS (24, 25), whereas our current findings and those of other studies (23, 26) may reflect transient increases in ROS during limited periods of physical exercise only.
This notion is further supported by the fact that most negative effects of antioxidant supplements observed in the current study occur irrespective of previous training status. While most effects appear quantitatively more pronounced within the previously untrained study group (i.e., Left arms of Figs. 1, 2, and S1), the data do not support the assumption that antioxidant supplement intake is less detrimental in previously trained subjects. ANOVA, which considered covariables as stated in Methods, indicates that most effects of antioxidants are similar in both pretrained and untrained individuals (Fig. S3 and Fig. S4) and that there was no significant interaction of vitamins by pretraining status with the exception of SOD1 and GPx1 expression, where antioxidants had more pronounced effects in previously untrained subjects than in trained subjected following the 4 week exercise intervention. Hence, the negative effects of antioxidants on exercise training with regard to insulin sensitivity are similar in untrained and pretrained individuals.
If transient increases in oxidative stress are capable of counteracting insulin resistance in humans, it is possible that preventing the formation of ROS by, for example, antioxidants might actually increase, rather than decrease, the risk of type 2 diabetes. While this remains to be determined, one metaanalysis of previously published studies (27) suggests that high dietary intake of fruits and vegetables, a source of antioxidants but also of numerous other bio-active compounds, may actually decrease the risk for type 2 diabetes. Nevertheless, and as stated by Hamer and Chida (27), all larger intervention trials evaluating the diabetes-preventive potential of defined antioxidant supplements have been unable to find any positive effects of supplementation (28–30). Moreover, antioxidant use in type 2 diabetics has been linked to increased prevalence of hypertension (31) and use of antioxidant supplements has recently been proposed to increase overall mortality in the general population (32). Taken together, these previously published findings tentatively suggest that fruits and vegetables may exert health-promoting effects despite their antioxidant content and possibly due to other bio-active compounds. However, it should be noted that the current study applied comparably high doses of oral antioxidants, which have been tested in healthy young men only.
Free radicals causing oxidative stress are an inevitable by-product of mitochondrial metabolism and have been proposed to exert repetitive damage to individual cells of the body promoting increased disease prevalence and aging (33). However, and in specific regard to exercise, antioxidants were incapable of further extending exercise-induced lifespan extension in rats (26). Repeated exposure to sublethal stress has been proposed to cumulate in enhanced stress resistance and ultimately increased survival rates due to a process named hormesis. By analogy, for sublethal ROS-dependent processes emanating from the mitochondria, the term “mitohormesis” was recently proposed on a hypothetical basis (34). Evidence for this novel concept has been provided in model organisms such as nematodes (15) and rats (17), and the current study would extend the concept of mitohormesis to the amelioration of insulin resistance in humans, suggesting that potential harmful ROS may exert health promoting effects via defined molecular intermediates (Fig. 3). In humans, this mitohormetic induction of GIR is paralleled by, and may in part be due to, ROS-related induction of PPARγ, PGC1α, and PGC1β, which then secondarily increase expression of ROS-detoxifying enzymes, including SOD1, SOD2, and GPx1. In this way exercise-induced ROS itself could increase endogenous ROS defense capacity in skeletal muscle, producing a mitohormetic state (Fig. 3) as observed in nematodes (15) and rats (17).
Taken together, we find that antioxidant supplements prevent the induction of molecular regulators of insulin sensitivity and endogenous antioxidant defense by physical exercise. Consistent with the concept of mitohormesis, we propose that transiently increased levels of oxidative stress reflect a potentially health-promoting process at least in regards to prevention of insulin resistance and type 2 diabetes mellitus.
Methods
The present study was approved by the ethics committee of the University of Leipzig, Leipzig, Germany. All study participants gave written informed consent before initiation of the study. The study design was registered at ClinicalTrials.gov registration number NCT00638560.
Forty healthy males participated in a prospective randomized 4 week intensive training intervention study. The study design is depicted in Fig. S1. Subjects were selected from a computer-based volunteer database based on sex, age, body-mass index, physical training status, availability, and additional criteria as listed at the end of this paragraph. Sex was prespecified to be male, age was prespecified to be 25 to 35 years; BMI was prespecified to be below 27 kg/m2, and the training status used is defined below. All subjects included in the study also needed to fulfill the following inclusion criteria: (i) absence of any acute or chronic inflammatory disease, (ii) absence of any metabolic disease including diabetes mellitus of any type, (iii) no medical history of hypertension and systolic blood pressure <140 mmHg and diastolic blood pressure <85 mmHg, (iv) no clinical evidence of cardiovascular or peripheral artery disease, (v) no thyroid dysfunction, (vi) no concomitant medication intake, (vii) no alcohol, nicotine, or drug abuse.
The study consisted of 2 parts (Fig. S1). The first part was performed as an open-label study and included 16 individuals; all of these terminated the study (no withdrawals). Based on the effects observed during this first part, the second part was initiated and subsequently performed as a double blind placebo-controlled study and included 24 individuals. Twenty-three participants completed the second part of study (one subject withdrew after baseline clamp for personal reasons, Fig. S1). None of the subjects participating in study part 2 had participated in study part 1.
Twenty of the 40 subjects enrolled were previously untrained, defined as performing less than 2 hours of exercise (including daily life activities) per week before the study was initiated. The remaining 20 subjects were considered pretrained, i.e., performed more than 6 hours of exercise per week. The different pretraining status was further verified by a significantly higher fitness level (measured as highest oxygen uptake per minute reached during a standardized graded bicycle test) in the pretrained compared to the untrained group.
Subjects of these 2 differentially pretrained groups were assigned by the randomization function of the statistical software package into an antioxidant treatment (n = 10 per subgroup) and a control group (n = 10 per subgroup). Participants in the antioxidant treatment groups (n = 20 each, out of which n = 10 were untrained and n = 10 were pretrained) received 500 mg vitamin C (ascorbic acid, Jenapharm) twice a day and 400 IU vitamin E (RRR-/D-α-tocopherol, Jenapharm) once a day orally. Optically matched placebo pills were provided by the Clinical Pharmacy Department of the University of Leipzig hospital.
All 40 subjects were subjected to supervised physical training, which consisted of training sessions on 5 consecutive days of the week for 4 weeks, i.e., 20 sessions in total. Each session included 20 min of biking or running, 45 min of circuit training, and 20 min periods for warming up and cooling down. All subjects completed a graded bicycle test to volitional exhaustion and had maximal oxygen uptake measured with an automated open circuit gas analysis system at baseline. The highest oxygen uptake per minute reached was defined as the maximal oxygen uptake (VO2 maximum), and subjects subsequently trained at their individual submaximal heart rate using heart rate monitors. Basal percentage fat-free mass was measured by dual x-ray absorptiometry.
At baseline and after 4 weeks of training, but 7 days after the last training session, blood samples were obtained in the fasting state. All baseline and postintervention blood samples and skeletal muscle samples were collected between 8–10 a.m. after an overnight fast. Plasma glucose concentrations were determined by the hexokinase method using an Immulite automated analyzer (Diagnostic Products Corporation). Plasma insulin concentrations were determined by an enzyme immunometric assay on the same instrument. Plasma adiponectin concentrations were determined by RIA (Linco Research) as previously described (35). Serum and skeletal muscle TBARS concentrations were determined fluorometrically according to standard procedures. Hyperinsulinemic euglycemic clamps were performed as previously described (18).
Skeletal muscle biopsies were obtained under local anesthesia from the right vastus lateralis muscle and immediately snap-frozen in liquid nitrogen. Biopsies before (“pre”, Fig. S1) and after intervention (“post”, Fig. S1) were obtained from all 39 study subjects; biopsies for the “early” time-point (Fig. S1) were obtained from 4 placebo-taking and 5 vitamin-treated individuals, while the remaining 3 individuals refused to undergo this additional biopsy. Human PGC-1α, PGC-1β, PPARγ, SOD1, SOD2, GPx1, and CAT gene expression was measured by quantitative real-time (RT)-PCR in a fluorescent temperature cycler using the TaqMan assay, and fluorescence was detected on an ABI PRISM 7000 sequence detector (Applied Biosystems). Total RNA was isolated from skeletal muscle samples using TRIzol (Life Technologies), and 1 μg RNA was reversely transcribed with standard reagents (Life Technologies) employing standard procedures. From each RT-PCR, 2 μl were amplified in a 26 μl PCR using the Brilliant SYBR Green QPCR Core Reagent Kit from Stratagene according to the manufacturer's instructions. Samples were incubated in the ABI PRISM 7000 sequence detector for an initial denaturation at 95 °C for 10 min, followed by 40 PCR cycles, each cycle consisting of 95 °C for 15 s, 60 °C for 1 min, and 72 °C for 1 min. The following primers were used: human PGC-1α: 5′TGCCCTGGATTGTTGACATGA (sense) and 5′TTTGTCAGGCTGGGGGTAGG (antisense); human PGC 1β: 5′TTGAGGAGTGCGAGGTGCTG (sense) and 5′ATCTGGGCCAGCAGAAGTGC (antisense), human PPARγ: 5′AGGCGAGGGCGATCTTGACAG (sense) and 5′GATGCGGATGGCCACCTCTTT (antisense); human superoxide dismutase 1 (SOD1): 5′GGTGTGGCCGATGTGTCTATT (sense) and 5′CTGCTTTTTCATCGACCACCA (antisense); human SOD2: 5′TGCTGCTTGTCCAAATCAGG (sense) and 5′CACACATCAATCCCCAGCAGT (antisense); human GPx1: 5′GCGGCGGCCCAGTCGGTGTA (sense) and 5′GAGCTTGGGGTCGGTCATAA (antisense); human catalase: 5′TCCGGGATCTTTTTAACGCCATTG (sense) and 5′TCGAGCACGGTAGGGACAGTTCAC (antisense); human 18S rRNA: 5′TGCCATGTCTAAGTACGCACG (sense); 5′TTGATAGGGCAGACGTTCGA (antisense). SYBR Green I fluorescence emissions were monitored after each cycle. Expression of PGC-1α, PGC-1β, PPARγ, SOD1, SOD2, GPx1, CAT, and 18S rRNA were quantified by using the second derivative maximum method of the TaqMan Software (Applied Biosystems) determining the crossing points of individual samples by an algorithm which identifies the first turning point of the fluorescence curve. Amplification of specific transcripts was confirmed by melting curve profiles (cooling the sample to 68 °C and reheating slowly up to 95 °C with parallel measurements of fluorescence) at the end of each PCR. The specificity of the PCR was further verified by subjecting the amplification products to agarose gel electrophoresis.
Statistical Analysis.
All data collection processes and subsequent statistical analyses were performed with SPSS, Version 15.0. Variables were tested for normal distribution using the Kolmogorov-Smirnov test. Nonnormally distributed variables were log transformed to approximate a normal distribution before applying t test or general linear modeling statistics. P-values of less than 0.05 were considered significantly different.
Group comparisons (Table 1) were made using a 2-sided unpaired Student's t tests. Within previously untrained (n = 20) or pretrained (n = 20) subgroups, interindividual effects of antioxidant treatment between treatment groups at baseline, as well as after intervention, were compared with two-sided unpaired Student's t tests. For comparing intra-individual effects of exercise within treatment groups (pre- vs. postexercise), 2-sided paired Student's t tests were used (Table 1 and Figs. 1 and 2).
To determine putatively differential effects of initial training status (previously untrained vs. pretrained), multivariate analyses were performed using a general linear model approach on the delta values (postvalues minus prevalues) of all 39 individuals. The ANOVA statistics for effects of vitamin supplements as dependent variable are given after adjustment for the covariates “open” versus “blinded”, and “initial training status” (previously untrained vs. pretrained). A significant P value would indicate that vitamin supplementation has an effect on outcome measurements irrespective of initial training status. This specific test has been denominated “ANOVA” throughout the Results and Discussion sections.
We also performed an additional ANOVA including an interaction term (training status by supplement) again using delta values (postvalues minus prevalues) and adjusting for the above covariates. A significant P value would indicate that the effect of vitamin supplementation is dependent on initial training status. This test has been denominated “interaction of vitamins by pretraining-status by ANOVA” throughout the Results and Discussion sections.
Supplementary Material
Acknowledgments.
The authors thank all 40 study subjects for their voluntary participation. This work was supported by Deutsche Forschungsgemeinschaft (German Research Association) Grant RI 1076/1–3 to M.R. and Grant KFO 152 “Atherobesity” (and specifically BL 833/1–1) to M. Blüher and M.S.
Footnotes
The authors declare no conflict of interest.
Data deposition: The study design described in this paper has been deposited at ClinicalTrials.gov (registration no. NCT00638560).
This article contains supporting information online at www.pnas.org/cgi/content/full/0903485106/DCSupplemental.
References
- 1.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 2.Kahn CR. Banting Lecture: Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43:1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- 3.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 4.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: The evidence. Can Med Ass J. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James DE, Kraegen EW, Chisholm DJ. Effect of exercise training on whole-body insulin sensitivity and responsiveness. J Appl Physiol. 1984;56:1217–1222. doi: 10.1152/jappl.1984.56.5.1217. [DOI] [PubMed] [Google Scholar]
- 6.Duncan GE, et al. Exercise training, without weight loss, increases insulin sensitivity and postheparin plasma lipase activity in previously sedentary adults. Diabetes Care. 2003;26:557–562. doi: 10.2337/diacare.26.3.557. [DOI] [PubMed] [Google Scholar]
- 7.Pan XR, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 8.Kelley DE, Goodpaster BH. Effects of physical activity on insulin action and glucose tolerance in obesity. Med Sci Sports Exerc. 1999;31:S619–S623. doi: 10.1097/00005768-199911001-00021. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zierath JR. Exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol. 2002;93:773–781. doi: 10.1152/japplphysiol.00126.2002. [DOI] [PubMed] [Google Scholar]
- 11.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–171. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- 12.Powers SK, Jackson MJ. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClung JP, et al. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci USA. 2004;101:8852–8857. doi: 10.1073/pnas.0308096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein BJ, Kalyankar M, Wu X. Redox paradox: Insulin action is facilitated by insulin-stimulated reactive oxygen species with multiple potential signaling targets. Diabetes. 2005;54:311–321. doi: 10.2337/diabetes.54.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulz TJ, et al. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Birringer M, et al. Improved glucose metabolism in mice lacking alpha-tocopherol transfer protein. Eur J Nutr. 2007;46:397–405. doi: 10.1007/s00394-007-0679-2. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Cabrera MC, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 18.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 19.Spranger J, et al. Adiponectin and protection against type 2 diabetes mellitus. Lancet. 2003;361:226–228. doi: 10.1016/S0140-6736(03)12255-6. [DOI] [PubMed] [Google Scholar]
- 20.St. Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Patti ME, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mootha VK, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 23.Higuchi M, Cartier LJ, Chen M, Holloszy JO. Superoxide dismutase and catalase in skeletal muscle: adaptive response to exercise. J Gerontol. 1985;40:281–286. doi: 10.1093/geronj/40.3.281. [DOI] [PubMed] [Google Scholar]
- 24.Rudich A, et al. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3–L1 adipocytes. Diabetes. 1998;47:1562–1569. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 25.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 26.Holloszy JO. Longevity of exercising male rats: Effect of an antioxidant supplemented diet. Mech Ageing Dev. 1998;100:211–219. doi: 10.1016/s0047-6374(97)00140-1. [DOI] [PubMed] [Google Scholar]
- 27.Hamer M, Chida Y. Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: Systematic review and meta-analysis. J Hypertens. 2007;25:2361–2369. doi: 10.1097/HJH.0b013e3282efc214. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, et al. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: A randomized controlled trial. J Am Med Assoc. 1999;282:1073–1075. doi: 10.1001/jama.282.11.1073. [DOI] [PubMed] [Google Scholar]
- 29.Czernichow S, et al. Antioxidant supplementation does not affect fasting plasma glucose in the Supplementation with Antioxidant Vitamins and Minerals (SU.VI.MAX) study in France: Association with dietary intake and plasma concentrations. Am J Clin Nutr. 2006;84:395–399. doi: 10.1093/ajcn/84.1.394. [DOI] [PubMed] [Google Scholar]
- 30.Liu S, et al. Vitamin E and risk of type 2 diabetes in the women's health study randomized controlled trial. Diabetes. 2006;55:2856–2862. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
- 31.Ward NC, et al. The effect of vitamin E on blood pressure in individuals with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. J Hypertens. 2007;25:227–234. doi: 10.1097/01.hjh.0000254373.96111.43. [DOI] [PubMed] [Google Scholar]
- 32.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: Systematic review and meta-analysis. J Am Med Assoc. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 33.Harman D. Aging: A theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 34.Tapia PC. Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: “Mitohormesis” for health and vitality. Med Hypotheses. 2006;66:832–843. doi: 10.1016/j.mehy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Oberbach A, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.