Abstract
Hunter syndrome (mucopolysaccharidosis-II) is caused by deficiency of the lysosomal enzyme iduronate-2-sulfatase. The assay of this sulfatase requires the use of α-L-iduronate glycosides containing a sulfate at the 2-position. We report a simple, three-step procedure for introduction of sulfate at the 2-position starting with the methyl ester of α-L-iduronate glycosides. The procedure involves protection of the 2- and 4-hydroxyl groups of the iduronate moiety as the dibutyl stannylene acetal, selective sulfation with sulfur trioxide–trimethylamine, and deprotection of the methyl ester to afford the desired 2-sulfate in 61% overall yield.
Keywords: sulfation, iduronic acid, mucopolysaccharidosis-II, Hunter syndrome
The development of new technology for the newborn screening for Hunter syndrome (mucopolysaccharidosis-II) is warranted because of the development of treatments that are most effective when started early in life.1 This lysosomal storage disease is caused by deficiency of the enzyme iduronate-2-sulfatase, which is needed for the degradation of dermatan sulfate and heparan sulfate, two components of cellular glycosaminoglycans. Synthetic substrates used to assay iduronate-2-sulfatase in vitro are usually disulfated disaccharides derived from the nitrous acid degradation of heparin.2,3 Such substrates have been useful for the development of a tandem mass spectrometry assay for the newborn screening for Hunter syndrome.3 However, more recently it has become apparent that the scale-up synthesis using nitrous acid degradation of heparin is impractical to obtain the amount of material needed to support worldwide newborn screening for Hunter syndrome. Thus, we became interested in developing a new method for the total synthesis of appropriate substrates that can be used at a scale of tens of grams per year.
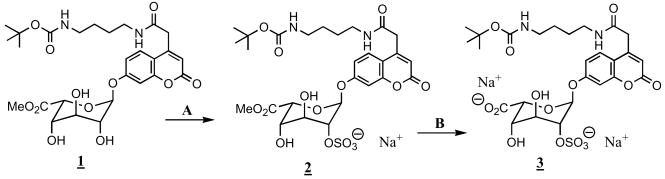
Our target molecule 3 is shown in Scheme 1. This molecule can be used to assay iduronate-2-sulfatase using either a flurometric assay or via tandem mass spectrometry with electrospray ionization. The former is made possible by the presence of the umbelliferryl moiety. In this case the assay mixture is supplemented with the enzyme α-L-iduronidase, which cleaves the glycosidic linkage to release the fluorescent coumarin only after the iduronate-2-sulfatase removes the 2-sulfate.4 For the tandem mass spectrometric assay, the α-L-iduronidase coupling enzyme is not needed. In this case, the desulfated α-L-iduronate glycoside is detected directly by tandem mass spectrometry. The presence of the BOC group directs the stability of the parent ion so that the fragmentation proceeds exclusively by cleavage of the carbamate (loss of 100 Da).5
Scheme I.
Reagents and conditions: A. SO3 NMe3–DMF. B. NaOH–H2O
There are a number of reports of the synthesis of sulfated saccharide building blocks that have been used to prepare heparin and heparan sulfate fragments, but to the best of our knowledge there are no reports on the facile incorporation of sulfate at the 2-position of α-L-iduronate glycosides. Our sulfation method is shown in Scheme 1. The route starts with the α-L-iduronate glycoside methyl ester 1, which we have prepared previously by total synthesis.5 Treatment of 1 with 1.5 equivalents of dibutyltin oxide in anhydrous methanol under reflux protects the 2- and 4-hydroxyl groups as the 2,4-stannylene acetal. The latter was used without further purification. It was dissolved in anhydrous N,N-dimethylformamide and treated with 1.5 equivalents of sulfur trioxide–trimethylamine complex for 24 h at 55 °C. The crude product was submitted to cation-exchange chromatography to convert the trimethylammonium salt of the sulfate to the sodium salt. The latter was purified by flash chromatography over silica gel to give compound 2.
Compound 2 was solubilized in methanol–water and treated with incremental amounts of aqueous sodium hydroxide to saponify the methyl ester. The crude product was purified by flash chromatography over silica gel to give the desired 3 in 96% purity (61% overall yield from 1). The structure was confirmed by 1H NMR spectroscopy and electrospray-ionization mass spectrometry. The former analysis shows that 96% of the product is sulfated at the 2-position and 4% at the 4-position. Since there is no enzyme known to be able to hydrolyze the sulfate at the 4-position, the removal of the trace amount of 4-sulfate is not necessary prior to enzyme assay for Hunter syndrome. The development of a newborn screening assay for Hunter syndrome based on iduronate-2-sulfatase substrate 3 will be reported elsewhere.
It is not clear why sulfation of the dibutyl stannylene acetal proceeds selectively at the 2-positon versus the 4-position. Earlier studies have shown that analogus dibutyl stannylene acetals can be acetylated or benzoylated selectively at the 2-positon.6,7
1. Experimental
1.1 General methods
Reactions were carried out in dry solvents in oven-dried glassware under an N2 atmosphere. Thin-layer chromatography (TLC) was carried out on silica plates (Silica Gel 60, F-254 (0.25 mm)). 1H NMR chemical shifts are reported in parts per million (δ) using the methanol peak as the internal standard (3.31 ppm). Electrospray-ionization mass spectra were acquired on a Bruker Esquire LC00066 ion-trap spectrometer. Flash chromatography was carried out with silica gel (40–63 μm).
1.2. Synthesis of compound 2
Starting material 1 (164.5 mg, 0.28 mmol, 1 equiv), prepared as described previously5, was solubilized in anhyd MeOH (16 mL), and dibutyltin(IV) oxide (106 mg, 0.42 mmol, 1.5 equiv, Aldrich) was added. The reaction mixture was heated under reflux for 40 min, after which time the dibutyltin oxide was completely dissolved. The reaction mixture was allowed to cool and was concentrated under vacuum. The residue was co-evaporated once with toluene to remove traces of water.
The residue was solubilized in anhyd N,N-dimethylformamide (16 mL). Sulfur trioxide–trimethylamine complex (59.1 mg, 0.42 mmol, 1.5 equiv, Aldrich) was added, and the reaction mixture was heated at 55 °C for 24 h. The reaction mixture was allowed to cool, and the reaction was then quenched with MeOH. The mixture was then concentrated under vacuum. To convert the product from the trimethylammonium salt to the sodium salt, the residue was submitted to cation-exchange chromatography [Dowex 50WX8-400 (Na+), 1 × 4 cm] using MeOH as the eluent. The sodium salt was purified by column chromatography on silica using 5:8:1 MeOH–CHCl3–H2O to give compound 2. TLC (silica, 5:8:1 MeOH–CHCl3–H2O): Rf 0.6. 1H NMR (300 MHz, CD3OD): 1.43 (s, 9H, t-butyl); 1.50 (m, 4H, CH2CH2); 3.04 (m, 2H, CH2N); 3.21 (t, 2H, CH2N); 3.74 (brs, 2H, CH2CO); 3.76 (s, 3H, CO2Me); 3.99 (brt, 1H, H-4); 4.19 (brt, 1H, H-3); 4.50 (m, 1H, H-2); 4.81 (d, 1H, H-5); 6.00 (brs, 1H, H-1); 6.28 (s, 1H, coumarin vinyl CH); 7.16–7.19 (m, 2H, coumarin CH); 7.70 (d, 1H, coumarin CH). The 1H NMR spectrum is provided as Supplementary data (Figs. S1 and S2).
1.3. Synthesis of compound 3
Compound 2 was solubilized in 1:1 methanol–water (15.4 mL) at room temperature. Aq 0.1 M NaOH was added in increments of 0.1 equiv of NaOH (283 μL, 0.03 mmol) until the pH of the solution reached approximately 8 (pH paper). The pH was maintained by incremental additions of the 0.1 M NaOH solution as the reaction proceeded (every 15–30 min). The reaction mixture was stirred for 5.5 h (1.3 equiv NaOH added), after which it was concentrated under vacuum to remove MeOH and finally lyophilized overnight. The residue was purified by column chromatography on silica using 5:8:1 MeOH–CHCl3–H2O to give compound 3 (96% 2-sulfated, 4% 4-sulfated by 1H NMR spectroscopy) with 61% overall yield from compound 1. TLC (silica, 5:8:1 MeOH–CHCl3–H2O): Rf 0.2. TLC analysis indicated a single spot. 1H NMR (300 MHz, CD3OD): 1.43 (s, 9H, t-butyl); 1.50 (m, 4H, CH2CH2); 3.04 (t, 2H, CH2N); 3.21 (t, 2H, CH2N); 4.07 (brs, 1H, H-4); 4.17 (brs, 1H, H-3); 4.48 (brs, 1H, H-2); H-5 under water peak; 6.14 (brs, 01H, H-1); 6.17 (s, 1H, coumarin vinyl CH); 7.07–7.12 (m, 2H, coumarin CH); 7.53 (d, 1H, coumarin CH). ESIMS: (negative-ion mode) (M-H)−1, Calcd 645.2, found 645.3. The 1H NMR spectrum is provided as Supplementary data (Figs. S1 and S2). The COSY 1H NMR spectrum confirmed that the sulfate is at the 2-position (not shown).
Supplementary Material
Acknowledgments
This work was support by grants from the National Institutes of Health (DK67869) and from Genzyme, Corp.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi: xxxxxxxx
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Muenzer J, Lamsa JC, Garcia A, Dacosta J, Garcia J, Treco DA. Acta Paediatr. 2002;(Suppl 91):98–99. doi: 10.1111/j.1651-2227.2002.tb03115.x. [DOI] [PubMed] [Google Scholar]
- 2.Hopwood JJ. Carbohydr Res. 1979;69:203–216. doi: 10.1016/s0008-6215(00)85765-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Wood T, Sadilek M, Scott CR, Turecek F, Gelb MH. Clin Chem. 2007;53:137–140. doi: 10.1373/clinchem.2006.077263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keulemans JLM, Sinigerska I, Garritsen VH, Huijmans JGM, Voznyi YV, van Diggelen OP, Kleijer W. J Prenat Diagn. 2002;22:1016–1022. doi: 10.1002/pd.457. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Clin Chem. 2008;54:2067–2070. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gavard O, Hersant Y, Alais J, Duverger V, Dilhas A, Bascou A, Bonnaffe D. Eur J Org Chem. 2003:3603–3620. [Google Scholar]
- 7.de Paz JL, Ojeda R, Reichardt N, Lomas-Martin M. Eur J Org Chem. 2003:3308–3324. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.