Abstract
Chemical-induced seizures up-regulated brain-derived neurotrophic factor (BDNF) mRNA expression. Intracerebroventricular (i.c.v.) administration of endogenous opioids preferentially activating μ opioid receptor (MOR) could also increase BDNF mRNA expression. The aim of this study was to determine to what extent i.c.v. administration of synthetic MOR-selective agonists in rats can modulate both seizure activity and up-regulation of BDNF mRNA expression. Effects and potencies of i.c.v. administration of morphine and [D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), were directly investigated by scoring behavioral seizures and measuring BDNF mRNA expression. In addition, effects of the opioid receptor antagonist naloxone and antiepileptic drugs, diazepam, phenobarbital, and valproate, on i.c.v. MOR agonist-induced behavioral seizures and up-regulation of BDNF mRNA expression were determined. A single i.c.v. administration of morphine (10–100 μg) or DAMGO (0.15–1.5 μg) dose-dependently elicited behavioral seizures and increased BDNF mRNA expression in the widespread brain regions. However, subcutaneous administration of MOR agonists neither produced behavioral seizures nor increased BDNF mRNA expression. Pretreatment with naloxone 1 mg/kg significantly reduced behavioral seizure scores and the up-regulation of BDNF mRNA expression elicited by i.c.v. morphine or DAMGO. Similarly, diazepam 10 mg/kg and phenobarbital 40 mg/kg significantly blocked i.c.v. MOR agonist-induced actions. Pretreatment with valproate 300 mg/kg only attenuated behavioral seizures, but it did not affect morphine-induced increase of BDNF mRNA expression. This study provides supporting evidence that seizure activity plays an important role in the up-regulation of BDNF mRNA expression elicited by central MOR activation and that decreased inhibitory action of GABAergic system through the modulation on GABA receptor synaptic function by central MOR activation is involved in its regulation of BDNF mRNA expression.
Keywords: neurotrophins, opioids, behavioral seizures, in situ hybridization, hippocampus, frontal cortex
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, is widely expressed in the adult mammalian brain regions, especially in the cerebral cortex, hippocampus, and amygdala complex (Pezet and Malcangio, 2004). After binding to the tropomyocin receptor kinase B on the cell surface of neurons, BDNF regulates neuronal survival, promotes neurite outgrowth, and maintains synaptic connectivity in the adult nervous system. Increasing bodies of evidence indicated that BDNF is involved in the pathophysiology of various neurodegenerative and neuropsychiatric disorders (Duman and Monteggia, 2006; Martinowich et al., 2007; Zuccato and Cattaneo, 2007).
BDNF gene expression can be modulated by a variety of physiological signals and insults in the central nervous system. It has been demonstrated that the up-regulation of BDNF mRNA expression occurs following the induction of neuronal activity such as seizures. Numerous studies have shown that BDNF mRNA levels were significantly increased by seizure activities elicited by systemic administration of kainic acid (Dugich-djordjevic et al., 1992), pentylenetetrazol (Humpel et al., 1993), pilocarpine (Poulsen et al., 2004), electrical kindling (Ernfors et al., 1991) or hilus lesion (Rocamora et al., 1992). The elevated BDNF mRNA expression was found not only after long-lasting or recurrent seizures, but also after brief episodes of hippocampal epileptiform activity (Ernfors et al., 1991). In addition, the seizure-induced BDNF gene up-regulation occurred in the widespread regions of the brain, especially in the hippocampus, cerebral cortex, amygdaloid complex, and piriform cortex (Ernfors et al., 1991; Humpel et al., 1993; Dias et al., 2003).
Early studies have shown that central administration of opioid receptor agonists participates in regulation of the seizure activity. For example, intracerebroventricular (i.c.v.) administration of morphine, met- and leu-enkephalin or β-endorphin produced epileptiform discharges (Urca et al., 1977; Urca and Frenk, 1982; Snead and Bearden, 1982). However, previous observations demonstrated that δ opioid receptor agonist-induced BDNF mRNA expression in some brain regions was independent of seizure activity (Torregrossa et al., 2004; Zhang et al., 2006). Given that i.c.v. administration of peptidic μ opioid receptor (MOR) agonists could up-regulate BDNF mRNA expression (Zhang et al., 2006), it is important to know whether and to what extent central administration of MOR agonists can modulate both the seizure activity and the up-regulation of BDNF mRNA expression.
Therefore, this study directly compared the effect and potency of i.c.v. administration of two MOR agonists, morphine and DAMGO, on the seizure activities and BDNF mRNA expression. Drug-induced seizures were quantified by the behavioral seizure scale and BDNF mRNA expression was determined by in situ hybridization. In addition, the present study further compared the effects of pretreatment with the MOR antagonist naloxone and various anticonvulsants, including diazepam, phenobarbital, and valproate, on the actions elicited by i.c.v. MOR agonists. By measuring both endpoints (i.e., behavioral seizures and up-regulation of BDNF mRNA expression), this study was conducted to determine if up-regulation of BDNF mRNA expression induced by centrally administered MOR agonists results from seizure activity.
EXPERIMENTAL PROCEDURES
Animals
Male Sprague–Dawley rats (250–275 g) were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA) and were housed in groups of three rats per cage. All animals were allowed ad libitum access to food and water, and were maintained on a 12 h light/dark cycle with lights on at 06:30 h in a room kept at a temperature of 21–22 °C. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. The experimental protocols were approved by the University Committee on the Use and Care of Animals at the University of Michigan.
Intracerebroventricular (i.c.v.) surgery
Rats were anesthetized by intramuscular injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed in a stereotaxic device. Each rat was prepared with a 23-gage stainless steel cannula (Small Parts, Miami Lakes, FL, USA) extending into the right lateral cerebral ventricle (coordinated from bregma, AP: 0.8 mm, ML: 1.5 mm, DV: 4.2 mm; Paxinos & Watson, 1986). The guide cannula was fixed in place with dental cement applied to the surface of the skull. Animals were allowed 6 or 7 days to recover from surgery. Each animal’s i.c.v. cannula placement was verified after experiment by injecting methylene blue and checking for distribution. Only data obtained from animals with good i.c.v. cannula placement were used for data analysis.
Drug administration
Morphine sulfate (Mallinckrodt, St. Louis, MO, USA), naloxone, fentanyl, etorphine (National Institute on Drug Abuse, Bethesda, MD, USA), DAMGO, diazepam, valproate, and phenobarbital (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile water. Morphine (10, 30, and 100 μg) or DAMGO (0.15, 0.5, and 1.5 μg) was administered i.c.v. via the guide cannula in volumes of 10 μL using a 25-μL Hamilton syringe attached via a polyethylene PE20 tube (Plastics One, Roanoke, VA, USA) to a 30-gage needle (Becton Dickinson, Franklin Lakes, NJ, USA). Solution was injected over a period of 60 sec and the needle was left within the guide cannula for an additional 30 sec to prevent reflux. Sterile water was administered as a vehicle for the control injection. The doses of i.c.v. MOR agonists were chosen based on previous studies showing that they were active in variety of behavioral assays, especially in electroencephalographic seizures and antinociceptive actions (Snead and Bearden, 1982; Tortella et al., 1987; Gogas et al., 1996; Fraser et al., 2000). Morphine (10 mg/kg), fentanyl (0.1 mg/kg) or etorphine (0.003 mg/kg) was administrated subcutaneously. The doses of s.c. MOR agonists were selected based on previous studies showing that they produced maximal antinociceptive effects (Walker et al., 1994; Vissers et al., 2006). For the antagonist study, animals were chosen randomly from the same batch and were injected with sterile water. Naloxone (1 mg/kg) was given subcutaneously 15 min prior to an i.c.v. administration of vehicle or MOR agonist. Diazepam (10 mg/kg, i.m.) was administered 15 min before i.c.v. MOR agonist. Valproate sodium (300 mg/kg, i.p.) and phenobarbital (40 mg/kg, i.p.) was administered 1 h before i.c.v. MOR agonist. The dose and pretreatment time for naloxone and antiepileptic drugs were chosen based on previous studies (Snead and Bearden, 1980; Berman and Adler, 1984; Tortella et al., 1987).
Characterization of behavioral seizures
During the observation period, behavioral seizures were scored according to a modified scale (Golarai et al., 1992; French et al., 1999). Score 0: No detectable motor manifestation; score 1: arrest of motion; score 2: monoclonic jerks of the head and neck; score 3: whole body bilateral activity (wet-dog shakes); score 4: forelimb clonus and rearing; score 5: generalized clonic tonic activity and loss of postural tone. Each animal was scored according to the highest class of behavioral manifestation observed during a 1 h observation period.
In situ hybridization histochemistry
At each time point, experimentally naïve animals were killed by decapitation, and their brains were rapidly removed, frozen in isopentane at −40 °C, and stored at −80 °C. Brains were sectioned at 20 μm on a cryostat and were thaw mounted onto poly-L-lysine-subbed slides. Slides were stored at −80 °C until they were processed for in situ hybridization, which was performed as described previously (Zhang et al., 2006). BDNF mRNA expression levels were determined by a double label in situ hybridization with a [35S]-labeled BDNF cRNA probe as described previously (Zhang et al., 2006). The rat BDNF cDNA (Isackson et al., 1991) was donated by Drs. Gall and Lauterborn (University of California, Irvine, CA, USA). The probe was radioactively labeled in a reaction containing 1 μg BDNF antisense linearized plasmid DNA, 5 × transcription buffer, 125 μCi each of [35S]UTP and [35S]CTP, 150 μM each of ATP and GTP, 12.5 mM dithiothreitol, 20 U RNase inhibitor and 6 U of T3 polymerase. The [35S]-labeled BDNF probe was hybridized to brain sections in hybridization buffer containing 1.5 million cpms radiolabeled probe per 80 μL. The slides were exposed on Kodak XAR film (Eastman Kodak, Rochester, NY) for 14 days.
Quantification of radioactive signal
BDNF mRNA levels were quantified using NIH Image software (Scion Image, Frederick, MD, USA). BDNF mRNA expression was examined in the frontal cortex, CA1, CA3 and dentate gyrus (DG) regions of hippocampus, the basolateral amygdaloid complex; the endopiriform nucleus and primary olfactory cortex. Each brain region was analyzed by creating an outline around the region and measuring both the left and right sides of the brain, and from rostral–caudal sections 100–200 μm apart. At least six sections per region per rat were quantified. The signal measurements were corrected for background and were determined as the mean radioactive intensity per pixel for that region. These signal values for each section were then averaged to obtain the mean signal for each region in each rat. These data points were then averaged per group and compared statistically.
Statistical analysis
Behavioral data were expressed as mean ± S.E.M. and were analyzed by using a Kruskal-Wallis ANOVA on ranks test. BDNF signals from in situ hybridization were expressed as mean percent of vehicle ± S.E.M. Statistical analysis was performed using one-way ANOVA with Dunnett’s post hoc test to compare differences between groups where P < 0.05 was considered significant.
RESULTS
Effects of i.c.v. administration of morphine and DAMGO on behavioral seizures and BDNF mRNA expression
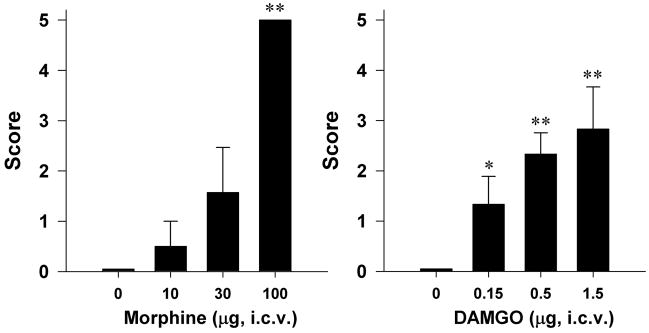
Both morphine and DAMGO dose-dependently increased behavioral seizure scores following i.c.v. administration (Figure 1). The dose of i.c.v. morphine 30 μg produced detectable seizure behaviors in 2 rats with score of 5. Following i.c.v. administration of morphine 100 μg, all rats displayed the generalized clonic tonic activity and loss of postural tone which was scored as 5. Most of the convulsions occurred 5–30 min after administration and was short-lasting (i.e., several sec to 1 min). The convulsions produced by i.c.v. morphine were recurrent in some rats. After i.c.v. administration of DAMGO, the average behavioral seizure scores in rats were 1.3±0.6, 2.3±0.4, and 2.8±0.8 at the dose of 0.15, 0.5, and 1.5 μg, respectively. The i.c.v. DAMGO-induced seizures occurred quickly and last shortly. Apparent seizure-like behaviors induced by i.c.v. DAMGO were wet-dog shakes. They happened within the first 15 min after i.c.v. administration of DAMGO and there was no generalized clonic tonic activity following i.c.v. administration of DAMGO.
Figure 1.
Effects of i.c.v. administration of morphine and DAMGO on the behavioral seizure score in rats. Each animal was scored according to the highest class of behavioral manifestation observed during the 1 h observation period. Each value represents mean ± S.E.M. (n=6). *, P<0.05 and **, P<0.01 compared with the vehicle condition.
Figure 2 illustrates that both i.c.v. morphine and DAMGO dose-dependently increased BDNF mRNA expression in several brain regions when compared with the vehicle condition. Post hoc comparisons indicate that i.c.v. morphine 100 μg significantly increased BDNF mRNA in the frontal cortex, CA1, CA3 and dentate gyrus regions of hippocampus, the basolateral amygdaloid complex, the endopiriform nucleus and primary olfactory cortex. Similarly, i.c.v. DAMGO 1.5 μg significantly increased BDNF mRNA expression in the subregions of hippocampus and amygdala, but the magnitude of up-regulation of BDNF mRNA expression by DAMGO is less than that of morphine (Figure 2, bottom panels).
Figure 2.
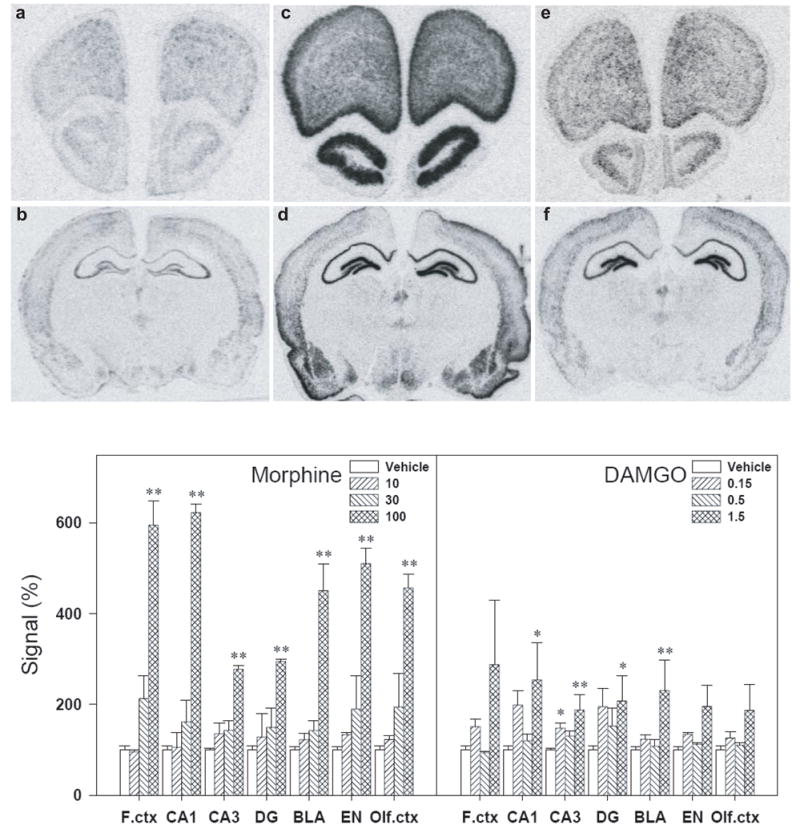
Representative autoradiograms of BDNF mRNA expression in the rat brain regions (a–f, top panels). BDNF mRNA expression was determined 3 h after i.c.v. administration of drugs. (a&b) Sections taken from a vehicle-treated animal. (c&d) Sections taken from an animal treated with morphine (100 μg, i.c.v.). (e&f) Sections taken from an animal treated with DAMGO (1.5 μg, i.c.v.). Quantification of BDNF mRNA signals in different brain regions are represented as mean percent of vehicle ± S.E.M. (bottom panels). *, P<0.05 and **, P<0.01 compared with vehicle. See Materials and Methods for other details.
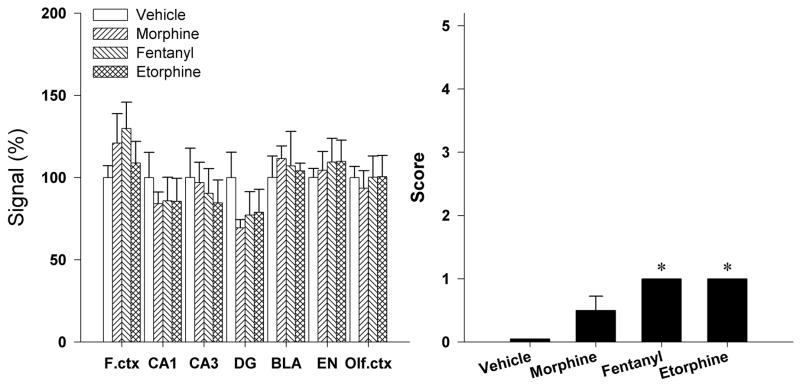
Effects of s.c. administration of morphine, fentanyl, and etorphine on behavioral seizures and BDNF mRNA expression
Figure 3 shows the effect of s.c. administration of non-peptidic MOR agonists on behavioral seizures and BDNF mRNA expression. None of the MOR agonists, morphine (10 mg/kg), fentanyl (0.1 mg/kg), and etorphine (0.003 mg/kg) produced obvious seizure-like behaviors. These MOR agonists only produced immobility which was scored as 1. In situ hybridization results showed that there was no significant difference on BDNF mRNA expression between MOR agonist-treated groups and the vehicle-treated group in all brain regions tested.
Figure 3.
Effects of s.c. administration of morphine (10 mg/kg), fentanyl (0.1 mg/kg), and etorphine (0.003 mg/kg) on the behavioral seizure score and the brain BDNF mRNA expression. Each animal was scored according to the highest class of behavioral manifestation observed during the 1 h observation period. BDNF mRNA expression was determined 3 h after drug administration. Quantification of BDNF mRNA signals in different brain regions are represented as mean percent of vehicle. Each value represents mean ± S.E.M. (n=4–6). *, P<0.05 compared with vehicle.
Effects of naloxone on behavioral seizures and BDNF mRNA expression increased by i.c.v. MOR agonists
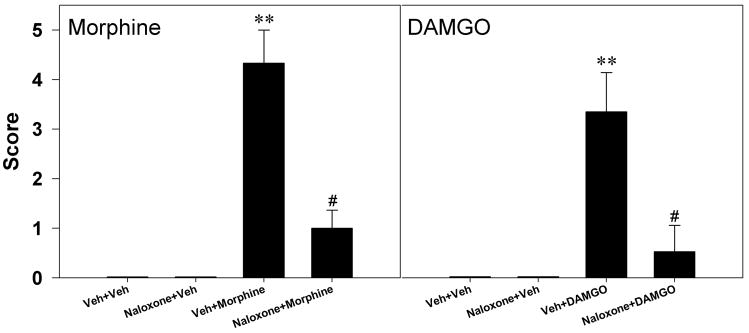
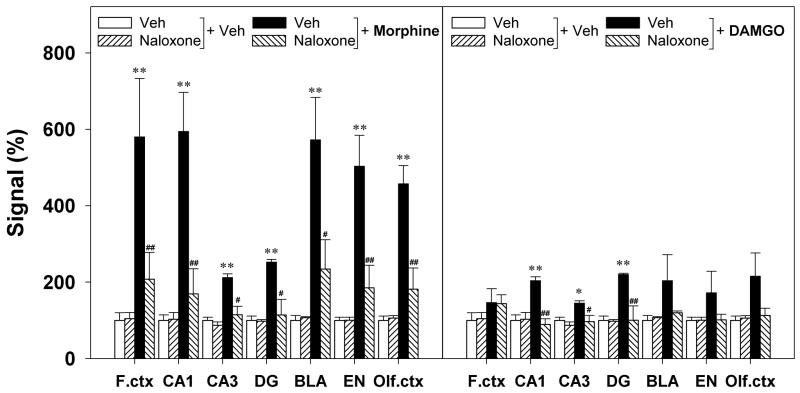
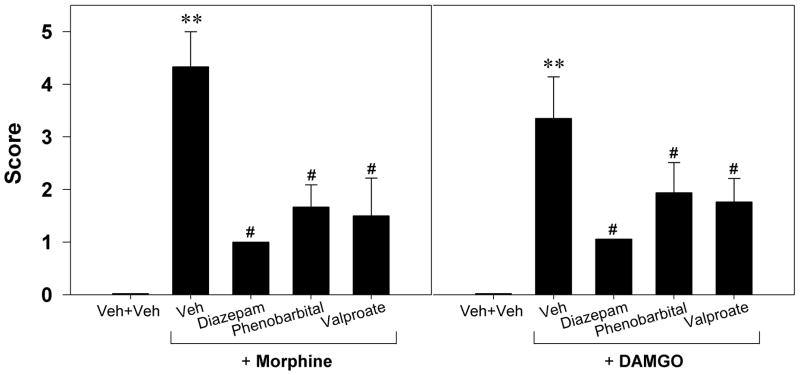
In order to determine whether MORs mediate behavior seizures and up-regulation of BDNF-mRNA expression elicited by i.c.v. administration of MOR agonists, naloxone (1 mg/kg, s.c.) was administered prior to administration of the MOR agonist. Following the vehicle pretreatment, the average behavioral seizure scores for rats receiving i.c.v. morphine (100 μg) and DAMGO (1.5 μg) were 4.3±0.7 and 3.2±0.8, respectively. Naloxone at the dose of 1 mg/kg had no effect on seizure behavior when given alone. However, pretreatment with naloxone significantly attenuated the behavioral seizure scores increased by i.c.v. morphine or DAMGO (Figure 4). Figure 5 illustrates that the vehicle pretreatment did not change the up-regulation of BDNF mRNA expression induced by i.c.v. morphine (100 μg) or DAMGO (1.5 μg). In contrast, pretreatment with naloxone 1 mg/kg significantly blocked the increases in BDNF mRNA expression induced by i.c.v. morphine in all tested regions (left panel). In addition, pretreatment with naloxone also prevented i.c.v. DAMGO-induced up-regulation of BDNF mRNA in the CA1, CA3, and dentate gyrus of hippocampus (right panel).
Figure 4.
Effects of naloxone on the behavioral seizure score increased by i.c.v. morphine 100 μg or i.c.v. DAMGO 1.5 μg. Naloxone 1 mg/kg was administered subcutaneously 15 min before administration of morphine or DAMGO. Each animal was scored according to the highest class of behavioral manifestation observed during the 1 h observation period. Each value represents mean ± S.E.M. (n=6).**, P<0.01 compared with the Veh+Veh condition; #, P<0.05 compared with Veh+(morphine or DAMGO).
Figure 5.
Effects of naloxone on the brain BDNF mRNA expression increased by i.c.v. morphine 100 μg or i.c.v. DAMGO 1.5 μg. Naloxone 1 mg/kg was administered subcutaneously 15 min before administration of morphine or DAMGO. Levels of BDNF mRNA expression were determined 3 h after i.c.v. administration of drugs. Quantification of BDNF mRNA signals in different brain regions are represented as mean percent of the vehicle+vehicle group. *, P<0.05 and **, P<0.01 compared with Veh+Veh; #, P<0.05 and ##, P<0.01 compared with Veh+(morphine or DAMGO). See Figure 4 for other details.
Effects of anticonvulsants on behavioral seizures and BDNF mRNA expression increased by i.c.v. MOR agonists
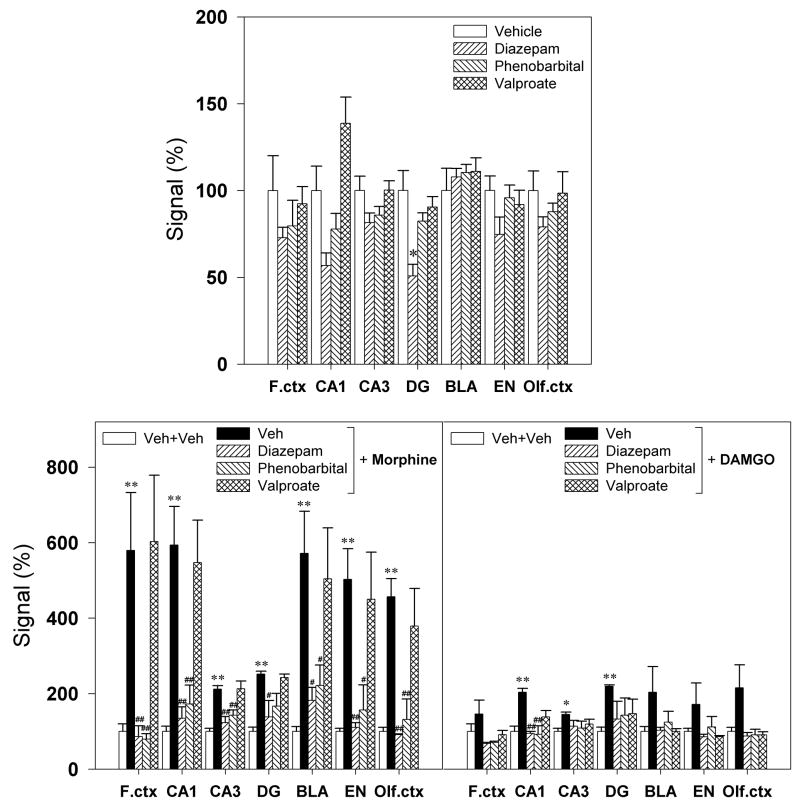
Anticonvulsants were used to determine whether the GABAergic system could modulate behavioral seizures and BDNF mRNA expression increased by i.c.v. MOR agonists. Diazepam (10 mg/kg), phenobarbital (40 mg/kg), or valproate (300 mg/kg) alone had no effect on the behavioral seizure score (data not shown). However, pretreatment with these anticonvulsants significantly attenuated the seizure-related behavioral changes induced by i.c.v. MOR agonists (Figure 6). In situ hybridization results showed that there was no significant change in BDNF mRNA expression following systemic administration of these anticonvulsants alone, except diazepam slightly decreased BDNF mRNA expression in the dentate gyrus of hippocampus (Figure 7, top panel). In addition, bottom panels in figure 7 illustrate that both diazepam and phenobarbital significantly blocked the increases in BDNF mRNA expression induced by morphine in all tested regions. Similarly, diazepam and phenobarbital prevented DAMGO-induced up-regulation of BDNF mRNA in the hippocampus. Valproate, on the other hand, failed to attenuate the up-regulation of BDNF mRNA expression elicited by i.c.v. morphine.
Figure 6.
Effects of systemic administration of diazepam (10 mg/kg, i.m.), phenobarbital (40 mg/kg, i.p.), and valproate (300 mg/kg, i.p.) on the behavioral seizure score increased by i.c.v. morphine 100 μg or i.c.v. DAMGO 1.5 μg. Diazepam was administered 15 min before administration of morphine or DAMGO. Phenobarbital or valproate was administered 1 h before administration of morphine or DAMGO. Each animal was scored according to the highest class of behavioral manifestation observed during the 1 h observation period. Each value represents mean ± S.E.M. (n=6).**, P<0.01 compared with the Veh+Veh condition; #, P<0.05 compared with Veh+(morphine or DAMGO).
Figure 7.
Effects of anticonvulsants, diazepam, phenobarbital, and valproate, on the brain BDNF mRNA expression increased by i.c.v. morphine 100 μg or i.c.v. DAMGO 1.5 μg. Effects of these anticonvulsants alone on the BDNF mRNA expression are presented in the top panel. Quantification of BDNF mRNA signals in different brain regions are represented as mean percent of the vehicle+vehicle group. *, P<0.05 and **, P<0.01 compared with Veh+Veh; #, P<0.05 and ##, P<0.01 compared with Veh+(morphine or DAMGO). See Figures 5&6 for other details.
DISCUSSION
The present study showed that central, not systemic, administration of MOR agonists, produced behavioral seizures and increased brain BDNF mRNA expression in rats. The behavioral seizures and up-regulation of BDNF mRNA elicited by i.c.v. morphine and DAMGO were completely blocked by an opioid receptor antagonist naloxone, demonstrating central MOR activation involved in these actions. Furthermore, antiepileptic drugs, diazepam and phenobarbital, attenuated both behavioral seizures and up-regulation of BDNF mRNA expression induced by i.c.v. MOR agonists. Interestingly, another antiepileptic drug valproate alleviated behavioral seizures, while failed to attenuate the up-regulation of BDNF mRNA expression. This study provides the first evidence that seizure activity plays an important role in the up-regulation of BDNF mRNA expression evoked by central administration of MOR agonists. The differential actions of antiepileptic drugs indicate that the decreased inhibitory action of the γ-aminobutyric acid (GABA)ergic system, specifically the modulation on GABAergic synaptic function to GABA receptors, by central MOR activation mainly participated in the up-regulation of BDNF mRNA expression.
The up-regulation of BDNF mRNA expression has been reported in animal models of seizures and in human epilepsy (Binder, 2004; Koyama and Ikegaya, 2005). Interestingly, MOR agonists, such as morphine, β-endorphin, and DAMGO, produced characteristic epileptiform seizures only when they were given intracerebroventrically (Urca and Frenk, 1982; Snead and Bearden, 1982; Tortella et al., 1987). However, it was not clear whether MOR activation increased BDNF gene expression and to what extent such increased BDNF expression resulted from MOR agonist-induced seizure activity. The present study showed that i.c.v. administration of doses of morphine and DAMGO that produced maximal seizure activity also increased expression of BDNF mRNA. In particular, the highest dose of i.c.v. morphine 100 μg tested herein produced large increases in both BDNF mRNA expression and behavioral seizure scores. The magnitude of increase in BDNF mRNA expression by morphine is similar to that produced by electroconvulsive shock (i.e., a 4–6 fold increase from the baseline) (Dias et al., 2003; Conti et al., 2007). It is interesting to note that electroconvulsive seizure therapy represents an effective treatment for depressed patients and the up-regulation of BDNF mRNA has been suggested as one of mechanisms underlying the actions of antidepressants (Duman and Monteggia, 2006; Martinowich et al., 2007). Nevertheless, i.c.v. administration of MOR agonists could up-regulate BDNF mRNA expression, but could not produce antidepressant-like effects (Broom et al., 2002; Zhang et al., 2006). These findings collectively indicate that BDNF is not a factor sufficient to explain therapeutic effects of antidepressant treatments.
Both i.c.v. morphine and DAMGO produced different magnitudes of increases in behavioral seizures and BDNF mRNA expression. It is important to note that morphine, not DAMGO, could not cause rapid internalization of MORs (Keith et al., 1996) and that there are differential actions between morphine and DAMGO on activation of G protein α-subunits (Saidak et al., 2006; Rodriguez-Munoz et al., 2007). In addition, it seems likely that different degrees of increased BDNF mRNA expression between morphine and DAMGO are due to different magnitudes of behavioral seizures elicited by both compounds (i.e., overt convulsions, generalized clonic tonic activity by morphine versus non-convulsive wet-dog shakes by DAMGO). It is worth noting that other opioids preferentially activating δ opioid receptors (DOR) only produced non-convulsive wet-dog shakes following i.c.v. administration (Urca et al., 1977; Tortella et al., 1978; Frenk, 1983). More importantly, i.c.v. administration of DOR agonists produced large increases of BDNF mRNA expression (i.e., 2–4 fold increase from the baseline), and non-peptidic and peptidic DOR agonists elicited such increases in different specific brain subregions (e.g., the frontal cortex versus hippocampus) (Torregrossa et al., 2004; Zhang et al., 2006). Given that up-regulation of BDNF mRNA expression by i.c.v. morphine and DAMGO occurred in the widespread regions of the brain, these findings contrast different functions of central MOR versus DOR in modulating the seizure activity and BDNF gene expression.
On the other hand, s.c. administration of morphine and other MOR agonists, fentanyl and etorphine, did not increase behavioral seizures and BDNF mRNA expression. Doses of MOR agonists for i.c.v. and s.c. administration were selected based on previous findings showing that they produced full antinociceptive effects in various animal pain models (Walker et al., 1994; Gogas et al., 1996; Fraser et al., 2000; Vissers et al., 2006). Different effects between administration routes (i.e., central versus systemic delivery) of MOR agonists on behavioral seizures and BDNF mRNA expression may lie in the rate and pattern of diffusion from the injection site and the location of sites of MOR actions in relation to the injection site (Urca and Frenk, 1982; Adler et al., 1984). Nevertheless, effects following systemic administration integrate the overall physiological outcome of supraspinal, spinal, and peripheral receptor activation. The unique action of i.c.v. MOR agonists in eliciting behavioral seizures and up-regulating BDNF mRNA expression illustrates the differences between central and systemic administration routes. Furthermore, present findings showed that changes in both behavioral seizures and BDNF mRNA expression by i.c.v. MOR agonists could be completely reversed by pretreatment with naloxone. It may suggest that central MOR activation is the first step in the pathway of i.c.v. MOR agonist-induced behavioral seizures and up-regulation of BDNF mRNA expression.
Antiepileptic drugs diazepam, phenobarbital, and valproate were effective in reducing behavioral seizure scores increased by i.c.v. MOR agonists. This finding, along with previous studies, indicated that the GABAergic inhibition played an important role in behavioral seizures induced by central administration of MOR agonists (Snead and Bearden, 1982; Frenk, 1983). More importantly, both diazepam and phenobarbital attenuated the up-regulation of BDNF mRNA expression elicited by i.c.v. MOR agonists. GABA receptor antagonists, such as bicuculline, CGP 56999A and picrotoxin, increased the levels of BDNF mRNA in the brain in vivo and in vitro (Zafra et al., 1991; Heese et al., 2000; Marmigère et al., 2003; Metsis et al., 1993). These results together may indicate a decreased inhibitory action of GABAergic system by central MOR activation involved in its up-regulation of BDNF mRNA expression.
Interestingly, valproate attenuated behavioral seizures, but failed to affect i.c.v. morphine-, not DAMGO-, induced up-regulation of BDNF mRNA expression. In contrast to that DAMGO is a highly MOR-selective agonist, morphine often exerts its action by activating other opioid receptor subtypes such as DOR (Suarez-Roca and Maixner, 1992). In addition, differential MOR internalizations between morphine and DAMGO caused distinct morphological changes in dendritic spines which may also contribute to the variation of both ligands’ effects (Liao et al., 2007). Nevertheless, valproate seems to have different blocking effectiveness against morphine’s robust versus DAMGO’s modest effects on BDNF mRNA levels. The antiepileptic mechanism of valproate is different from that of the barbiturates and benzodiazepines, which act directly on the GABAA receptor complex. Although it does increase the GABAergic transmission, valproate has no affinity for GABAA receptors complex. Its action is mainly through the direct increase of GABA levels as a result of the reduction of GABA metabolism or the promotion of GABA secretion (Owens and Nemeroff, 2003). Different actions of these antiepileptic drugs on BDNF mRNA expression may imply that up-regulation of BDNF mRNA expression evoked by i.c.v. MOR agonists is more related to the GABAergic inhibition through its modulation on GABAergic synaptic function onto the GABA receptors or other signal mechanisms. This viewpoint is supported by the evidence that MOR activation could enhance paired-pulse excitatory activity within the dendrites of CA1 pyramidal neurons and the augmentation was exclusively due to the inhibition of GABAergic synaptic transmission onto GABAA receptors (McQuiston and Saggau, 2003). Nevertheless, it is possible that some effects independent of seizures may be involved in MOR agonist-induced up-regulation of BDNF gene expression. A recent study demonstrated that morphine-induced enhancement of BDNF gene expression in vitro may be mediated through the phosphorylation of cAMP response element-binding protein and the subsequent activation of extracellualr signal-regulated kinase 1/2 pathway (Takayama and Ueda, 2005).
Naloxone and diazepam were the effective antiepileptic drugs that inhibited behavioral seizures and BDNF mRNA expression elicited by centrally administrated MOR agonists. This finding provides the supporting evidence that pretreatment with naloxone or benzodiazepines can attenuate the epileptic symptoms. In fact, it has been reported that the epilepticus state caused by overdose of centrally administered morphine could be successfully treated with naloxone or diazepam (Kulkarni and Nagrath, 1983; Sauter et al., 1994; Groudine et al., 1995). Collectively, this study demonstrates that both behavioral seizures and up-regulation of BDNF mRNA expression induced by i.c.v. morphine and DAMGO were naloxone-sensitive. Differential actions of antiepileptic drugs suggest that there may be additional mechanisms involved in the interaction between seizures and up-regulation of BDNF gene expression. The rapid onset of BDNF mRNA up-regulation by i.c.v. administration of MOR agonists may provide insight on the relationship between seizures and the overactivity of MOR or/and BDNF.
Acknowledgments
This work was supported by the US National Institutes of Health grants DA07281, DA13685, & MH42251, and Taiwan National Science Council Grants NSC-96-2413-H-004-019 & NSC-97-2628-H-004-089-MY2. The authors thank Ms. Alisha Diggs for her assistance with the editing of manuscript.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- MOR
mu opioid receptors
- DAMGO
[D-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- i.c.v.
intracerebroventricular
- DG
dentate gyrus
- F.ctx
frontal cortex
- BLA
basolateral amygdaloid complex
- EN
endopiriform nucleus
- Olf.ctx
primary olfactory cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler MW, Rowan CH, Geller EB. Intracerebroventricular vs. subcutaneous drug administration: apples and oranges? Neuropeptides. 1984;5:73–76. doi: 10.1016/0143-4179(84)90030-1. [DOI] [PubMed] [Google Scholar]
- Berman EF, Adler MW. The anticonvulsant effect of opioids and opioid peptides against maximal electroshock seizures in rats. Neuropharmacology. 1984;23:367–371. doi: 10.1016/0028-3908(84)90200-4. [DOI] [PubMed] [Google Scholar]
- Binder DK. The role of BDNF in epilepsy and other diseases of the mature nervous system. Adv Exp Med Biol. 2004;548:34–56. doi: 10.1007/978-1-4757-6376-8_3. [DOI] [PubMed] [Google Scholar]
- Broom DC, Jutkiewicz EM, Folk JE, Traynor JR, Rice KC, Woods JH. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- Conti B, Maier R, Barr AM, Morale MC, Lu X, Sanna PP, Bilbe G, Hoyer D, Bartfai T. Region-specific transcriptional changes following the three antidepressant treatments electro convulsive therapy, sleep deprivation and fluoxetine. Mol Psychiatry. 2007;12:167–189. doi: 10.1038/sj.mp.4001897. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Dugich-Djordjevic MM, Tocco G, Lapchak PA, Pasinetti GM, Najm I, Baudry M, Hefti F. Regionally specific and rapid increases in brain-derived neurotrophic factor messenger RNA in the adult rat brain following seizures induced by systemic administration of kainic acid. Neuroscience. 1992;47:303–315. doi: 10.1016/0306-4522(92)90246-x. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Bengzon J, Kokaia Z, Persson H, Lindvall O. Increased levels of messenger RNAs for neurotrophic factors in the brain during kindling epileptogenesis. Neuron. 1991;7:165–176. doi: 10.1016/0896-6273(91)90084-d. [DOI] [PubMed] [Google Scholar]
- Fraser GL, Gaudreau GA, Clarke PBS, Menard DP, Perkins MN. Antihyperalgesic effects of δ opioid agonists in a rat model of chronic inflammation. Br J Pharmacol. 2000;129:1668–1672. doi: 10.1038/sj.bjp.0703248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Humby T, Horner CH, Sofroniew MV, Rattray M. Hippocampal neurotrophin and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Frenk H. Pro- and anticonvulsant actions of morphine and the endogenous opioids: involvement and interactions of multiple opiate and non-opiate systems. Brain Res. 1983;287:197–210. doi: 10.1016/0165-0173(83)90039-5. [DOI] [PubMed] [Google Scholar]
- Gogas KR, Cho HJ, Botchkina GI, Levine JD, Basbaum AI. Inhibition of noxious stimulus-evoked pain behaviors and neuronal fos-like immunoreactivity in the spinal cord of the rat by supraspinal morphine. Pain. 1996;65:9–15. doi: 10.1016/0304-3959(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Golarai G, Cavazos JE, Sutula TP. Activation of the dentate gyrus by pentylenetetrazol evoked seizures induces mossy fiber synaptic reorganization. Brain Res. 1992;593:257–264. doi: 10.1016/0006-8993(92)91316-7. [DOI] [PubMed] [Google Scholar]
- Groudine SB, Cresanti-Daknis C, Lumb PD. Successful treatment of a massive intrathecal morphine overdose. Anesthesiology. 1995;82:292–295. doi: 10.1097/00000542-199501000-00035. [DOI] [PubMed] [Google Scholar]
- Heese K, Otten U, Mathivet P, Raiteri, Marescaux C, Bernasconi R. GABAB receptor antagonists elevate both mRNA and protein levels of the neurotrophins nerve growth factor (NGF) and brainderived neurotrophic factor (BDNF) but not neurotrophin-3 (NT-3) in brain and spinal cord of rats. Neuropharmacology. 2000;39:449–462. doi: 10.1016/s0028-3908(99)00166-5. [DOI] [PubMed] [Google Scholar]
- Humpel C, Wetmore C, Olson L. Regulation of brain-derived neurotrophic factor messenger RNA and protein at the cellular level in pentylenetetrazol-induced epileptic seizures. Neuroscience. 1993;53:909–918. doi: 10.1016/0306-4522(93)90476-v. [DOI] [PubMed] [Google Scholar]
- Isackson PJ, Huntsman MM, Murray KD, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- Keith DE, Murray SR, Zaki PA, Chu PC, Lissin DV, Kang L, Evans CJ, von Zastrow M. Morphine activates opioid receptors without causing their rapid internalization. J Biol Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- Koyama R, Ikegaya Y. To BDNF or not to BDNF: that is the epileptic hippocampus. Neuroscientist. 2005;11:282–287. doi: 10.1177/1073858405278266. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Nagrath A. Modification by GABA-ergic agents and clonidine of morphine-induced convulsions and toxicity in rats. Clin Exp Pharmacol Physiol. 1983;10:125–129. doi: 10.1111/j.1440-1681.1983.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Liao D, Grigoriants OO, Wang W, Wiens K, Loh HH, Law PY. Distinct effects of individual opioids on the morphology of spines depend upon the internalization of mu opioid receptors. Mol Cell Neurosci. 2007;35:456–469. doi: 10.1016/j.mcn.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigère F, Rage F, Tapia-Arancibia L. GABA-glutamate interaction in the control of BDNF expression in hypothalamic neurons. Neurochem Int. 2003;42:353–358. doi: 10.1016/s0197-0186(02)00100-6. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Saggau P. Mu-opioid receptors facilitate the propagation of excitatory activity in rat hippocampal area CA1 by disinhibition of all anatomical layers. J Neurophysiol. 2003;90:1936–1348. doi: 10.1152/jn.01150.2002. [DOI] [PubMed] [Google Scholar]
- Metsis M, Timmusk T, Arenas E, Persson H. Differential usage of multiple brain-derived neurotrophic factor promoters in the rat brain following neuronal activation. Proc Natl Acad Sci U S A. 1993;90:8802–8806. doi: 10.1073/pnas.90.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Pharmacology of valproate. Psychopharmacol Bull. 2003;37 Suppl 2:17–24. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; San Diego, CA: 1986. [Google Scholar]
- Pezet S, Malcangio M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin Ther Targets. 2004;8:391–399. doi: 10.1517/14728222.8.5.391. [DOI] [PubMed] [Google Scholar]
- Poulsen FR, Lauterborn J, Zimmer J, Gall CM. Differential expression of brain-derived neurotrophic factor transcripts after pilocarpine-induced seizure-like activity is related to mode of Ca2+ entry. Neuroscience. 2004;126:665–676. doi: 10.1016/j.neuroscience.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Rocamora N, Palacios JM, Mengod G. Limbic seizures induce a differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor and neurotrophin-3, in the rat hippocampus. Brain Res Mol Brain Res. 1992;13:27–33. doi: 10.1016/0169-328x(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, de la Torre-Madrid E, Sanchez-Blazquez P, Garzon J. Morphine induces endocytosis of neuronal mu-opioid receptors through the sustained transfer of Galpha subunits to RGSZ2 proteins. Mol Pain. 2007;3:19. doi: 10.1186/1744-8069-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidak Z, Blake-Palmer K, Hay DL, Northup JK, Glass M. Differential activation of G-proteins by mu-opioid receptor agonists. Br J Pharmacol. 2006;147:671–680. doi: 10.1038/sj.bjp.0706661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter K, Kaufman HH, Bloomfield SM, Cline S, Banks D. Treatment of high-dose intrathecal morphine overdose. Case report. J Neurosurg. 1994;81:143–146. doi: 10.3171/jns.1994.81.1.0143. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Bearden LJ. Anticonvulsants specific for petit mal antagonize epileptogenic effect of leucine enkephalin. Science. 1980;210:1031–1033. doi: 10.1126/science.6254150. [DOI] [PubMed] [Google Scholar]
- Snead OC, 3rd, Bearden LJ. The epileptogenic spectrum of opiate agonists. Neuropharmacology. 1982;21:1137–1144. doi: 10.1016/0028-3908(82)90171-x. [DOI] [PubMed] [Google Scholar]
- Suarez-Roca H, Maixner W. Morphine produces a multiphasic effect on the release of substance P from rat trigeminal nucleus slices by activating different opioid receptor subtypes. Brain Res. 1992;579:195–203. doi: 10.1016/0006-8993(92)90051-a. [DOI] [PubMed] [Google Scholar]
- Takayama N, Ueda H. Morphine-induced chemotaxis and brain-derived neurotrophic factor expression in microglia. J Neurosci. 2005;25:430–435. doi: 10.1523/JNEUROSCI.3170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortella FC, Moreton JE, Khazan N. Electroencephalographic and behavioral effects of D-ala2-methionine-enkephalinamide and morphine in the rat. J Pharmacol Exp Ther. 1978;206:636–643. [PubMed] [Google Scholar]
- Tortella FC, Robles L, Mosberg HI. Evidence for mu opioid receptor mediation of enkephalin-induced electroencephalographic seizures. J Pharmacol Exp Ther. 1987;240:571–577. [PubMed] [Google Scholar]
- Torregrossa MM, Isgor C, Folk JE, Rice KC, Watson SJ, Woods JH. The delta-opioid receptor agonist (+)BW373U86 regulates BDNF mRNA expression in rats. Neuropsychopharmacology. 2004;29:649–659. doi: 10.1038/sj.npp.1300345. [DOI] [PubMed] [Google Scholar]
- Urca G, Frenk H. Systemic morphine blocks the seizures induced by intracerebroventricular (i.c.v.) injections of opiates and opioid peptides. Brain Res. 1982;246:121–126. doi: 10.1016/0006-8993(82)90148-2. [DOI] [PubMed] [Google Scholar]
- Urca G, Frenk H, Liebeskind JC, Taylor AN. Morphine and enkephalin: analgesic and epileptic properties. Science. 1977;197:83–86. doi: 10.1126/science.867056. [DOI] [PubMed] [Google Scholar]
- Vissers KCP, Greenen F, Biermans R, Meert TF. Pharmacological correlation between the formalin test and the neuropathic pain behavior in different species with chronic constriction injury. Pharmacol Biochem Behav. 2006;84:479–486. doi: 10.1016/j.pbb.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci U S A. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, Shi YG, Rice KC, Woods JH, Watson SJ, Ko MCH. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and mu-opioid receptors independent of antidepressant-like effects. Eur J Neurosci. 2006;23:984–994. doi: 10.1111/j.1460-9568.2006.04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol. 2007;81:294–330. doi: 10.1016/j.pneurobio.2007.01.003. [DOI] [PubMed] [Google Scholar]