Abstract
Ovotestis development in B6-XYPOS mice provides a rare opportunity to study the interaction of the testis- and ovary-determining pathways in the same tissue. We studied expression of several markers of mouse fetal testis (SRY, SOX9) or ovary (FOXL2, Rspo1) development in B6-XYPOS ovotestes by immunofluorescence, using normal testes and ovaries as controls. In ovotestes, SOX9 was expressed only in the central region where SRY is expressed earliest, resulting in testis cord formation. Surprisingly, FOXL2-expressing cells also were found in this region, but individual cells expressed either FOXL2 or SOX9, not both. At the poles, even though SOX9 was not upregulated, SRY expression was down-regulated normally as in XY testes, and FOXL2 was expressed from an early stage, demonstrating ovarian differentiation in these areas. Our data (1) show that SRY must act within a specific developmental window to activate Sox9; (2) challenge the established view that SOX9 is responsible for down-regulating Sry expression; (3) disprove the concept that testicular and ovarian cells occupy discrete domains in ovotestes; and (4) suggest that FOXL2 is actively suppressed in Sertoli cell precursors by the action of SOX9. Together these findings provide important new insights into the molecular regulation of testis and ovary development.
Keywords: Sry, Sox9, FoxL2, sex determination, gonad differentiation
Introduction
Sex determination is one of the most variable developmental processes in the animal kingdom. In mammals, sex is determined by the inheritance of a Y or an X chromosome from the father. One single gene on the Y chromosome, Sry, is necessary and normally sufficient to initiate testicular development (Berta et al., 1990; Jäger et al., 1990; Koopman et al., 1991). Sry induces a cascade of gene expression that regulates the differentiation of the bipotential genital ridges into testicular tissue. In the absence of Sry, or if SRY function is impaired, the genital ridges develop into ovaries. Subsequently, hormones produced by the testes and ovaries direct the differentiation of all secondary sexual characteristics (for review see (Wilhelm and Koopman, 2006).
Sry encodes a SOX-type high mobility group (HMG) transcription factor thought to regulate transcription of downstream target genes. Recently, it has been shown that the expression of Sox9, encoding another HMG box transcription factor from the same family as SRY is directly regulated by SRY (Sekido and Lovell-Badge, 2008). Sox9 expression is up-regulated immediately after Sry expression in the supporting cells of the developing testis and marks their differentiation into Sertoli cells (Sekido et al., 2004; Wilhelm et al., 2005). Subsequently, Sox9 activates the expression a number of genes, such as Amh (encoding anti-Müllerian hormone), Vnn-1 (encoding vanin-1), and Pgds (encoding prostaglandin-D-synthetase), that are known to be involved in testis differentiation (Arango et al., 1999; de Santa Barbara et al., 1998; Wilhelm et al., 2007; Wilson et al., 2005). Like Sry, the activity of Sox9 is both necessary and sufficient to induce testis development in the genital ridges (Barrionuevo et al., 2006; Chaboissier et al., 2004; Foster et al., 1994; Vidal et al., 2001; Wagner et al., 1994).
Interestingly, cases exist of XY sex reversal even in the presence of a normal Sry gene. A classic example in mice is B6-YDOM sex reversal, characterized by the combination of a Y chromosome from some Mus domesticus subspecies, such as Mus domesticus poschiavinus (YPOS), with the inbred C57BL/6 (B6) genetic background (Eicher et al., 1982). The severity of the phenotype depends on the M. domesticus subspecies. Y chromosomes from M. d. poschiavinus (YPOS) and M. d. tirano (YTIR) mice cause the most severe B6-YDOM sex reversal, with all XY mice developing as females with two ovaries, or hermaphrodites with either two ovotestes or one ovary and one ovotestis (Eicher and Washburn, 2001; Eicher et al., 1982). Each ovotestis consists of ovarian tissue at one or both poles of the gonad and testicular tissue in the centre. In contrast, YAKR and YRF/J chromosomes cause a delay in B6 mice testis cord formation that resolves by 15.5 dpc, whereas YFVB and YSJL chromosomes induce normal testis differentiation (Eicher and Washburn, 1986; Nagamine et al., 1987; Washburn and Eicher, 1983).
The B6-YDOM phenomenon suggests that the mere presence of the Y chromosome, i.e., the Sry gene, is not always sufficient to induce normal testis differentiation. Therefore, a number of studies have addressed the possibility that Sry structure and/or expression might vary between M. domesticus Y chromosomes. DNA sequence analysis and RT-PCR expression studies of the various M. domesticus Sry genes did reveal genetic variants, but the differences found could not be correlated with the observed spectrum of gonadal phenotypes caused by the various Sry genes on a B6 genetic background (Albrecht et al., 2003; Lee and Taketo, 1994; Lee and Taketo, 2001; Nagamine et al., 1999; Palmer and Burgoyne, 1991; Taketo et al., 1991).
Recently, the possible contribution of timing defects to B6-YPOS sex reversal was studied using whole mount in situ hybridisation (Bullejos and Koopman, 2005). Normally, Sry expression starts around 10.5 days post coitum (dpc) in the centre of the XY genital ridge, expands to the anterior and then posterior pole to reach a maximum at 11.5 dpc, before extinguishing in the same wave-like pattern centre to anterior pole and posterior pole (Albrecht and Eicher, 2001; Bullejos and Koopman, 2001; Wilhelm et al., 2005). By comparing the characteristics of this wave of expression in several strains of mice including B6 XYPOS, Bullejos and Koopman (Bullejos and Koopman, 2005) established delayed expression of Sry, and downstream genes such as Sox9, as a likely cause for B6-YPOS sex reversal.
B6 XYPOS fetuses provide a powerful system in which to study the molecular basis of mammalian testis and ovary differentiation within the same gonad. Although gonads of 6-week-old B6 XYPOS females contain follicles with germ cells, it is unknown whether normal ovarian differentiation was initiated within these gonads. In the present study we used immunohistochemical staining to identify the molecular events that are impaired in fetal B6 XYPOS gonads. We found that even though SRY is expressed along the whole length of the genital ridge, SOX9 up-regulation and subsequent testis differentiation takes place only in the centre, confirming that a critical function of SRY is to induce SOX9 expression, and that SOX9, not SRY, is responsible for inducing Sertoli cell differentiation and thus orchestrating testis development. Furthermore, our findings indicate that an active pathway of ovarian development normally begins in the early fetal stage, and that ovotestis development in B6 XYPOS mice is caused by a localized failure of testis-determining genes to completely suppress the competing ovarian pathway.
Results
The present study was based on B6 XYPOS mice, representing the most severely affected class of B6-YDOM sex reversal. Most B6 XYPOS fetuses generated in our breeding regime contained ovotestes, providing an opportunity to study the molecular basis of mammalian testis and ovary differentiation within the same gonad.
Morphology of developing B6 XYPOS gonads
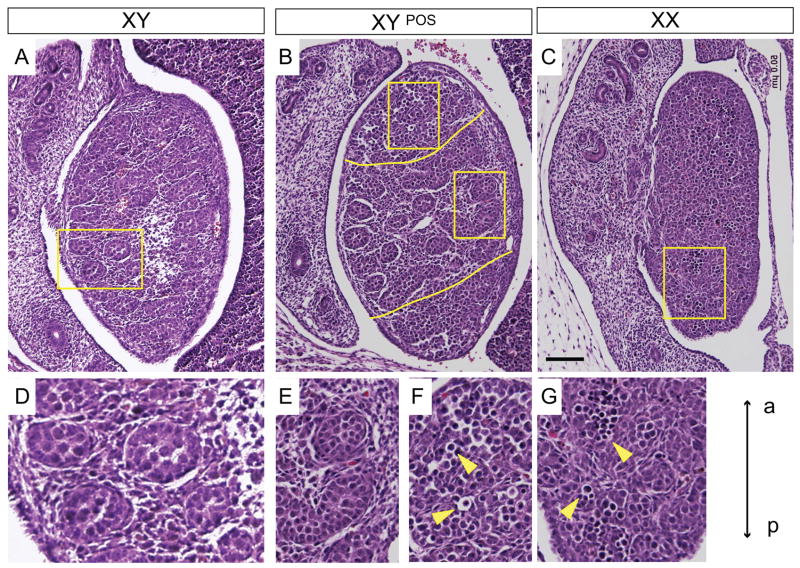
Before analysing in detail the molecular events of the development of B6 XYPOS sex reversed gonads, we performed histological studies using haematoxylin/eosin-stained sagittal sections of normal 14.5 dpc B6 XX, B6 XY, and B6 XYPOS fetuses. As expected, B6 XY gonads contained testis cords comprised of clusters of germ cells (round cells with large nuclei and annular cytoplasm), surrounded by Sertoli cells (irregular-shaped cells interdigitating with germ cells and with nuclei concentrated towards the periphery of the cords) and a layer of peritubular myoid cells (flattened, smooth muscle-like cells surrounding the basement membrane of the cord) (Fig. 1A and D). In contrast, B6 XX ovaries were less organized histologically, but contained meiotic germ cells recognizable by their round shape and dark-staining chromosomal condensations (Fig. 1C and G). B6 XYPOS gonads presented as ovaries or as ovotestis with testicular cords in the centre and unorganized (Fig. 1B and E), ovarian-like tissue at the poles (Fig. 1B and F).
Figure 1. Histology of B6 XYPOS ovotestes.
Sagittal sections of paraffin-embedded B6 XY, B6 XYPOS and B6 XX fetuses at 14.5 dpc were stained with haematoxylin and eosin. (A) B6 XY gonad shows typical testicular morphology with testis cords enclosing the germ cells. (B) The B6 XYPOS ovotestis contained testicular cords in the centre and seemingly undifferentiated tissue at the poles (separated by yellow lines). (C) B6 XX gonad displays ovarian morphology with germ cells that have entered meiosis. (D) Enlargement of the region marked with a rectangle in (A) shows testis cords. (E) Enlargement of the region marked with a rectangle in (B, right) shows testis cords in the central region of the ovotestis. (F) Enlargement of the region marked with a rectangle in (B, left) shows meiotic germ cells (yellow arrowheads) at the anterior pole. (G) Enlargement of the region marked with a rectangular in (C) shows meiotic germ cells. Images are oriented so that anterior pole is at the top (a, anterior, p, posterior). Scale bar, 50 μm.
We analysed 32 B6 XYPOS fetuses in the course of this study. None developed even a single testis. Eight (25%) displayed complete XY gonadal sex reversal, i.e. both gonads contained no testicular tissue and were identical to ovaries of B6 XX mice. Of the remaining 24, 12 displayed an ovotestis on one side and an ovary on the other, while 12 had two ovotestes. Of the 36 ovotestes analysed, 34 had ovarian tissue at both poles, one had ovarian tissue only at the anterior pole and one had ovarian tissue only at the posterior pole.
Down-regulation of SRY protein is delayed in B6 XYPOS gonads
Under normal circumstances, SRY protein expression follows closely the expression of Sry mRNA starting in the centre of the genital ridge at around 10.75 dpc (12 tail somite [ts] stage), extending to the poles by 11.5 dpc (18 ts), before disappearing from the centre and the anterior pole, with the last cells remaining positive for SRY at 12.5 dpc at the posterior end (Wilhelm et al., 2005). In B6 XYPOS genital ridges, Sry transcription is initiated later than normal and reaches the poles shortly before 12.0 dpc (22 ts, (Bullejos and Koopman, 2005). To examine if this mRNA is translated into functional protein throughout the gonads, we used an antibody specific for SRY protein (Wilhelm et al., 2005) and performed immunofluorescence on sagittal sections of B6 XX, B6 XY and B6 XYPOS fetuses at 11.5 dpc. For comparison, different marker antibodies were used on consecutive sections of the same sample (Figs. 2 to 4).
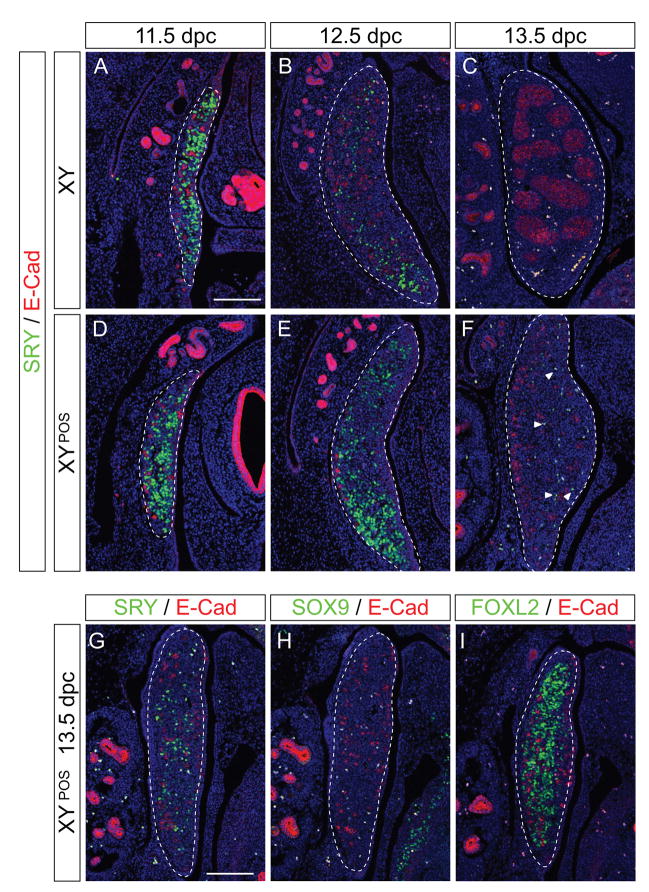
Figure 2. Temporal and spatial expression of SRY in B6 XYPOS gonads.
Immunofluorescence of sagittal sections of paraffin-embedded B6 XY (A–C) and B6 XYPOS (D–F) fetuses from 11.5 dpc to 13.5 dpc with antibodies specific for SRY (green) and E-cadherin (red), which is expressed in germ cells and in the mesonephric tubules of the mesonephros. SRY expression is similar in B6 XYPOS to B6 XY gonads at 11.5 dpc (A and D). At 12.5 dpc the down-regulation of SRY is delayed in B6 XYPOS compared to B6 XY gonads (B and E) and at 13.5 dpc a few SRY-positive cells are detectable only in B6 XYPOS gonads (F, arrowheads). Sections are adjacent sections to those shown in Figures 3 and 4. (G–I) SRY down-regulation is delayed in B6 XYPOS ovaries. Immunofluorescence of sagittal sections of a paraffin-embedded B6 XYPOS fetus at 13.5 dpc to with antibodies specific for SRY (green, G), SOX9 (green, H) and FOXL2 (green, I) together with E-cadherin (red in all three panels), which is expressed in germ cells and in the mesonephric tubules of the mesonephros. FOXL2 is expressed along the whole length of the gonad, whereas no SOX9 expression was detected. SRY expression remains detectable at 13.5 dpc, a stage at which it is normally completely shut down. Pictures are oriented so that anterior pole is at the top. DAPI staining (blue) marks cell nuclei. Scale bar for all panels, 100 μm. Dotted lines encompass gonads.
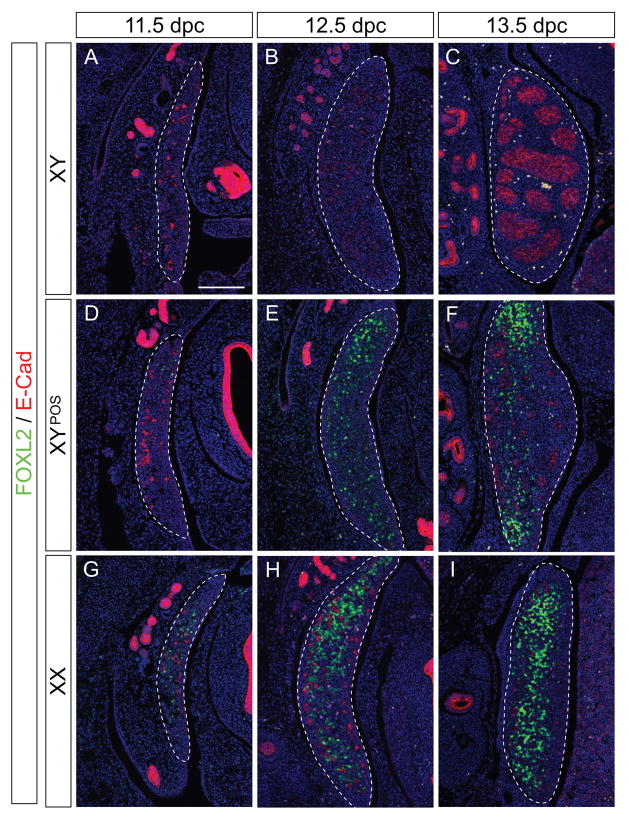
Figure 4. Analysis of the female marker FOXL2 in B6 XYPOS gonads.
Immunofluorescence of sagittal sections of paraffin-embedded B6 XY (A–C), B6 XYPOS (D–F), and B6 XX (G–I) fetuses from 11.5 dpc to 13.5 dpc with antibodies specific for FOXL2 (green) and E-cadherin (red). FOXL2 is expressed in B6 XYPOS gonads from 11.5 dpc until 13.5 dpc (D–F) in an area overlapping with SOX9 expression (E and F, compare with Fig. 3E and F). No FOXL2 expression was detectable in B6 XY gonads (A–C), and strong expression was seen in B6 XX gonads from 11.5 dpc (G–I). Sections are adjacent to those shown in Figures 2 and 3. DAPI staining (blue) marks cell nuclei. Pictures are oriented so that the anterior pole is at the top. Scale bar for all panels, 100 μm. Dotted lines encompass gonads.
At 11.5 dpc, SRY expression was observed at similar levels along the whole length of all B6 XY and B6 XYPOS genital ridges (Fig. 2A and D). At 12.5 dpc, SRY protein was present in only a few cells throughout the B6 XY testis, mostly at the posterior pole (Fig. 2B), whereas all (8 out of 8 investigated) B6 XYPOS ovotestes retained more SRY-expressing cells (Fig. 2E). Even at 13.5 dpc, SRY-positive cells were detected in 12 out of 12 B6 XYPOS ovotestes (Fig. 2F) and ovaries (Fig. 2G–I), a stage at which SRY expression is already completely down-regulated in B6 XY testes (Fig. 2C). By 14.5 dpc, all 16 B6 XYPOS gonads had completely extinguished SRY expression (data not shown). We conclude that not only the onset (Bullejos and Koopman, 2005) but also the entire program of SRY protein expression is delayed in B6 XYPOS gonads relative to their B6 XY counterparts.
Expression of SOX9 is restricted in B6 XYPOS gonads
The mechanism by which SRY expression is abruptly down-regulated at 12.5 dpc has not been established, but one hypothesis is that SOX9 is directly or indirectly responsible for repressing SRY expression. In support of this hypothesis, the timing of Sox9 up-regulation and Sry down-regulation are closely correlated, and XY gonads in mouse fetuses with a targeted deletion of Sox9 show persistent Sry expression (Barrionuevo et al., 2006; Chaboissier et al., 2004). Thus, if SOX9 represses Sry expression, the observation that SRY is down-regulated in all regions of the B6 XYPOS gonads requires that SOX9 is likewise expressed along the whole length of the these gonads.
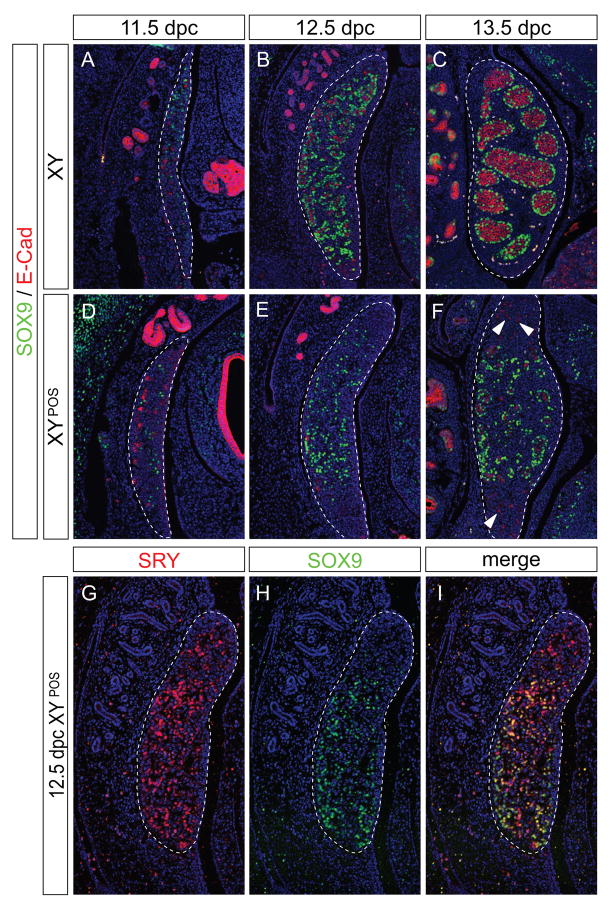
To test this possibility, we examined the dynamics of SOX9 protein expression by immunofluorescence. In agreement with previous studies (Wilhelm et al., 2005), SOX9 protein was first noted in Sertoli cell-precursors in the centre/anterior pole of the B6 XY genital ridge at 11.5 dpc (Fig. 3A). By 12.5 dpc, the first signs of testis cord formation are visible in the B6 XY testis; Sertoli cells expressing SOX9 were found to enclose E-cadherin (E-cad) positive germ cells (Fig. 3B). Over the next two days, all SOX9-positive cells were incorporated into testis cords (Fig. 3C) and the testis increased in size primarily due to an expansion of the interstitial region.
Figure 3. Temporal and spatial expression of SOX9 in B6 XYPOS gonads.
Immunofluorescence of sagittal sections of paraffin-embedded B6 XY (A–C) and B6 XYPOS (D–F) fetuses from 11.5 dpc to 13.5 dpc with antibodies specific for SOX9 (green) and E-cadherin (red). SOX9 is expressed in Sertoli cells in B6 XY gonads throughout development (A–C), whereas SOX9 expression is induced and maintained in the B6 XYPOS gonads only in the centre (D–F). Sections are adjacent to those shown in Figures 2 and 4. (G–I) Double immunofluorescence of 12.5 dpc B6 XYPOS fetus with antibodies specific to SRY (red) and SOX9 (green). Red channel only (G), green channel only (H) and merged channels (I). DAPI staining (blue) marks cell nuclei. Pictures are oriented so that anterior pole is at the top. Scale bar for all panels, 100 μm. Dotted lines encompass gonads.
The onset of SOX9 expression at 11.5 dpc in B6 XYPOS genital ridges (n=7) was similar to that noted in normal B6 XY genital ridges (Fig. 3D). However, by 12.5 dpc, in contrast to the situation in B6 XY genital ridges, SOX9 protein expression in B6 XYPOS genital ridges was either not up-regulated (n=6) or was present in the centre but not at the poles (n=8; Fig. 3E). SOX9-positive cells in the central region assembled into testicular cords, enclosing only those germ cells resident in this region (Fig. 3F). For better comparison of SRY and SOX9 expression in these samples, we performed two-colour immunofluorescence (Fig. 3G–I). This analysis clearly showed that although SRY was expressed throughout the whole length of the gonad, SOX9 was up-regulated only in the centre, and in some samples at the posterior (Fig. 3H) or anterior pole. Polar tissue that did not express SOX9 appeared undifferentiated, with germ cells distributed throughout, as observed in B6 XX ovaries at this stage of development. From these observations, we conclude that SOX9 expression is not induced at the poles of B6 XYPOS gonads, despite SRY expression in these regions, and that testis cord formation correlates with the domain of SOX9 expression and not the domain of SRY expression.
The ovarian program is activated at the poles
Although the partially sex-reversed gonads of B6 XYPOS fetuses are referred to as ovotestes, no evidence exists that the presumptive ovarian regions at the poles have, in fact, embarked on a differentiation pathway characteristic of ovarian development at the stages described in this study, rather than merely remaining undifferentiated. For this reason, we investigated if and when the ovarian program is initiated in B6 XYPOS ovotestes using an antibody specific for FOXL2, a member of the forkhead transcription factor family that is expressed in the undifferentiated ovarian granulosa cells (Schmidt et al., 2004). These granulosa cells belong to the same supporting cell lineage that in testes gives rise to Sertoli cells (Albrecht and Eicher, 2001). In addition we performed in situ hybridization (ISH) for Rspo1, another ovarian-specific marker that has been shown to be crucial for female development (Chassot et al., 2008; Parma et al., 2006; Tomizuka et al., 2008).
Both markers displayed essentially the same expression pattern (Fig. 4 and Suppl. Fig. 1) and we focussed on characterizing FOXL2 expression in more detail. As expected, FOXL2 protein was expressed in normal B6 XX gonads from 11.5 dpc onwards (Fig. 4G–I), but not in normal B6 XY gonads at any stage investigated (Fig. 4A–C). In contrast, in B6 XYPOS ovotestes, FOXL2 was detected from 11.5 dpc onwards. FOXL2 expression was present throughout all gonads that did not express SOX9 (Fig. 2H and I). In contrast, FOXL2 was expressed predominantly at the poles in all 29 ovotestes investigated, demonstrating that the ovarian pathway indeed had been initiated (Fig. 4D–F).
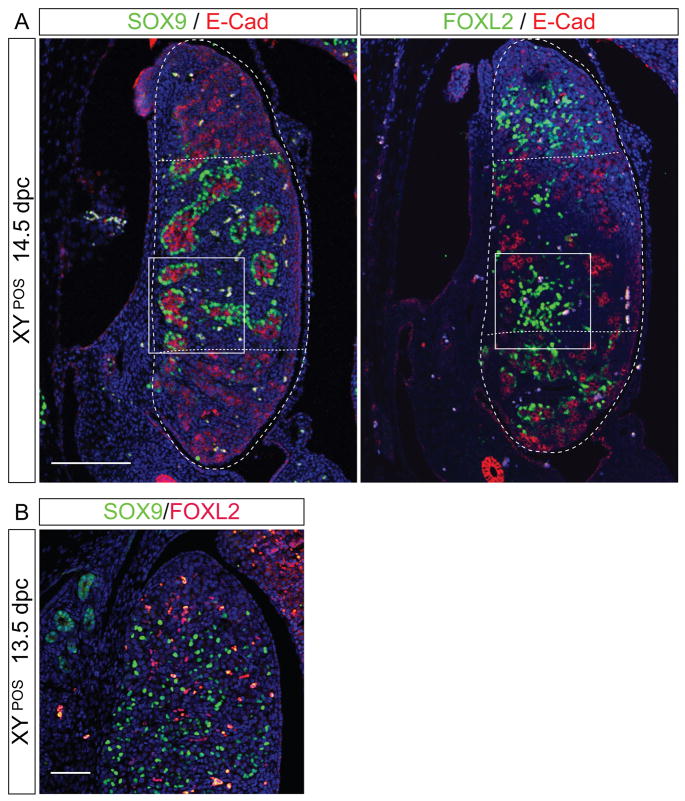
Intriguingly, in 14 of 29 ovotestes, FOXL2 expression was not completely excluded from the centre of the genital ridge where the testicular program was active, as shown by SOX9 expression and testis cord formation (compare Fig. 3E, F, 4E, F and Fig. 5). Analysis of serial sections of ovotestes indicated that FOXL2 was expressed in interstitial regions between SOX9-positive testis cords (Fig. 5A). To exclude the possibility that SOX9 and FOXL2 were expressed within the same cells, we performed double immunofluorescence for SOX9 and FOXL2 on the same section. No double-positive cells, which would appear yellow in the images, were detected in three independent samples (Fig. 5B). The few cells in which we observed yellow staining were autofluorescent blood cells. We conclude that the testicular and ovarian developmental pathways can occur in the same region, but are mutually exclusive at a cellular level, confirming that individual support cell precursors initiate differentiation as either ovarian granulosa or testicular Sertoli cells (Hersmus et al., 2008) (Albrecht and Eicher, 2001; Cocquet et al., 2002; Schmidt et al., 2004).
Figure 5. FOXL2 and SOX9 are expressed in overlapping regions in B6 XYPOS ovotestes, but are mutually exclusive at the cellular level.
(A) Immunofluorescence of adjacent sagittal sections of a paraffin-embedded B6 XYPOS fetus at 14.5 dpc with antibodies specific for SOX9 (green, left panel) and FOXL2 (green, right panel) together with E-cadherin (red in both panels). Dotted lines delineate ovarian tissue at the poles from testicular tissue in the centre of the gonad. FOXL2 is detectable even within morphological testicular tissue (see rectangle). Scale bar, 100 μm. (B) Double immunofluorescence of a sagittal section of B6 XYPOS ovotestis at 14.5 dpc with antibodies specific for SOX9 (green) and FOXL2 (red) indicates that these two genes are not co-expressed in individual cells. DAPI staining (blue) marks cell nuclei; yellow staining is due to autofluorescence of blood cells. Scale bar, 100 μm.
SF1 is not the limiting factor
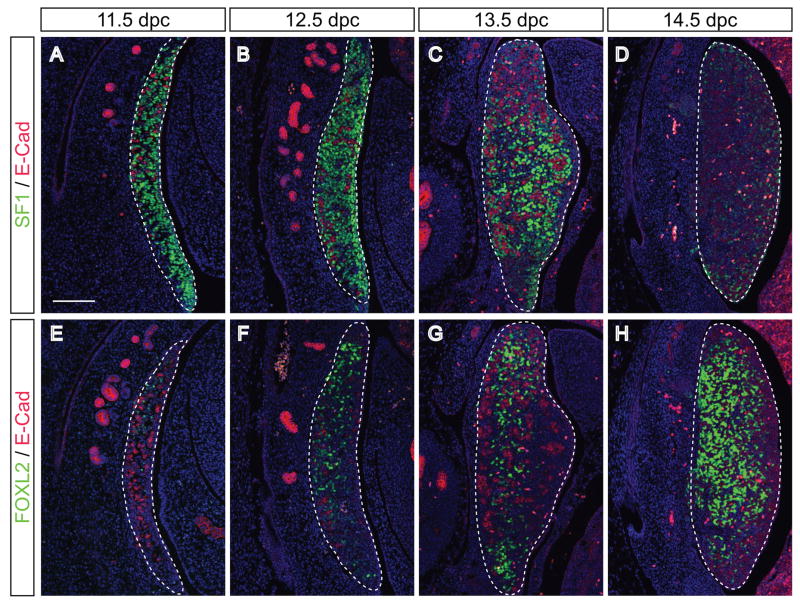
The failure of Sox9 up-regulation at the poles and the induction of the female program even though SRY is present could be due to a missing co-factor or partner protein in these regions. One factor that has recently been shown to be necessary for the induction of Sox9 expression together with SRY is steroidogenic factor 1 (SF1, (Sekido and Lovell-Badge, 2008)). To test if SF1 is the limiting factor, we performed IF on B6 XYPOS embryos from 11.5 to 14.5 dpc. To confirm the activation of the female program at the poles, we used the antibody specific for FOXL2 on adjacent sections (Fig. 6E–H). SF1 was strongly expressed throughout the genital ridge at 11.5 and 12.5 dpc (Fig. 6A and B), ruling out SF1 as the limiting factor for SOX9 expression. Interestingly, SF1 expression was downregulated thereafter in regions that had committed to the ovarian fate (Fig. 6C and D).
Figure 6. SF1 is expressed by somatic cells throughout B6 XYPOS ovotestes.
Immunofluorescence of sagittal sections of paraffin-embedded B6 XYPOS fetuses from 11.5 to 13.5 dpc with antibodies specific for (A–D) SF1 (green) and E-cadherin (red) and adjacent sections (E–H) for FOXL2 (green) and E-caherin (red). SF1 is strongly expressed by somatic cells in the B6 XYPOS gonads along the whole length of the gonad at 11.5 (A) and 12.5 dpc (B) also in regions in which the ovarian pathway was initiated as shown by FOXL2 expression (E and F). From 13.5 dpc onwards SF1 expression is decreased in FOXL2-positive regions (C and D, compare with G and H). DAPI staining (blue) marks cell nuclei. Pictures are oriented so that anterior pole is at the top. Scale bar for all panels, 100 μm. Dotted lines encompass gonads.
Discussion
Ovotestes provide a unique opportunity to study two opposite differentiation pathways, testicular and ovarian, that are not normally observed together in the same developing gonad. B6-YDOM sex reversal in mice in particular represents a tractable model for studying the interplay between these pathways because the frequency with which ovotestes can be experimentally generated is high (Eicher et al., 1982), the spatial distribution of testicular and ovarian material in B6 XYDOM gonads is consistent (Eicher et al., 1995; Eicher et al., 1982; Nagamine et al., 1999), and the root cause of the gonadal sex reversal is clearly identifiable as defective interaction of the B6 autosomal/X genes with the Mus domesticus poschiavinus allele of the gene that initiates the testis-determining pathway, Sry (Biddle and Nishioka, 1988; Eicher and Washburn, 1986; Eicher et al., 1982).
Our analysis of B6-YPOS ovotestes provides support for a number of conclusions derived from previous studies of the development of testes and ovaries in wild type mice, and the study of various transgenic and targeted gene knockout mouse lines. For example, our data confirm that levels of SRY expression are critical for SOX9 expression (Albrecht et al., 2003), that Sox9 is epistatic to Sry and is therefore the key determinant of Sertoli cell fate and hence testis differentiation (Vidal et al., 2001), that an active ovarian-determining pathway operates from an early stage in gonadal development (Eicher et al., 1996), and that compromise of the testis-determining pathway can tip the balance in favour of ovary development (Kim et al., 2006).
In addition, our analysis has yielded important new insights into the nature of the testis- and ovary-determination programs and their mutual antagonism. These are:
SRY must act within a specific developmental window to activate Sox9;
Repression and extinction of Sry expression may not be due to Sox9 expression as previously thought;
Testicular and ovarian tissues can occupy the same spatial domain, disproving morphologically-derived paradigm that they are mutually exclusive; and
Individual cells express either SOX9 or FOXL2 but not both, suggesting that the ovarian pathway is actively suppressed in Sertoli cell precursors at or above the level of FOXL2 by the action of SOX9.
These findings are elaborated in more detail below.
Sry must reach a critical threshold within a defined window of competence to up-regulate Sox9 expression in supporting cells
B6 XYPOS sex reversed mice represent a classic example of a phenotype resulting from the genetic incompatibility between genes required for initiating normal testicular development. In this case, the incompatibility is between the Sry carried by the M. d. poschiavinus Y chromosome (SryPOS), and autosomal/X-linked genes carried by mice of the B6 inbred strain (Eicher et al., 1982). A number of studies have aimed to identify structural, expression level, or expression timing idiosyncrasies correlating with the ability of SryPOS to elicit sex reversal in B6 XYPOS mice (Albrecht et al., 2003; Lee and Taketo, 1994; Lee and Taketo, 2001; Nagamine et al., 1999; Palmer and Burgoyne, 1991; Taketo et al., 1991), culminating in the observation that Sry is expressed later than normal (Bullejos and Koopman, 2005). However this story is by no means complete and the present study clarifies several remaining key questions. At the poles of B6 XYPOS gonads, SRY is evidently expressed at robust levels equivalent to those found in the central region of these gonads. Our data indicate that SRY expression at the poles of B6 XYPOS gonads occurs too late to activate Sox9 expression there, showing that a window of competence is required for supporting cells to respond to SRY. This conclusion is in agreement with recent data showing that experimental activation of an Sry transgene must be induced before 11.3 dpc in order to initiate testis differentiation (Hiramatsu et al., 2009). This window of competence is likely defined at the molecular level by the presence or activity of a partner protein or co-factor for SRY as suggested previously (Kidokoro et al., 2005). Such factors are thought to be involved in the activity and target specificity of most, if not all, SOX proteins (Kamachi et al., 1999; Kamachi et al., 2000; Wilson and Koopman, 2002), and we propose that SRY is typical of SOX transcription factors in this regard. Several possible partner proteins have been reported for SRY (Li et al., 2006; Matsuzawa-Watanabe et al., 2003; Oh et al., 2005; Poulat et al., 1997) but their expression profiles have not been studied in detail and their requirement for SRY function has not been substantiated. One co-factor recently suggested to be important for Sox9 initiation by SRY is SF1 (Sekido and Lovell-Badge, 2008). However, SF1 expression is present at the poles of B6 XYPOS gonads at 11.5 dpc, making it unlikely that SF1 is the limiting factor. Our present data therefore implicate other partner proteins in SRY’s ability to up-regulate Sox9.
Another possibility is that FOXL2 represses Sox9 transcription. This hypothesis is based on the goat PIS model and the mouse Foxl2−/− in which the loss of Foxl2 results in the up-regulation of Sox9 (Ottolenghi et al., 2007). The early expression of FOXL2 we observed at the poles of B6 XYPOS gonads might inhibit the positive influence of SRY on Sox9 expression. Further experiments are needed to investigate this hypothesis on a molecular level.
Are levels of SRY expression important for activating Sox9 transcription? Sex determination in XX and XY individuals involves a number of transcription factors that act in dosage-sensitive pathways (Bardoni et al., 1994; Huang et al., 1999; Jordan et al., 2001; Kim et al., 2006), so it would be reasonable to suggest that the ability of SRY to activate Sox9 transcription, whether directly or indirectly, relies on sufficient levels of SRY expression. Indeed, previous studies indicate that expression levels of SRY are critical for testis-determining function (Burgoyne et al., 2001; Capel et al., 1993; Nagamine et al., 1999; Sekido et al., 2004; Swain et al., 1998). Our present study provides compelling evidence that levels of SRY expression are important for Sox9 activation. In the central region of B6 XYPOS gonads, SRY is clearly expressed within the required temporal window to activate Sox9 transcription in a subset of supporting cells. However, other supporting cells instead activate the ovarian program of development, indicated by FOXL2 expression, implying that a threshold level of SRY activity is not reached in these cells. In other words, the central region of B6 XYPOS gonads contains a mixture of supporting cells in which SRY is expressed above a certain threshold for Sox9 activation, and other supporting cells in which SRY threshold expression is not reached and the ovarian pathway is activated.
Down-regulation of SRY expression
In mice, SRY is expressed in a short window of time encompassing only a few days before being abruptly down-regulated at 12.5 dpc (Bullejos and Koopman, 2001; Sekido et al., 2004; Wilhelm et al., 2005). The molecules responsible for SRY down-regulation have not been identified, but one hypothesis — indeed the only hypothesis put forward to date — is that SOX9 acts directly or indirectly to suppress Sry transcription. Two transgenic mouse models support this idea. First, XX transgenic mice that express a reporter gene consisting of the enhanced green fluorescence protein (EGFP) under the control of the Sry regulatory region continue to express EGFP beyond the normal time of when Sry is down-regulated, possibly because these mice do not express Sox9 in the genital ridge (Albrecht and Eicher, 2001). Second, in XY mouse fetuses harbouring a null-mutation for Sox9, the expression of endogenous Sry is not down-regulated but persists until at least 13.5 dpc (Barrionuevo et al., 2006; Chaboissier et al., 2004).
We found that SRY expression occurs throughout the length of B6 XYPOS gonads whereas SOX9 is up-regulated only in the central region. Despite this, SRY expression is down- regulated even in the regions where SOX9 is not expressed, although with some delay. One possible explanation is that down-regulation of SRY in B6 XYPOS gonads occurs only at the protein level. However, in situ hybridisation studies of B6 XYPOS ovotestes suggested that Sry mRNA also is down-regulated (data not shown). The latest stage at which Sry expression was studied in the Sox9 null mice was 13.5 dpc (Barrionuevo et al., 2006; Chaboissier et al., 2004). We detected SRY expression in 13.5 dpc ovotestes, in agreement with published data, but found that expression disappeared by 14.5 dpc. Further study of the Sox9-null mice at later stages is required to establish the dynamics of SRY down-regulation in these mouse models. However, our present data clearly indicate that either SOX9 expression is not responsible for repressing Sry or that other mechanisms, such as the induction of the female program, can cause down-regulation of Sry expression.
Antagonism and co-existence of testis and ovarian differentiation
The discovery of the B6-YDOM sex reversal phenomenon focused the attention of geneticists on the concept of a molecular tussle between the testicular and ovarian pathways within the supporting cell precursor lineage of the fetal genital ridge (Eicher et al., 1982). If the testis-determining gene is able to exert its influence, these cells will differentiate as Sertoli cells, testis cords will form, and testis development will ensue. An early explanation for B6-YDOM sex reversal was posited in the timing mismatch hypothesis (Eicher and Washburn, 1986; Palmer and Burgoyne, 1991). According to this hypothesis, activation of the Sry gene (then designated Tdy) carried on the M. d. poschiavinus Y chromosome occurs too late so that the testis-determining pathway would “miss the boat” and an ovary-determining program would proceed. This hypothesis has turned out to be correct in many respects. First, it predicts late action of Sry in B6 XYPOS mice, which has been shown to be the case (Bullejos and Koopman, 2005). Second, several autosomal loci corresponding to putative ovarian-determining genes have been mapped genetically in studies involving B6-YPOS sex reversal (Eicher et al., 1996), supporting the concept of an active ovarian-determining pathway. Third, this hypothesis predicts that the ovary-determining pathway is able to engage very early, long before morphological signs of ovary differentiation emerge. Several microarray expression profiling studies (Beverdam and Koopman, 2006; Jorgensen and Gao, 2005; Nef et al., 2005) indicate that ovarian genes are expressed as early as 11.5 dpc. Using double immunofluorescence of SOX9 with FOXL2 we found that the expression of SOX9 and FOXL2 is mutually exclusive at a cellular level as has been described for humans (Hersmus et al., 2008), confirming that supporting cells that fail to activate SOX9 in the central region of B6 XYPOS ovotestes, rapidly activate the female differentiation pathway as evidenced by FOXL2 expression. This in turn suggests that the ovarian pathway is actively suppressed in Sertoli cell precursors by the action of SOX9. It remains to be determined whether SOX9 suppresses Foxl2 directly, or whether intermediate steps might be involved.
A surprising aspect of the B6-YPOS sex reversal model emerging from our data is the coexistence of cells undertaking two opposing differentiation pathways, testicular and ovarian, not only in distinct parts of the same tissue, but also intermingled in the central region of the ovotestes. Previous studies have identified two paracrine recruitment mechanisms based on PGD2 and FGF9 that allow SOX9-negative supporting cells to be recruited by adjacent SOX9-expressing cells to become SOX9-positive and differentiate as Sertoli cells (Kim et al., 2006; Wilhelm et al., 2005). It is possible that these paracrine mechanisms are not sufficiently robust or are activated too late to completely recruit all neighbouring SOX9-negative supporting cells to differentiate into Sertoli cells during ovotestis development. Further studies are required to follow the fate of these “ovarian” cells within the testicular tissue, so as to determine whether these cells are recruited at a later stage, i.e. trans-differentiate into Sertoli cells, or alternatively undergo apoptosis.
The fate of germ cells in B6 XYPOS gonads
The co-existence of testicular and ovarian cells within the same spatial domain in B6 XYPOS ovotestes allows important observations regarding the behaviour of germ cells within these two tissue types. In the central regions of these ovotestes, even though both pre-Sertoli and pre-granulosa cells were present, all germ cells were associated with Sertoli cells and became incorporated into testis cords. This suggests that pre-Sertoli cells but not pre-granulosa cells are able to actively attract germ cells or that pre-Sertoli cells are extremely efficient in doing this in comparison to pre-granulosa cells. Evidence for such an attraction mechanism has not been presented previously. Evidently, this mechanism has a limited reach — perhaps even being a juxtacrine mechanism — because germ cells in the polar, ovarian regions are not drawn into cords even if they are relatively close to developing testicular cords.
Summary and conclusions
Careful study of the classic B6-YPOS sex reversal model has allowed us to confirm previous data and also draw several novel conclusions regarding the molecular and cellular control of sex determination. In particular it was possible to clarify the relationship between SRY and SOX9 expression, a relationship that previously has been difficult to disentangle, and the relationship between the testicular and ovarian pathways that compete in determining the fate of the supporting cell lineage and thus the fate of the gonads as ovaries or testes. The finding that testicular and ovarian-fated cells co-occupy the central region of B6 XYPOS ovotestes provides impetus for further studies of this model system to determine in close detail the molecular antagonism between testicular and ovarian pathways.
An important outcome of the present study is that both the timing and levels of Sry expression contribute to the phenomenon of B6-YPOS sex reversal, settling an issue that has exercised geneticists for the last 25 years. This in turn suggests that mutations, which affect either the timing or the level of SRY expression, but not the actual structure of the SRY protein, may be an important, hitherto unrecognised cause of human idiopathic XY gonadal dysgenesis.
Experimental Procedures
Mice
Fetuses were collected from timed matings involving normal B6 females mated to B6 XYPOS hermaphrodites, with noon of the day a mating plug was observed designated 0.5 days post coitum (dpc). For more accurate staging, the tail somite (ts) stage of the fetus was determined by counting the number of somites posterior to the hind limb, with 11.5 dpc corresponding to 18 ts, and 12.5 dpc to 30 ts. The presence of the Y chromosome was determined by using multiplex genotyping PCR assay on tissue lysate as described previously (Capel et al., 1999). All husbandry and experimental procedures involving mice used in this research project were reviewed and approved by the Institutional Animal Care and Use Committee of The Jackson Laboratory.
Immunofluorescence
Fetuses were fixed in 4% paraformaldehyde in PBS at 4°C overnight, rinsed three times with PBTx (PBS containing 0.1% Triton X-100), dehydrated and embedded in paraffin. Section immunofluorescence of 7 μm paraffin sections was performed as described previously (Wilhelm et al., 2005) with the following antibodies: rabbit anti-SRY (1:100, Wilhelm et al., 2005) rabbit anti-SOX9 (1:300, Wilhelm et al., 2005), rabbit anti-FOXL2 (1:500, (Cocquet et al., 2002), rabbit anti-SF1 (1:1000, kind gift of Dr. Ken-Ichirou Morohashi, Okazaki, NIBB, Japan) and mouse anti-E-Cadherin (1:200, BD Biosciences). For double immunofluorescence, a sheep anti-mouse SOX9 antibody was raised against the same epitope as that raised in rabbit. All secondary antibodies (goat anti-rabbit Alexa Fluor 488, goat anti-rabbit Alexa Fluor 594, goat anti-mouse Alexa Fluor 488, goat anti-mouse Alexa Fluor 594 and goat anti-sheep Alexa Fluor 488) were obtained from Molecular Probes (Invitrogen) and used at 1:200 dilution.
Section in situ hybridization (ISH)
Section ISH was performed as described previously (Wilhelm et al., 2007) with probes for Rspo1 (Parma et al., 2006) and Scc (Yao et al., 2002).
Supplementary Material
Supplementary Figure 1. Rspo1 is expressed at the poles of B6 XYPOS ovotestes. In situ hybridisation (ISH) of adjacent sagittal sections of a paraffin-embedded B6 XYPOS fetus at 13.5 dpc for Rspo1 (middle panel) and Scc to highlight testicular area (right panel). Rspo1 IΣH οφ α B6 ΞΞ φετυσ ισ σηοων φορ χομ παρισον (λεφτ πανελ) Πιχτυρεσ αρε ο ριεντεδ σο τηατ αντεριορ πολε ισ ατ τηε τοπ. Δοττεδ λινεσ δε λινεατε γονδσ. Σχαλε βαρ, 100 μm
Acknowledgments
We are grateful to Lisa Somes for conducting PCR assays for determining the presence of the Y chromosome, Dr. Ken-Ichirou Morohashi (Okazaki, NIBB, Japan) for the SF1 antibody and the histology facility of The Jackson Laboratory for help with processing the samples. This work was supported by a travel grant from the Network in Genes and Environment in Development (NGED) and research grants from the Australian Research Council (ARC) and National Health and Medical Research Council of Australia to PK and the National Institutes of Health (USA) to DW (HD049431) and EE (GM20919 and RR01183). PK is a Federation Fellow of the ARC. Confocal microscopy was performed at the Australian Cancer Research Foundation/Institute for Molecular Bioscience Dynamic Imaging Facility for Cancer Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albrecht K, Eicher E. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Albrecht KH, Young M, Washburn LL, Eicher EM. Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics. 2003;164:277–88. doi: 10.1093/genetics/164.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango N, Lovell-Badge R, Behringer R. Targeted mutagenesis of the endogenous mouse Mis gene promoter: In vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- Bardoni B, Zanaria E, Guioli S, Floridia G, Worley KC, Tonini G, Ferrante E, Chiumello G, McCabe ER, Fraccaro M, et al. A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet. 1994;7:497–501. doi: 10.1038/ng0894-497. [DOI] [PubMed] [Google Scholar]
- Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous Inactivation of Sox9 Causes Complete XY Sex Reversal in Mice. Biol Reprod. 2006;74:195–201. doi: 10.1095/biolreprod.105.045930. [DOI] [PubMed] [Google Scholar]
- Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the male sex determining gene. Nature. 1990;348:448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Hum Mol Genet. 2006;15:417–31. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Biddle FG, Nishioka Y. Assays of testis development in the mouse distinguish three classes of domesticus-type Y chromosome. Genome. 1988;30:870–8. doi: 10.1139/g88-140. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn. 2001;221:201–205. doi: 10.1002/dvdy.1134. [DOI] [PubMed] [Google Scholar]
- Bullejos M, Koopman P. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-YDOM sex reversal. Dev Biol. 2005;278:473–81. doi: 10.1016/j.ydbio.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Burgoyne PS, Lovell-Badge R, Rattigan A. Evidence that the testis determination pathway interacts with a non-dosage compensated, X-linked gene. Int J Dev Biol. 2001;45:509–12. [PubMed] [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mech Dev. 1999;84:127–31. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Capel B, Rasberry C, Dyson J, Bishop CE, Simpson E, Vivian N, Lovell-Badge R, Rastan S, Cattanach BM. Deletion of Y chromosome sequences located outside the testis determining region can cause XY female sex reversal. Nat Genet. 1993;5:301–7. doi: 10.1038/ng1193-301. [DOI] [PubMed] [Google Scholar]
- Chaboissier MC, Kobayashi A, Vidal VI, Lutzkendorf S, van de Kant HJ, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development. 2004;131:1891–901. doi: 10.1242/dev.01087. [DOI] [PubMed] [Google Scholar]
- Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, de Rooij DG, Schedl A, Chaboissier MC. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17:1264–77. doi: 10.1093/hmg/ddn016. [DOI] [PubMed] [Google Scholar]
- Cocquet J, Pailhoux E, Jaubert F, Servel N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M, Veitia RA. Evolution and expression of FOXL2. J Med Genet. 2002;39:916–21. doi: 10.1136/jmg.39.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Südbeck P, Scherer G, Poulat F, Berta P. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol. 1998;18:6653–6665. doi: 10.1128/mcb.18.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher EM, Shown EP, Washburn LL. Sex reversal in C57BL/6J-YPOS mice corrected by a Sry transgene. Philos Trans R Soc Lond B Biol Sci. 1995;350:263–8. doi: 10.1098/rstb.1995.0160. discussion 268–9. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Genetic control of primary sex determination in mice. Annu Rev Genet. 1986;20:327–60. doi: 10.1146/annurev.ge.20.120186.001551. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL. Does one gene determine whether a C57BL/6J-YPOS mouse will develop as a female or as an hermaphrodite? J Exp Zool. 2001;290:322–6. doi: 10.1002/jez.1072. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Schork NJ, Lee BK, Shown EP, Xu X, Dredge RD, Pringle MJ, Page DC. Sex-determining genes on mouse autosomes identified by linkage analysis of C57BL/6J-YPOS sex reversal. Nat Genet. 1996;14:206–9. doi: 10.1038/ng1096-206. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Washburn LL, Whitney JB, 3rd, Morrow KE. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science. 1982;217:535–7. doi: 10.1126/science.7089579. [DOI] [PubMed] [Google Scholar]
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Hersmus R, Kalfa N, de Leeuw B, Stoop H, Oosterhuis JW, de Krijger R, Wolffenbuttel KP, Drop SL, Veitia RA, Fellous M, Jaubert F, Looijenga LH. FOXL2 and SOX9 as parameters of female and male gonadal differentiation in patients with various forms of disorders of sex development (DSD) J Pathol. 2008;215:31–8. doi: 10.1002/path.2335. [DOI] [PubMed] [Google Scholar]
- Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, Morohashi K, Wilhelm D, Koopman P, Kanai Y. A critical time window of Sry action in gonadal sex determination in mice. Development. 2009;136:129–38. doi: 10.1242/dev.029587. [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb A, Bartley J. Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet. 1999;87:349–353. doi: 10.1002/(sici)1096-8628(19991203)87:4<349::aid-ajmg13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Jäger RJ, Anvret M, Hall K, Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990;348:452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–9. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen JS, Gao L. Irx3 is differentially up-regulated in female gonads during sex determination. Gene Expr Patterns. 2005;5:756–62. doi: 10.1016/j.modgep.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Kamachi Y, Cheah KS, Kondoh H. Mechanism of regulatory target selection by the SOX high-mobility-group domain proteins as revealed by comparison of SOX1/2/3 and SOX9. Mol Cell Biol. 1999;19:107–20. doi: 10.1128/mcb.19.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet. 2000;16:182–7. doi: 10.1016/s0168-9525(99)01955-1. [DOI] [PubMed] [Google Scholar]
- Kidokoro T, Matoba S, Hiramatsu R, Fujisawa M, Kanai-Azuma M, Taya C, Kurohmaru M, Kawakami H, Hayashi Y, Kanai Y, Yonekawa H. Influence on spatiotemporal patterns of a male-specific Sox9 activation by ectopic Sry expression during early phases of testis differentiation in mice. Dev Biol. 2005;278:511–25. doi: 10.1016/j.ydbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Lee CH, Taketo T. Normal onset, but prolonged expression, of Sry gene in the B6. YDOM sex-reversed mouse gonad. Dev Biol. 1994;165:442–52. doi: 10.1006/dbio.1994.1266. [DOI] [PubMed] [Google Scholar]
- Lee CH, Taketo T. Low levels of Sry transcripts cannot be the sole cause of B6-YTIR sex reversal. Genesis. 2001;30:7–11. doi: 10.1002/gene.1026. [DOI] [PubMed] [Google Scholar]
- Li Y, Oh HJ, Lau YF. The poly(ADP-ribose) polymerase 1 interacts with Sry and modulates its biological functions. Mol Cell Endocrinol. 2006:257–258. 35–46. doi: 10.1016/j.mce.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Watanabe Y, Inoue J, Semba K. Transcriptional activity of testis-determining factor SRY is modulated by the Wilms’ tumor 1 gene product, WT1. Oncogene. 2003;22:7900–4. doi: 10.1038/sj.onc.1206717. [DOI] [PubMed] [Google Scholar]
- Nagamine CM, Morohashi K, Carlisle C, Chang DK. Sex reversal caused by Mus musculus domesticus Y chromosomes linked to variant expression of the testis-determining gene Sry. Dev Biol. 1999;216:182–94. doi: 10.1006/dbio.1999.9436. [DOI] [PubMed] [Google Scholar]
- Nagamine CM, Taketo T, Koo GC. Studies on the genetics of tda-1 XY sex reversal in the mouse. Differentiation. 1987;33:223–31. doi: 10.1111/j.1432-0436.1987.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–77. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Li Y, Lau YF. Sry associates with the heterochromatin protein 1 complex by interacting with a KRAB domain protein. Biol Reprod. 2005;72:407–15. doi: 10.1095/biolreprod.104.034447. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16:2795–804. doi: 10.1093/hmg/ddm235. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne PS. The Mus musculus domesticus Tdy allele acts later than the Mus musculus musculus Tdy allele: a basis for XY sex-reversal in C57BL/6-YPOS mice. Development. 1991;113:709–14. doi: 10.1242/dev.113.2.709. [DOI] [PubMed] [Google Scholar]
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, Valentini S, Guerra L, Schedl A, Camerino G. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet. 2006;38:1304–9. doi: 10.1038/ng1907. [DOI] [PubMed] [Google Scholar]
- Poulat F, Barbara PS, Desclozeaux M, Soullier S, Moniot B, Bonneaud N, Boizet B, Berta P. The human testis determining factor SRY binds a nuclear factor containing PDZ protein interaction domains. J Biol Chem. 1997;272:7167–72. doi: 10.1074/jbc.272.11.7167. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–42. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274:271–9. doi: 10.1016/j.ydbio.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–4. doi: 10.1038/nature06944. [DOI] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–7. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- Taketo T, Saeed J, Nishioka Y, Donahoe PK. Delay of testicular differentiation in the B6. YDOM ovotestis demonstrated by immunocytochemical staining for mullerian inhibiting substance. Dev Biol. 1991;146:386–95. doi: 10.1016/0012-1606(91)90240-4. [DOI] [PubMed] [Google Scholar]
- Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, Yoshitome A, Yamawaki K, Amagai M, Inoue A, Oshima T, Kakitani M. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17:1278–91. doi: 10.1093/hmg/ddn036. [DOI] [PubMed] [Google Scholar]
- Vidal V, Chaboissier M, de Rooij D, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79:1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Washburn LL, Eicher EM. Sex reversal in XY mice caused by dominant mutation on chromosome 17. Nature. 1983;303:338–40. doi: 10.1038/303338a0. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Hiramatsu R, Mizusaki H, Widjaja L, Combes AN, Kanai Y, Koopman P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J Biol Chem. 2007;282:10553–60. doi: 10.1074/jbc.M609578200. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Koopman P. The makings of maleness: towards an integrated view of male sexual development. Nat Rev Genet. 2006;7:620–31. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287:111–24. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Wilson M, Koopman P. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Curr Opin Genet Dev. 2002;12:441–6. doi: 10.1016/s0959-437x(02)00323-4. [DOI] [PubMed] [Google Scholar]
- Wilson MJ, Jeyasuria P, Parker KL, Koopman P. The transcription factors steroidogenic factor-1 and SOX9 regulate expression of Vanin-1 during mouse testis development. J Biol Chem. 2005;280:5917–23. doi: 10.1074/jbc.M412806200. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev. 2002;16:1433–40. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Rspo1 is expressed at the poles of B6 XYPOS ovotestes. In situ hybridisation (ISH) of adjacent sagittal sections of a paraffin-embedded B6 XYPOS fetus at 13.5 dpc for Rspo1 (middle panel) and Scc to highlight testicular area (right panel). Rspo1 IΣH οφ α B6 ΞΞ φετυσ ισ σηοων φορ χομ παρισον (λεφτ πανελ) Πιχτυρεσ αρε ο ριεντεδ σο τηατ αντεριορ πολε ισ ατ τηε τοπ. Δοττεδ λινεσ δε λινεατε γονδσ. Σχαλε βαρ, 100 μm