Abstract
The role of chemokines in immune function is clearly established. Recent evidence suggests that these molecules also play an important role in the CNS as modulators of neuronal activity. The chemokine CXCL12 has been identified in several regions of the adult rat brain including the substantia nigra, ventral tegmental area and caudate putamen. CXCR4, a receptor activated by CXCL12, is expressed by dopaminergic neurons in the substantia nigra. The present study tested the effects of intracranial injections of CXCL12 on cocaine-induced locomotion and stereotypic activity in adult male Sprague Dawley rats. Results demonstrate that intracerebroventricular administration of CXCL12 (25 ng/4 μl) 15 minutes prior to cocaine (20 mg/kg IP) produced a significant potentiation of both ambulatory and stereotypic activity as compared to cocaine alone. The effects of CXCL12 were blocked by administration of the selective CXCR4 antagonist, AMD 3100. Administration of CXCL12 into specific brain regions was performed to further understand the site of action of CXCL12. Bilateral administration of CXCL12 (25 ng/0.5 μl) into the ventral tegmental area 15 minutes prior to cocaine (20 mg/kg IP) significantly potentiated cocaine-induced ambulatory activity, whereas microinjections of CXCL12 into the caudate putamen selectively increased stereotypy. Conversely, administration of CXCL12 into the lateral shell of the nucleus accumbens resulted in an inhibition of cocaine-stimulated ambulatory activity. No alterations in ambulatory or stereotypic activity were observed following CXCL12 administration into the core of the nucleus accumbens. These results demonstrate that CXCL12 can modulate the behavioral effects produced by cocaine in a brain region-specific manner.
Keywords: chemokine, psychostimulant, CXCR4, dopamine, mesolimbic, nigrostriatal
Chemokines are defined as small (8–14 kDa) chemoattractant cytokines involved in a variety of developmental and pathological conditions. Recent reports propose that endogenous chemokines within the brain act in conjunction with neurotransmitters to modulate brain functions (Adler et al. 2005). The chemokines themselves have been hypothesized to act as transmitters or neuromodulators in neuronal communication and have been shown to alter the actions of neuronally-active pharmacological agents such as opioids and cannabinoids (Steele et al. 2002; Adler et al. 2005; Burbassi et al. 2008).
CXCL12, formerly known as stromal cell derived factor one alpha (SDF-1α), is a CXC category chemokine secreted by bone marrow stromal cells as well as populations of neuronal and non-neuronal cells within the adult rat brain (Stumm et al. 2002). CXCL12 is involved in embryogenesis, cell migration and development of the brain, heart and large blood vessels (Moepps et al. 2000; Kucia et al. 2004). Until recently, CXCL12 was thought to signal through a single receptor, CXCR4. Vital during pre-natal development, CXCL12 or CXCR4 knock-out animals develop lethal cardiovascular, neuronal and blood vessel defects and die soon after birth (Ma et al. 1998; Zou et al. 1998). A second receptor that binds CXCL12, CXCR7 (orphan receptor RDC1), was identified via radio-labeled CXCL12 binding in vitro (Balabanian et al. 2005; Burns et al. 2006). Both CXCR4 and CXCR7 are G-protein coupled receptors that contain highly conserved transmembrane regions.
Studies have demonstrated the presence of CXCR4 mRNA in cultured rat neurons, astrocytes, glia and microglia (Ohtani et al. 1998; Bajetto et al. 1999). Both CXCL12 and CXCR4 have been found in regions of the adult rat brain including the cerebral cortex, caudate putamen and white matter of the corpus callosum (Banisadr et al. 2003). CXCR4 has been shown via immunohistochemistry to be localized within the substantia innominata in the basal forebrain, the caudate putamen and globus pallidus within the basal ganglia, and the substantia nigra and ventral tegmental area within the midbrain (Banisadr et al. 2002), all areas rich in dopaminergic neurons. Of note, CXCR4 has not been detected in the nucleus accumbens (Banisadr et al. 2002), an area highly innervated by dopaminergic terminals. Double immunostaining for CXCR4 and tyrosine hydroxylase, the rate-limiting enzyme in the synthesis of dopamine, revealed that the majority of tyrosine hydroxylase-positive neurons in the substantia nigra were also immunoreactive for CXCR4, indicating that CXCR4 is expressed by dopaminergic neurons in this brain region (Banisadr et al. 2002). Two of the major dopaminergic tracks within the brain are the mesolimbic and nigrostriatal dopamine pathways. A major component of the mesolimbic dopamine system links the ventral tegmental area to the nucleus accumbens. The ventral tegmental area is comprised of dopamine-, GABA- and glutamate-containing neurons while the nucleus accumbens is comprised mainly (95%) of GABAergic medium spiny neurons. Most of the remaining neurons of the nucleus accumbens are cholinergic interneurons. The dopaminergic neurons projecting from the ventral tegmental area to the nucleus accumbens are involved in locomotor activity (Mogenson et al. 1979; Kalivas et al. 1981). The nucleus accumbens in conjunction with the ventral tegmental area are targets of psychostimulant drugs and have been widely implicated in mediating the rewarding and locomotor-stimulating actions of psychostimulants (Koob 1992). The nigrostriatal pathway consists of dopaminergic cell bodies in the substantia nigra which innervates the caudate putamen. Enhanced dopaminergic neurotransmission in the caudate putamen following psychostimulant administration is associated with hyperactivity, especially an increase in stereotypic behaviors (Asher and Aghajanian 1974; Kuczenski et al. 1991; Ushijima et al. 1995). Of particular relevance to the present study, administration of CXCL12 into the substantia nigra has been shown to increase both contralateral turning and extracellular dopamine within the caudate putamen (Skrzydelski et al. 2007).
Extensive research demonstrates the ability of cocaine to influence the activity of the mesolimbic and nigrostriatal dopamine systems. Cocaine blocks the dopamine transporter thereby inhibiting the reuptake of dopamine (Heikkila et al. 1975). By inhibiting the dopamine transporter, excess synaptic dopamine accumulates in the nucleus accumbens and caudate putamen thereby altering many functions including motivation, attention, reward and locomotor activity (Reith 1986; Ritz et al. 1987).
The present study investigated the potential of CXCL12, when injected into specific regions of the mesolimbic and nigrostriatal dopamine pathways, to modulate cocaine-induced behaviors. Results demonstrate site-specific actions of CXCL12 and its ability to modulate the locomotor- and stereotypic-activating effects of cocaine.
Experimental Procedures
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were utilized in all experiments. Animals were housed on a 12 h light-dark cycle (7 AM – 7 PM) with ad libitum access to food and water in groups of four per cage. Weight range upon arrival was 250–300 g and upon testing was 300–350 g. Animals were allowed to acclimate to the animal facility for one week prior to surgery. Following surgery to implant indwelling intracranial cannula, animals were individually housed. Experimental testing began three days after surgery. Separate groups of rats were used for each study conforming to a between-subjects design. No animal was tested more than once. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Experimental protocols were approved by the Institutional Animal Care and Use Committee of Temple University School of Medicine.
Drugs
Telazol (tiletamine/zolazepam) was purchased from Fort Dodge Animal Health (Overland Park, KS, USA). Recombinant mouse CXCL12/SDF-1α was obtained from R&D Systems (Minneapolis, MN, USA, 10 μg). Cocaine hydrochloride was generously supplied by the National Institute of Drug Abuse (Bethesda, MD, USA). Cocaine (20 mg/mL) and CXCL12 (12.5 – 200 ng/dose) were dissolved in sterile normal saline (0.9%) before use. AMD 3100 (2 μg/dose, octahydrochloride hydrate) was obtained from Sigma Aldrich (St. Louis, MO, 5 mg) and was dissolved in sterile normal saline (0.9%) before use.
Surgery
Rats were anesthetized with Telazol (40 mg/kg IP) and placed in a Kopf stereotaxic apparatus. Intracerebroventricular (ICV) cannulae were constructed of polyethylene tubing (PE 20, PE 10) and intracranial injection cannulae were constructed of stainless steel (22 GA guide, Plastics One, Roanoke, VA). A unilateral ICV cannula was inserted into the right lateral ventricle for the initial experiment. For subsequent experiments, cannulae were inserted bilaterally into the ventral tegmental area, caudate putamen, nucleus accumbens lateral shell or nucleus accumbens core. The following stereotaxic coordinates were utilized: ICV: A/P −0.9, M/L −1.4, V −4.0; caudate putamen: A/P 1.2, M/L ±2.4, V −4.7; lateral accumbens shell: A/P 1.3, M/L ±3.1, V −7.5; nucleus accumbens core: A/P 1.7, M/L ±1.5, V −6.8; ventral tegmental area: A/P −6.0, M/L ±0.7, V −8.0; and the incisor bar was adjusted to achieve a flat skull position (−3.9± 0.5) as determined according to the atlas of Paxinos and Watson (Paxinos and Watson 2007). The cannulae were secured with dental acrylic anchored to a stainless steel surgical screw inserted into the skull. Stainless steel stylets, 1.0 mm longer than the guide cannulae, were inserted to keep them free of debris. Cannula placements were verified at the conclusion of the experiment.
ICV and intracranial injections
Rats were placed into the activity monitors for 30 minutes prior to receiving an ICV or intracranial injection of either CXCL12 or normal saline. Injection volumes were 4 μl for ICV administration and 0.5 μl for intracranial injections. Injection cannulas were 28 GA and extended 1 mm below the 22 GA guide cannula (Plastics One, Roanoke, VA). Solutions were injected by hand at a rate of 1 μl/min with a Hamilton syringe connected to the cannula by polyethylene tubing. Rats were not restrained during injections. Fifteen minutes later, cocaine (20 mg/kg) or normal saline (1 mL/kg) was administered by the intraperitoneal (IP) route. Activity was recorded for 120 minutes post IP injection as described below. In one cohort of rats, AMD 3100 was administered via the ICV cannula (2 μg/4 μl) sixty minutes prior to an ICV injection of either CXCL12 or normal saline (Fricker et al. 2006). Fifteen minutes later, animals received an IP injection of either cocaine (20 mg/kg) or saline. Activity was measured for 120 minutes post IP injection as described below.
Behavioral activity
Activity was measured using a Digiscan D Micro System (Accuscan, Columbus, OH) and eight individual activity monitors. A single activity monitor consists of an aluminum frame equipped with 16 infrared light beams and detectors whereby a standard clear plastic animal cage (20 × 20 × 42 cm) is placed. As the animal moves within the plastic cage, light beams are broken and recorded by a computer connected to the Digiscan system. Activity was recorded as total activity, ambulatory activity and stereotypy. Total activity represents all beam breaks compiled by a single animal, and is the sum of the ambulatory and stereotypy counts. Ambulatory activity represents successive beam breaks while stereotypy counts are defined as bouts of repetitive beam breaks. Stereotypic counts do not identify specific stereotypic behavior, rather repeated breaks of the same beam indicative of a stationary animal engaged in a repetitive behavior as opposed to ambulation. Activity was measured over 120 minutes with 5 minute collection intervals.
Cannula placement
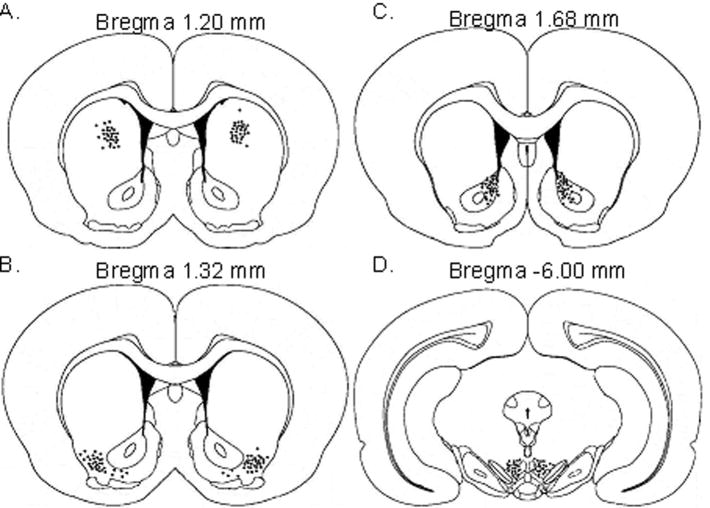
At the conclusion of each study, ICV cannula placements were verified by injecting 4 μL of methylene blue (Taylor Pharmaceuticals, Decatur, IL) into the cannula. Thirty minutes later, rats were decapitated and inspected for cannula placement. A positive result for correct ICV cannula placement was evident by even distribution of dye throughout the ventricles of the brain. For intracranial cannula placements, brains were excised, frozen and cut in 40 μM sections for determination of cannula placements. Final depths of individual cannula for all animals are marked by an enclosed circle in Figure 1. The rostral-caudal extent of cannula locations from bregma for each brain region investigated were as follows: caudate putamen 1.08 mm to 1.32 mm, lateral accumbens shell 1.22 mm to 1.44 mm, nucleus accumbens core 1.56 mm to 1.80 mm and ventral tegmental area −5.88 mm to −6.12 mm.
Figure 1. Cannula placements.
Injection sites for A) caudate putamen, B) lateral accumbens shell C) nucleus accumbens core, and D) ventral tegmental area. Locations of the tip of the cannulae are indicated by black circles. Figures reproduced from Paxinos and Watson (2007) with permission.
Data analysis
Data are expressed as the mean ± S.E.M. Data were examined for significance by repeated measures ANOVA followed by Bonferroni post-tests (GraphPad Prism V.4). Statistical significance was achieved when p<0.05.
Results
ICV administration of CXCL12 and cocaine-induced activity
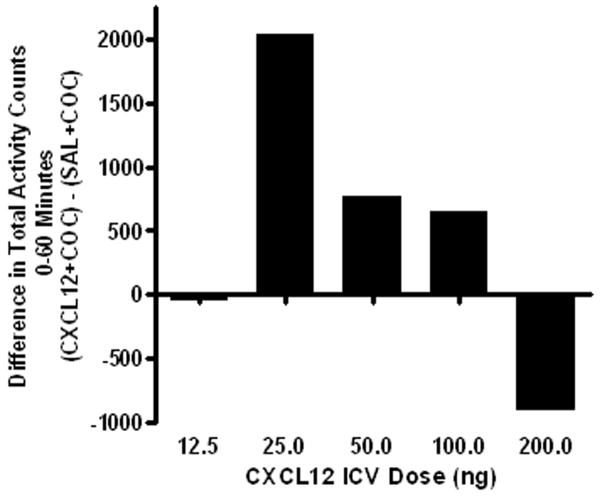
CXCL12 was administered ICV 15 minutes prior to an injection of cocaine (20 mg/kg). Using individual cohorts of rats, a range of doses for CXCL12 were evaluated including 12.5, 25, 50, 100 and 200 ng in 4 μL of sterile saline (0.9%). Differences in the mean total activity counts from 0 to 60 minutes between the rats receiving CXCL12 ICV plus cocaine and those receiving saline ICV plus cocaine for 5 doses of CXCL12 are shown in Figure 2. The differences between groups were calculated to determine which dose of CXCL12 provided the largest increase in cocaine-induced activity. The greatest potentiation of cocaine-induced activity was seen with the 25 ng dose of CXCL12.
Figure 2. Effect of ICV CXCL12 on cocaine-induced activity.
. CXCL12 (12.5 - 200 ng/4 μl) was administered ICV 15 minutes prior to cocaine (20 mg/kg IP), and activity was measured for 60 minutes. Data are expressed as the difference in total activity counts ([CXCL12 ICV + cocaine IP] minus [saline ICV + cocaine IP]). Results show that 25 ng of CXCL12 produced the greatest increase in cocaine-induced activity. N= 4 – 8 per treatment group.
CXCL12 potentiated cocaine-induced activity which was blocked by AMD 3100
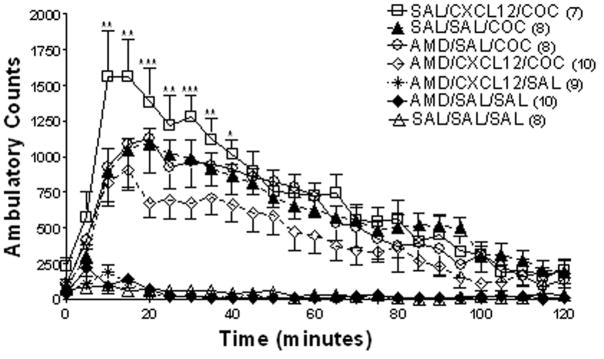
To establish that CXCL12 was producing its effects through CXCR4, the selective CXCR4 antagonist AMD 3100 (0.2 or 2 μg/4 μl ICV) was administered 60 minutes prior to CXCL12 (25 ng/4 μl ICV) followed by either cocaine (20 mg/kg IP) or saline. Repeated measures ANOVA showed a significant difference between groups (F(6,174) = 28.66, p<0.0001) (Fig. 3). Bonferroni post hoc analysis confirmed the ability of CXCL12 to potentiate the ambulatory activity of cocaine (SAL/CXCL12/COC vs. SAL/SAL/COC; p<0.05). ICV AMD 3100 (2 μg/4 μl ICV) prior to CXCL12 and cocaine administration inhibited the potentiation of cocaine-induced ambulatory activity (AMD3100/CXCL12/COC vs. SAL/CXCL12/COC; *p<0.05, **p<0.01, ***p<0.001, Fig. 3). Administration of the lower dose of AMD 3100 (0.2 μg/4 μl ICV) had no significant effect on activity alone (AMD/SAL/SAL vs. SAL/SAL/SAL p>0.05) or on cocaine-induced activity (AMD/SAL/COC vs. SAL/SAL/COC p>0.05). These results demonstrate that CXCL12, by activating CXCR4 receptors, can enhance cocaine-induced activity.
Figure 3. Effect of ICV AMD 3100 and CXCL12 on cocaine-induced ambulatory activity.
The selective CXCR4 antagonist AMD 3100 (2 μg/4 μl) was administered 60 minutes prior to administration of CXCL12 (25 ng/4 μl ICV), and then followed by saline or cocaine (20 mg/kg IP). Repeated measures ANOVA followed by Bonferroni post-hoc analysis showed a significant difference between the AMD/CXCL12/COC and the SAL/CXCL12/COC groups indicating that AMD 3100 blocked the CXCL12-induced increase in cocaine-stimulated ambulatory activity. Data are expressed as mean ± SEM beam breaks/five minute period. N= 7 – 10 per group as indicated in parentheses on the figure for each group. (AMD/CXCL12/COC vs. SAL/CXCL12/COC; *p<0.05, **p<0.01, ***p<0.001)
CXCL12 administration into the ventral tegmental area
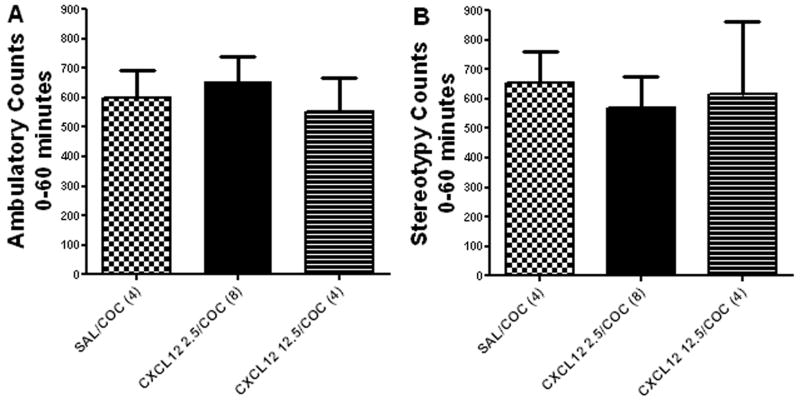
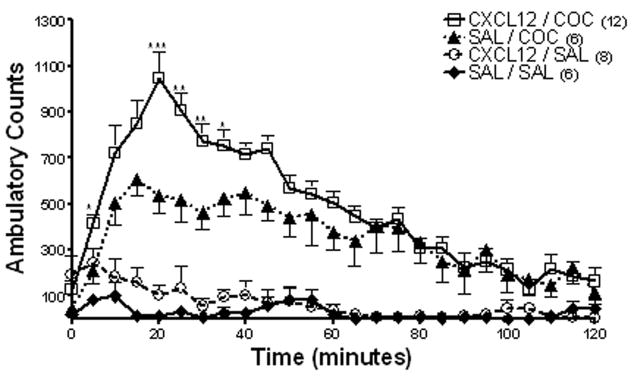
Microinjections of CXCL12 into the ventral tegmental area in conjunction with IP injections of cocaine were used to evaluate their interactions within the cell body region of the mesolimbic dopamine system. Doses of 2.5, 12.5 and 25 ng CXCL12 injected bilaterally into the ventral tegmental area were tested with and without cocaine (20 mg/kg IP). Results demonstrated that 2.5 and 12.5 ng CXCL12 failed to illicit a significant difference between cohorts receiving CXCL12 plus cocaine as compared to cocaine alone (Fig. 4; ANOVA: ambulatory activity F(2,15) = 0.27, p>0.05; or stereotypy F(2,15) = 0.09, p>0.05). However, 25 ng/0.5 μl CXCL12 injected bilaterally into the ventral tegmental area significantly enhanced cocaine-induced ambulatory activity. Repeated measures ANOVA showed a significant difference in ambulatory activity between the treatment groups (F(3,99) = 70.12, p<0.0001) (Fig. 5). Bonferroni post hoc analysis revealed that bilateral administration of CXCL12 (25 ng/0.5 μl) into the ventral tegmental area concomitantly with cocaine (20 mg/kg IP) resulted in significantly higher ambulatory activity as compared to cocaine alone (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001). CXCL12 alone had no effect on ambulatory activity (CXCL12/SAL vs. SAL/SAL; p>0.05).
Figure 4. Dose-response of CXCL12 administered into the ventral tegmental area.
Administration of CXCL12 (2.5 or 12.5 ng/0.5 μl) bilaterally into the ventral tegmental area concomitantly with cocaine failed to produce a significant increase in A) ambulatory activity or B) stereotypy when compared rats receiving cocaine alone. N = 4 – 8 per group as indicated for each in parentheses. (SAL/COC vs. CXCL12 2.5/COC or CXCL12 12.5/COC; p>0.05.)
Figure 5. Effect of CXCL12 administration into the ventral tegmental area on ambulatory activity.
Administration of CXCL12 (25 ng/0.5 μl) into the ventral tegmental area 15 minutes prior to cocaine (20 mg/kg IP) resulted in a significant increase in ambulatory activity as compared to cocaine alone. Data are expressed as mean ± SEM beam breaks/five minute period. N= 6 – 11 per group as indicated for each. (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001)
In contrast to its effect on ambulatory activity, CXCL12 injected directly into the ventral tegmental area did not alter cocaine-induced stereotypic activity. Stereotypy was significantly different between treatment groups (F(3,99) = 15.83, p<0.0001; data not shown). Administration of cocaine alone increased stereotypy counts as compared to saline controls (SAL/COC vs. SAL/SAL p<0.05). Post hoc analysis showed no significant difference in the stereotypic activity of rats administered CXCL12 (25 ng/0.5 μl) into the ventral tegmental area and cocaine (20 mg/kg IP) as compared to cocaine alone (CXCL12/COC vs. SAL/COC p>0.05). CXCL12 administered into the ventral tegmental area alone did not significantly alter stereotypy (CXCL12/SAL vs. SAL/SAL p>0.05).
CXCL12 administration into the caudate putamen
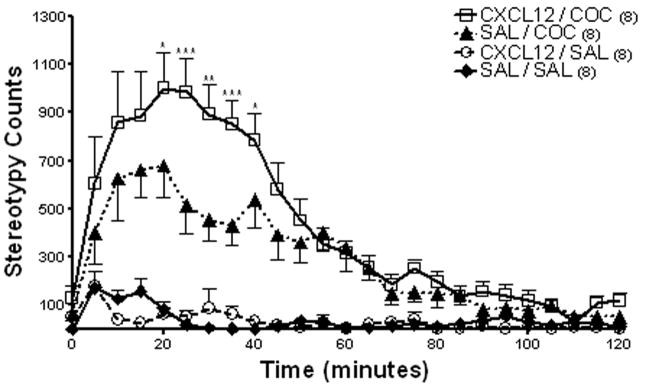
Bilateral administration of CXCL12 (25 ng/0.5 μl) into the caudate putamen 15 minutes prior to cocaine (20 mg/kg IP) resulted in a potentiation in stereotypy as compared to cocaine alone (Fig. 6). Repeated measures ANOVA showed a significant difference between treatment groups (F(3,99) = 36.31, p<0.0001). Bonferroni post hoc analysis showed that CXCL12 administration into caudate putamen prior to a systemic injection of cocaine produced greater stereotypy than that produced by cocaine alone (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001).
Figure 6. Effect of CXCL12 administration into the caudate putamen on stereotypy activity.
Administration of CXCL12 (25 ng/0.5 μl) into the caudate putamen 15 minutes prior to cocaine (20 mg/kg IP) resulted in significantly higher stereotypic activity as compared to cocaine alone. Data are expressed as mean ± SEM beam breaks/five minute period. N= 8 per group. (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001)
Ambulatory activity was significantly different between treatment groups (F(3,99) = 33.76, p<0.0001; data not shown). Administration of cocaine (20 mg/kg IP) produced a significant increase in ambulatory activity as compared to saline controls (SAL/COC vs. SAL/SAL p<0.05). Administration of CXCL12 (25 ng/0.5 μl) into the caudate putamen concomitantly with cocaine (20 mg/kg IP) produced a similar increase in ambulatory activity as that produced by cocaine (20 mg/kg IP) alone (CXCL12/COC vs. SAL/COC p>0.05). CXCL12 (25 ng/0.5 μl) administered into the caudate putamen alone did not significantly alter ambulatory or stereotypy activity as compared to saline controls (CXCL12/SAL vs. SAL/SAL p>0.05).
CXCL12 administration into the nucleus accumbens lateral shell
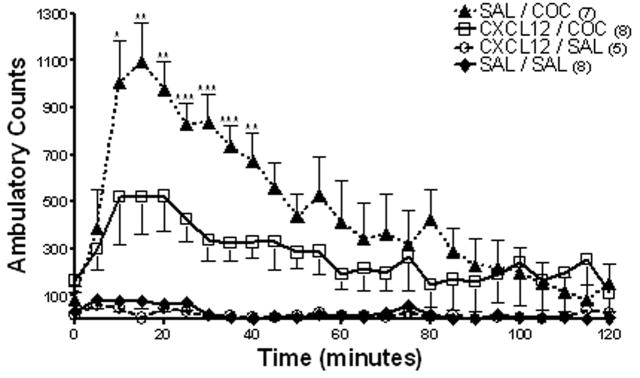
Bilateral administration of CXCL12 (25 ng/0.5 μl) into the lateral accumbens shell 15 minutes prior to cocaine (20 mg/kg IP) resulted in an inhibition in ambulatory activity as compared to cocaine alone (Fig. 7). Repeated measures ANOVA showed a significant difference in ambulatory activity between the groups (F(3,99) = 38.45, p<0.0001). Bonferroni post hoc analysis revealed that CXCL12 administration into the lateral accumbens shell prior to a systemic injection of cocaine produced significantly less ambulatory activity as compared to cocaine alone (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001).
Figure 7. Effect of CXCL12 administration into the lateral accumbens shell on ambulatory activity.
Administration of CXCL12 (25 ng/0.5 μl) into the lateral accumbens shell 15 minutes prior to cocaine (20 mg/kg IP) produced a significant inhibition in ambulatory activity as compared to cocaine alone. Data are expressed as mean ± SEM beam breaks/five minute period. N= 5 – 8 per group as indicated for each. (CXCL12/COC vs. SAL/COC; *p<0.05, **p<0.01, ***p<0.001)
CXCL12 injected into the lateral accumbens shell did not alter cocaine-induced stereotypy. Stereotypy was significantly different between treatment groups (F(3,99) = 36.04, p<0.0001; data not shown). Administration of cocaine (20 mg/kg IP) significantly increased stereotypy (SAL/COC vs. SAL/SAL p<0.05). Administration of CXCL12 (25 ng/0.5 μl) into the lateral accumbens shell concomitantly with cocaine (20 mg/kg IP) did not alter cocaine-induced stereotypy (CXCL12/COC vs. SAL/COC p>0.05). CXCL12 (25 ng/0.5 μl) administered into the lateral accumbens shell alone did not significantly alter ambulatory activity or stereotypy as compared to saline controls (CXCL12/SAL vs. SAL/SAL p>0.05).
CXCL12 administration into the core of the nucleus accumbens
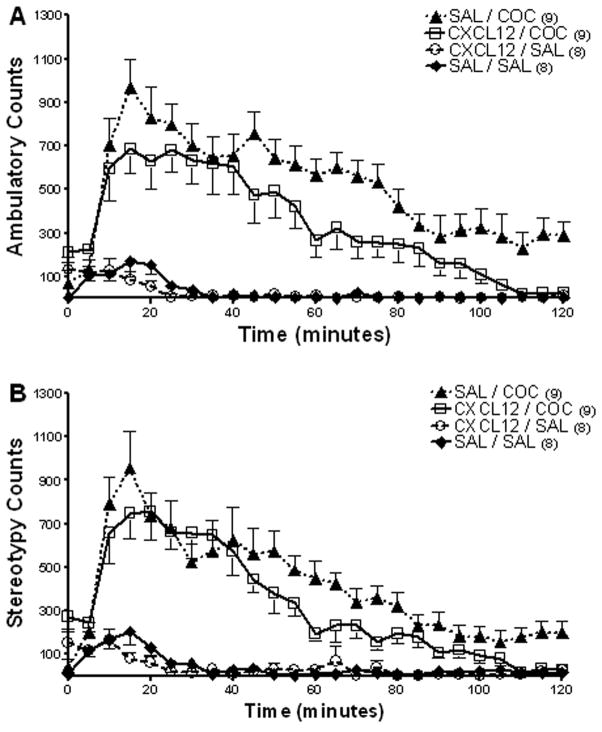
Bilateral administration of CXCL12 (25 ng/0.5 μl) into the core of the nucleus accumbens 15 minutes prior to cocaine (20 mg/kg IP) did not alter cocaine-induced ambulatory activity or stereotypy (Fig. 8A, 8B). Repeated measures ANOVA showed there was a significant difference in ambulatory activity between groups (F(3,99) = 73.48, p<0.0001). Bonferroni post hoc analyses of ambulatory activity showed CXCL12 administration into the core of the nucleus accumbens prior to a systemic injection of cocaine was not significantly different than cocaine alone (CXCL12/COC vs. SAL/COC; p>0.05, Fig. 8A). CXCL12 (25 ng/0.5 μl) administered into the core of the nucleus accumbens alone did not significantly alter ambulatory activity as compared to saline controls (CXCL12/SAL vs. SAL/SAL p>0.05).
Figure 8. Effect of CXCL12 administration into the core of the nucleus accumbens on ambulatory activity and stereotypy.
. Administration of CXCL12 (25 ng/0.5 μl) into the core of the nucleus accumbens 15 minutes prior to cocaine (20 mg/kg IP) did not significantly alter A) ambulatory activity or B) stereotypy as compared to the cocaine alone. Data are expressed as mean ± SEM beam breaks/five minute period. N= 8 – 9 per group as indicated for each. (CXCL12/COC vs. SAL/COC; p>0.05)
Repeated measures ANOVA showed a significant difference in stereotypy between the groups (F(3,99) = 30.32, p<0.0001). Bonferroni post hoc analyses showed cocaine (20 mg/kg IP) significantly increased stereotypy (SAL/COC vs. SAL/SAL p<0.05) however CXCL12 administration into the core of the nucleus accumbens did not significantly alter cocaine induced stereotypy (CXCL12/COC vs. SAL/COC; p>0.05, Fig. 8B). CXCL12 (25 ng/0.5 μl) administered into the core of the nucleus accumbens alone did not significantly alter stereotypy as compared to saline controls (CXCL12/SAL vs. SAL/SAL p>0.05).
Discussion
The present study evaluated the ability of CXCL12 to modulate the behavioral effects of cocaine. In the initial studies, CXCL12 was administered ICV. Ambulatory and stereotypic activities of rats administered concomitant ICV CXCL12 and IP cocaine were significantly higher than those given cocaine alone. The selective CXCR4 receptor antagonist AMD 3100 prevented CXCL12 from increasing cocaine-induced activity indicating that CXCL12 potentiated the effects of cocaine by activating CXCR4 receptors. AMD 3100 had no effect on baseline activity at the doses tested suggesting that CXCR4 does not tonically regulate activity under normal physiological conditions. AMD 3100 is a CXCR4 receptor antagonist and does not inhibit calcium influx, stimulate guanosine triphosphate binding or have any effects on chemotaxis (Fricker et al. 2006). Although AMD 3100 alone failed to alter baseline activity in the present study, it should be noted that baseline activity was low as it usually is when measured during the animals’ light cycle (inactive period), therefore any suppressant effect of AMD 3100 on activity could have been masked.
Hyperactivity produced by cocaine is due to its ability to enhance dopaminergic neurotransmission (Kuczenski et al. 1991). Therefore, one potential mechanism by which CXCL12 could potentiate cocaine-stimulated activity is through an interaction with dopaminergic neurons. Previous studies have demonstrated that CXCR4 is expressed by dopaminergic neurons within the substantia nigra (Banisadr et al. 2002; Banisadr et al. 2003). Here, cocaine-induced activity was used to broaden the understanding of CXCL12/CXCR4 pathways in the brain and investigate their interaction with dopaminergic neurons. Based upon the initial findings of a potentiation of cocaine-induced activity following ICV administration of CXCL12, subsequent studies investigated specific brain regions to determine the site of action of CXCL12. Using individual cohorts of animals, CXCL12 was injected bilaterally into the ventral tegmental area, caudate putamen, lateral accumbens shell and core of the nucleus accumbens both alone and in combination with cocaine, and the effects on activity determined. These findings are summarized in Table 1. It was somewhat surprising that the effective dose (i.e., 25 ng) of CXCL12 to enhance cocaine-stimulated activity was similar when administered ICV and into specific brain regions such as the ventral tegmental area. However, dose-response curves for both were performed which help to validate the findings. Further, the effective dose of CXCL12 when administered into the ventral tegmental area was similar to that published by Skrzydelski and colleagues. In their study, 50 ng CXCL12 was injected into the substantia nigra and this resulted in an increase in both contra-lateral turning and extracellular dopamine in the caudate putamen (Skrzydelski et al. 2007).
Table 1.
Modulation of cocaine-induced ambulatory and stereotypic activity by CXCL12 (25 ng)
| Brain Region | Ambulatory | Stereotypy |
|---|---|---|
| Lateral Ventricle | + | + |
| Ventral Tegmental Area | + | 0 |
| Caudate Putamen | 0 | + |
| Nucleus Accumbens | ||
| Core | 0 | 0 |
| Lateral Shell | − | 0 |
increase in cocaine-induced activity (p<0.05),
decrease in cocaine-induced activity (p<0.05); 0, no change in activity(p>0.05)
The ventral tegmental area has previously been shown to be an important site for mediating many of the behavioral effects of cocaine, including its reinforcing and locomotor-activating effects (Roberts and Koob 1982; Bozarth and Wise 1986). Dopaminergic cell bodies of the mesolimbic dopamine pathway originate in the ventral tegmental area and project to limbic forebrain regions including the nucleus accumbens. Positive CXCR4-immunoreactivity and CXCR4 mRNA have been identified in the ventral tegmental area (Banisadr et al. 2002). In the current study, a significant potentiation of cocaine-induced ambulatory activity was observed when CXCL12 was injected into the ventral tegmental area prior to cocaine. Cocaine-induced stereotypy was not affected by intra-ventral tegmental area injection of CXCL12. Although the mechanism by which CXCL12 could enhance the behavioral effects of cocaine has not been elucidated, it is possible that CXCL12 is activating CXCR4 receptors on dopaminergic neurons within the ventral tegmental area, stimulating a calcium current within these neurons and thus increasing dopamine release in the terminal regions. Another possibility involves the co-expression of CXCR4 on GABAergic interneurons of the ventral tegmental area (Banisadr et al. 2002). Activation of CXCR4 receptors could inhibit these GABAergic neurons, thus causing a disinhibition of the dopaminergic pathway.
The effects of CXCL12 administered directly into the terminal areas of the mesolimbic dopamine pathway were also evaluated. In contrast to its action in the ventral tegmental area, a significant decrease in cocaine-induced ambulatory activity was seen when CXCL12 was injected into the lateral shell of the nucleus accumbens. Stereotypy was unaltered by CXCL12 into the accumbens shell, similar to the ventral tegmental area. Preliminary results from our laboratory have provided evidence of CXCR4 immunoreactivity in the lateral shell of the nucleus accumbens (unpublished data). The nucleus accumbens contains a high percentage of GABAergic medium spiny neurons, some of which project back to the ventral tegmental area. If CXCR4 were present on these GABAergic neurons within the nucleus accumbens, activation of this receptor could cause feedback inhibition of dopaminergic neurons in the ventral tegmental area thus affecting subsequent release of dopamine (Guyon and Nahon 2007). In contrast to its effects in the lateral shell of the accumbens, administration of CXCL12 into the core of the nucleus accumbens did not affect cocaine-induced ambulatory or stereotypy activity. A previous report suggests that the core of the nucleus accumbens is devoid of CXCR4 receptors (Banisadr et al. 2002), and our preliminary immunohistochemistry data agree with this assessment (unpublished data). Thus, the present finding of no effect of CXCL12 when administered into the core of the nucleus accumbens agree with the lack of CXCR4 receptors in this brain region.
Previous research has shown that administration of CXCL12 into the substantia nigra induces contralateral turning and increases extracellular dopamine concentrations in the striatum (Skrzydelski et al. 2007), providing evidence that activation of CXCR4 can augment dopaminergic transmission in the nigrostriatal pathway. Studies have also shown that stereotypic activity is primarily controlled by the caudate putamen (Asher and Aghajanian 1974; Ushijima et al. 1995). In the present study, administration of CXCL12 into the terminal region of the nigrostriatal pathway, the caudate putamen, significantly potentiated cocaine-induced stereotypy.
CXCR4 is localized in the substantia nigra on both dopaminergic neurons and GABA axonal processes (Banisadr et al. 2002). CXCL12, by activating CXCR4, can influence the function of dopaminergic neurons through its actions on GABA- and glutamate transmission (Guyon et al. 2006) and can increase intracellular calcium via N-type calcium currents (Zheng et al. 1999; Guyon et al. 2005). Multiple pre- and post-synaptic effects of CXCL12 on dopamine neurons in the substantia nigra have been shown including (1) an increase in the frequency of GABAA postsynaptic events in response to CXCL12 produced by activation of CXCR4 receptors on GABAergic neurons of the substantia nigra, (2) a glutamatergic inward current likely due to glutamate release from non-neuronal cells, (3) CXCL12 activation of a G-protein activated inwardly rectifying K+ channel (GIRK) current via CXCR4 and (4) in increase in the amplitude of total high voltage-activated calcium currents through CXCR4 activation at low concentrations of CXCL12 (.1–10 nM) (Guyon et al. 2006; Guyon and Nahon 2007). Together, these effects of CXCL12 in the substantia nigra result in increased dopamine release in the striatum (Skrzydelski et al. 2007).
In the present investigation, a dose-response study was performed to determine the optimum dose of CXCL12 ICV to alter cocaine-induced activity. Results revealed an inverted U-shaped dose-response function. This is in agreement with previous studies that have documented dose-dependent actions of CXCL12. For example, CXCL12 at concentrations of 0.1 – 1.0 nM depolarizes dopaminergic neurons by inducing the stimulation of glutamate receptors, whereas high concentrations (≥10 nM) hyperpolarize dopaminergic neurons by stimulating GABA receptors (Guyon et al. 2006). CXCL12 has also been shown to exert a biphasic effect on high voltage-activated calcium currents of dopaminergic neurons: a potentiation at low concentrations (0.1–10 nM) and a depression at higher concentrations (50–100 nM) (Banisadr et al. 2005; Guyon et al. 2006). Results from the present study with CXCL12 (25 ng) are consistent with the effects seen at low concentrations in terms of potentiating calcium currents of dopaminergic neurons (Guyon et al. 2006). Our finding that higher doses of CXCL12 were not effective in potentiating cocaine-induced activity may be due to its reported depression of calcium currents at high concentrations or activation of CXCR7 in addition to CXCR4 at higher doses. It has been shown in cultures of dopaminergic neurons, that the effect of high concentrations of CXCL12 (up to 1 μM) are not blocked by AMD 3100 (Guyon and Nahon 2007). Other possibilities include differential binding to various sites on the CXCR4 receptor, truncation of CXCL12 by proteases leading to active peptides that could differentially bind CXCR4 or activation of the CXCR7 receptor. Recent data have identified CXCR7 mRNA expression within the shell of the accumbens and the caudate putamen (Schonemeier et al. 2008). Alternatively, evidence of desensitization and internalization of CXCR4 at high concentrations of CXCL12 (Haribabu et al. 1997; Stumm and Hollt 2007) could also help explain the inverted U-shaped dose-response curve.
These results presented herein demonstrate that CXCL12 can modulate the behavioral effects of cocaine differently based upon its site of action within the brain. CXCL12, acting through its receptor CXCR4, can influence the function and behavioral output of both the mesolimbic and nigrostriatal dopamine pathways. Furthermore, the ability of CXCL12 to increase stereotypy when injected into the caudate putamen but inhibit locomotion following administration into the lateral accumbens shell demonstrates that activation of CXCR4 on different neuronal populations inherently dictates its effect on behavior. The influence of CXCL12 on these dopaminergic systems provides further evidence of neurotransmitter/chemokine interactions in the brain. Future research into the central mechanisms of CXCL12 and both the CXCR4 and CXCR7 receptors is needed to elucidate the variety of cellular interactions and impact this chemokine has within the adult brain.
Acknowledgments
We would like to thank Dr. Martin W. Adler for his expert advice, and Dr. Khalid Benamar for his technical guidance. This work was supported by F31 DA024516 (JT), T32 DA07237 (EMU), P30 DA13429 (MW Adler/EMU) and PA Dept. of Health (EMU)
Abbreviations
- ANOVA
analysis of variance
- CXCL12
formally known as SDF-1α (stromal cell derived factor one alpha)
- GABA
gamma-aminobutyric acid
- ICV
intracerebral ventricular
- IP
intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Adler MW, Geller EB, Chen X, Rogers TJ. Viewing chemokines as a third major system of communication in the brain. AAPS J. 2005;7(4):E865–70. doi: 10.1208/aapsj070484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher IM, Aghajanian GK. 6-Hydroxydopamine lesions of olfactory tubercles and caudate nuclei: effect on amphetamine-induced stereotyped behavior in rats. Brain Res. 1974;82(1):1–12. doi: 10.1016/0006-8993(74)90888-9. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Piccioli P, Costa A, Florio T, Schettini G. Glial and neuronal cells express functional chemokine receptor CXCR4 and its natural ligand stromal cell-derived factor 1. J Neurochem. 1999;73(6):2348–57. doi: 10.1046/j.1471-4159.1999.0732348.x. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280(42):35760–6. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16(9):1661–71. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18(6):1593–606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines and brain functions. Curr Drug Targets Inflamm Allergy. 2005;4(3):387–99. doi: 10.2174/1568010054022097. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. NIDA Res Monogr. 1986;67:190–6. [PubMed] [Google Scholar]
- Burbassi S, Aloyo VJ, Simansky KJ, Meucci O. GTPgammaS incorporation in the rat brain: a study on mu-opioid receptors and CXCR4. J Neuroimmune Pharmacol. 2008;3(1):26–34. doi: 10.1007/s11481-007-9083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker SP, Anastassov V, Cox J, Darkes MC, Grujic O, Idzan SR, Labrecque J, Lau G, Mosi RM, Nelson KL, Qin L, Santucci Z, Wong RS. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72(5):588–96. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Guyon A, Rovere C, Cervantes A, Allaeys I, Nahon JL. Stromal cell-derived factor-1alpha directly modulates voltage-dependent currents of the action potential in mammalian neuronal cells. J Neurochem. 2005;93(4):963–73. doi: 10.1111/j.1471-4159.2005.03083.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 2006;96(6):1540–50. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38(3):365–76. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem. 1997;272(45):28726–31. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24(8):847–52. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Increase in spontaneous motor activity following infusion of neurotensin into the ventral tegmental area. Brain Res. 1981;229(2):525–9. doi: 10.1016/0006-8993(81)91016-7. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13(5):177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35(3):233–45. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS, Aizenstein ML. Amphetamine, cocaine, and fencamfamine: relationship between locomotor and stereotypy response profiles and caudate and accumbens dopamine dynamics. J Neurosci. 1991;11(9):2703–12. doi: 10.1523/JNEUROSCI.11-09-02703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95(16):9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moepps B, Braun M, Knopfle K, Dillinger K, Knochel W, Gierschik P. Characterization of a Xenopus laevis CXC chemokine receptor 4: implications for hematopoietic cell development in the vertebrate embryo. Eur J Immunol. 2000;30(10):2924–34. doi: 10.1002/1521-4141(200010)30:10<2924::AID-IMMU2924>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Wu M, Manchanda SK. Locomotor activity initiated by microinfusions of picrotoxin into the ventral tegmental area. Brain Res. 1979;161(2):311–9. doi: 10.1016/0006-8993(79)90072-6. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249(2–3):163–6. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- Paxinos George, Watson Charles. The rat brain in stereotaxic coordinates. Amsterdam; Boston: Elsevier; 2007. [Google Scholar]
- Reith ME. Effect of repeated administration of various doses of cocaine and WIN 35,065-2 on locomotor behavior of mice. Eur J Pharmacol. 1986;130(1–2):65–72. doi: 10.1016/0014-2999(86)90184-6. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–23. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17(5):901–4. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510(2):207–20. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem. 2007;102(4):1175–83. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Steele AD, Szabo I, Bednar F, Rogers TJ. Interactions between opioid and chemokine receptors: heterologous desensitization. Cytokine Growth Factor Rev. 2002;13(3):209–22. doi: 10.1016/s1359-6101(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Stumm R, Hollt V. CXC chemokine receptor 4 regulates neuronal migration and axonal pathfinding in the developing nervous system: implications for neuronal regeneration in the adult brain. J Mol Endocrinol. 2007;38(3):377–82. doi: 10.1677/JME-06-0032. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22 (14):5865–78. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushijima I, Carino MA, Horita A. Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacol Biochem Behav. 1995;52(4):737–41. doi: 10.1016/0091-3057(95)00167-u. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98(2):185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393(6685):595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]