Summary
Many of the obligate steps of physiology and disease are dynamic in time and space, and thus, end-point assays do not always provide a full understanding of these processes. Comprehensive understanding of the functional complexity of protein interactions and cell trafficking requires mapping of cellular and molecular function within complex systems over biologically relevant time scales. New approaches to bioluminescence imaging of cell migration, signaling pathways, drug action and interacting protein partners in vivo allow the study of biology and disease within the context of living animals.
Introduction
Genetically-encoded imaging reporters introduced into cells and transgenic animals enable noninvasive, longitudinal studies of dynamic biological processes in intact cells and living animals [1]. These reporters can produce signal intrinsically (e.g., fluorescent proteins), through enzymatic activation of an inactive substrate (luciferases), by enzymatic modification of an imagable (e.g., optical) substrate with selective retention in reporter cells, or by direct binding or import of an active (e.g., radiolabeled) reporter substrate or probe. Except in the context of gene therapy, genetically-encoded reporters are less likely to be used in humans, but possess a fundamental advantage in basic and pre-clinical research in that once validated, a single genetically-encoded reporter can theoretically be cloned into a variety of vectors to interrogate a broad array of regulatory pathways. Compared to injectable radiopharmaceuticals, for example, this eliminates constraints inherent to traditional routes of synthesizing, labeling and validating a new and different radioligand for every new receptor or protein of interest.
The most common reporters include firefly luciferase (bioluminescence imaging), green fluorescence protein (fluorescence imaging), transferrin receptor (magnetic resonance imaging), Herpes Simplex Virus-1 thymidine kinase (positron emission tomography) and variants with enhanced spectral and kinetic properties optimized for use in vivo. When cloned into promoter/enhancer sequences or engineered into fusion proteins, imaging reporters enable fundamental processes such as transcriptional regulation, signal transduction cascades, protein-protein interactions, protein degradation, oncogenic transformation, cell trafficking and targeted drug action to be temporally and spatially registered in vivo. Ideally, the magnitude and time course of reporter gene activity should parallel the strength and duration of endogenous target gene expression. Genetically-encoded imaging reporters also provide the potential for a stable source of signal enabling longitudinal studies in living organisms with high temporal and, in some cases, high spatial resolution.
Bioluminescence imaging (BLI) of luciferase reporters provides a relatively simple, robust, cost-effective and extremely sensitive means to image fundamental biological processes in vivo due to exceptionally high signal-to-noise levels. Nevertheless, bioluminescence remains dependent on substrate pharmacokinetics, except in the case of bacterial lux operons, and in general, is usually represented as planar imaging datasets, therefore imposing some positional uncertainty on the attained signal. There are many luciferases with matching substrates available. However, most are blue/green and therefore are less suitable for deep tissue imaging. The luciferases that have been found to be most useful for molecular imaging are firefly (Photinus pyralis) luciferase, Renilla luciferase, green or red click beetle (Pyrophorus plagiophthalamus) luciferases and Gaussia luciferase [2-4]. However, both Renilla and Gaussia luciferases emit blue light, which is highly attenuated in living tissue, and possess high bursting activity, therefore requiring care and precision in timing the readout. New mutants of Renilla luciferase were recently reported, and while favorably shifted approximately 66 nm, are still rather green for optimal use in vivo [5]. Moreover, the Renilla and Gaussia luciferase substrate, coelenterazine, has been shown to be transported by the multidrug resistance transporter P-glycoprotein [6] as well as to interact efficiently with superoxide anion and peroxynitrate in light-producing reactions [7], thereby complicating certain applications of Renilla and Gaussia luciferases in vivo. Nonetheless, the favorable attributes of luciferin-based imaging provide a versatile platform for studying biology in vivo.
Herein we will focus on regulatory and biochemical events in several of the major classes of diseases that can be interrogated with luciferases as the imaging reporter gene. Recent strategies to regulate genetically-encoded reporter activation and thereby detect and dynamically monitor various components of cell motility and cell machinery (transcriptional, post-transcriptional, translational and post-translational) in intact small animal models will be briefly highlighted in this review.
Imaging Cancer
The most common use of BLI in cancer research has been to assess mass and location of xenografted cells constitutively expressing a luciferase gene, providing a robust strategy to monitor effectiveness of anti-tumor drugs in vivo. Galkin and co-workers utilized BLI to study small molecule (TAE684) inhibition of the anaplastic lymphoma kinase (NPM-ALK) fusion protein unique to anaplastic large-cell lymphomas (ALCL) [8]. They assessed the dose-response of constitutively bioluminescent Karpas-299 lymphoma cells in a mouse xenograft model, and found that TAE684 exhibited potent and specific inhibition of lymphoma cell growth.
In basic cancer research, BLI combined with genetic alteration (gene knock-out or over-expression) has increased understanding of the consequences of specific genes (or gene combinations) on tumor load and metastasis. For instance, using a breast cancer metastasis model, Podsypanina and co-workers investigated whether primary mammary cells can be induced to form metastases in the absence of oncogene-driven transformation at the primary site. They harvested mammary tissue from a transgenic mouse expressing a mammary-specific transactivator driving a doxycycline-inducible cassette comprised of middle T antigen and luciferase separated by an IRES. Dissociated mammary cells from doxycycline-naive animals were injected via tail vein into recombination activation gene null (Rag-/-) mice maintained on a doxycyline diet. The presence of doxycyline was sufficient to cause tumor formation in the lungs, indicating that previously untransformed mammary cells can produce metastatic-like disease, once induced, without first undergoing transformation at a primary tumor site [9]. The authors further found that untransformed mammary cells could survive in the blood and lung for up to 17 weeks, however subsequent foci formation at a metastatic site required transformation.
Inhibition of angiogenesis is an increasingly important cancer treatment strategy since angiogenesis is a process that is critical to tumor growth. One study employed BLI to quantify angiogenesis, monitored on a long time-scale, after co-implantation of human umbilical vein endothelial cells (HUVEC) expressing firefly luciferase (FLuc) and bone marrow-derived human mesenchymal stem cells (HMSC) into immunodeficient mice [10]. HSMC+ implants demonstrated stable bioluminescent signal for up to 120 days; however, a marked decrease in photon emission was observed in the two weeks following treatment with a VEGFR tyrosine kinase inhibitor (SU5416), thus demonstrating the usefulness of BLI in ascertaining anti-angiogenic potential of therapeutic agents.
While chemotherapy using small molecules has been and remains the most effective method of systemic cancer treatment, future therapies may build on human genome sequence data coupled with safe and effective methods of delivering gene therapy. Toward this end, Xie and co-workers sought to find a highly specific and effective way to deliver a proapoptotic transgene that could be used in pancreatic cancer gene therapy [11]. They utilized BLI to aid generation and optimization of a gene delivery vector encoding the pancreatic-specific cholecystokinin type A receptor (CCKAR) promoter driving a Gal4-VP16 transactivator combined with a Gal4-response element driving a luciferase reporter. Addition of the post-transcriptional regulatory element from the woodchuck hepatitis virus (WPRE) further enhanced expression from this vector, while maintaining pancreatic-specificity, by comparison to a CMV-driven luciferase. Following luciferase-aided vector optimization, the authors replaced luciferase with the proapoptotic gene, Bcl-2-interacting killer (Bik), containing two phospho-mimetic mutations that activate Bik (BikDD). The expression vector bearing BikDD was then demonstrated to specifically and effectively reduce pancreatic tumors compared to a non-specific CMV-driven BikDD. Thus, the imaging vector facilitated the rapid design and implementation of an optimal therapeutic vector.
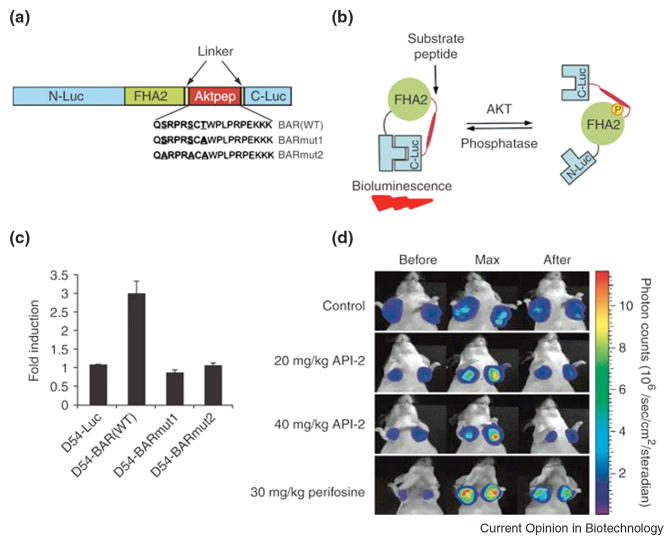
Beyond assessing the role that various genes have on tumor mass and location, BLI has furthered in vivo mechanistic studies especially through the ability to directly interrogate signal transduction processes. These studies typically involve luciferase reporter genes fused to promoter regions or gene chimera sequences of interest. For instance, Naik and Piwnica-Worms recently described an in vivo β-catenin-FLuc reporter system that enables direct visualization of β-catenin stabilization, a process that is frequently upregulated in many cancers [12]. Furthermore, split-luciferase reporter strategies aid assessment of the initial events in pathway activation, and in specific cases, has allowed interrogation of post-translational modifications. For instance, using a previously described split-luciferase reporter system [13] incorporated into a single-chain biosensor consisting of N- and C-terminal luciferase fragments flanking an FHA2 domain linked to a peptide sequence recognized by Akt (Aktpep), Zhang and co-workers have monitored in vivo activation of the serine/threonine kinase Akt [14]. Under basal conditions, the single-chain bioluminescence Akt kinase reporter (BAR) is flexible enough to allow complementation of the luciferase fragments (Figure 1). However, upon Akt activation, the binding of the FHA2 domain to the newly phosphorylated Aktpep sterically hinders complementation, which in turn attenuates bioluminescent signals. While limitations exist for single-chain biosensors as equilibrium reporters [15], molecule-specific pharmacodynamics of a kinase inhibitor were successfully monitored in living animals. Aberrant expression of epidermal growth factor receptor (EGFR), another kinase critical for growth of certain tumors, can be similarly monitored. Herein, the optimized split luciferase complementation system [13] was again used, now to monitor association of EGFR with its downstream binding partners Grb2 and Shc, by fusing each interacting protein to a luciferase fragment [16]. Upon association of interacting proteins, functional luciferase is reconstituted and can deliver accurate readout of radiotherapy-induced EGFR activity in the presence/absence of tyrosine kinase inhibitors in tumor models in vivo.
Figure 1.
Bioluminescence imaging of Akt kinase activity in vivo. (A) Domain structure of the single-chain bioluminescence Akt kinase reporter (BAR). Three versions of the reporter were developed: the wild-type (BARWT) molecule, which contains the wild-type Aktpep sequence; the BARmut1 molecule, which contains a threonine-to-alanine substitution at the primary phosphorylation site; and the BARmut2 molecule, which has all serine and threonine residues in the substrate sequence mutated to alanine. (B) The proposed mechanism of action for the BAR reporter involves Akt-dependent phosphorylation of the Aktpep domain (thick line), which results in its interaction with the FHA2 domain (right). In this form, the reporter is constrained and has minimal bioluminescence activity. In the absence of Akt activity, the N-Luc and C-Luc domains complement each other, restoring bioluminescence activity (left). (C) Stable cell lines expressing each of the reporters were treated with API-2 (40 μM), an Akt inhibitor, for 1 h. The change in bioluminescence activity compared with pretreatment values was plotted as fold induction. Data were derived from a minimum of five experiments. (D) Molecular imaging of Akt activity. Tumor-bearing mice were treated with vehicle control (20% DMSO in PBS), API-2 (20 mg/kg or 40 mg/kg) or perifosine (30 mg/kg), a phosphoinositide 3-kinase inhibitor. Images of representative mice are shown before treatment, during maximal luciferase signal upon treatment (Max), and after treatment. Adapted by permission from Macmillan Publishers Ltd: Zhang et.al., Nature Medicine, 13:1114-1119, copyright 2007.
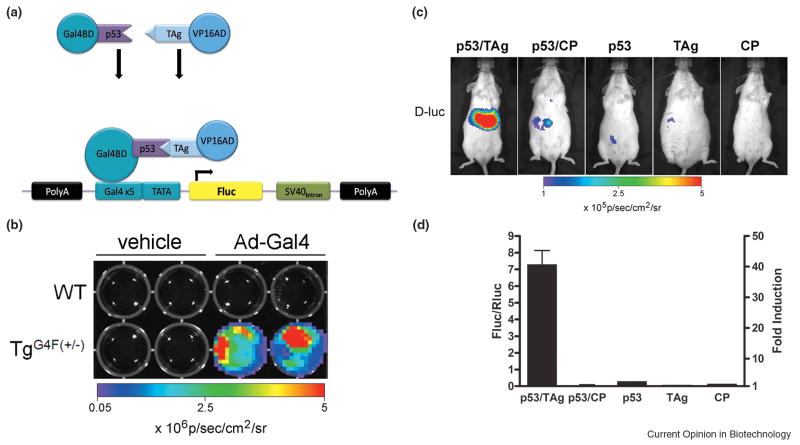
An alternative method to study in vivo protein-protein interactions is modeled after the two-hybrid transactivation system. Pichler and colleagues generated a transgenic Gal4-FLuc universal reporter mouse strain and tested its usefulness by characterizing the interaction between p53 and SV40 large T antigen (TAg) [17]. In this transactivation system, p53 was fused to a Gal4 DNA-binding domain and TAg was fused to the activator domain from VP16 (Figure 2). After hepatocellular somatic gene transfer of the interacting chimeras, only the reporter mice that were transfected with the interacting pair induced FLuc expression in the liver compared to a negative control. Beyond studies of signaling cascades in cancer, the future of this technique will likely find use in assessments of viral progression and preclinical experimental therapeutics.
Figure 2.
Use of a universal Gal4-Fluc transgenic reporter mouse to image protein-protein interactions in vivo. (A) Schematic representation of the transgene vector (G4F) that was injected into pronuclei of FVB oocytes, and schematic diagram indicating a two-protein interaction system transactivating expression of FLuc. (B) Mouse embryonic fibroblasts (MEF) of a negative embryo (WT) and a positive embryo (TgG4F(+/-)) were treated with either vehicle or 4×107 pfu of Ad5Gal4BD-VP16 (Ad-Gal4). Cells were imaged 24 h after infection. MEFs from the positive embryo were induced 3-log fold when compared with the uninducible negative MEFs. (C) Imaging protein–protein interactions in vivo using a Gal4-Fluc reporter mouse. In this transactivation system, p53 was fused to a Gal4 DNA binding-domain and TAg was fused to the activator domain from VP16. Hepatocytes of TgG4F(+/-) mice were transfected in vivo by hydrodynamic somatic gene transfer with different plasmid combinations of pGal4BDp53 together with pVP16-TAg or off-target pVP16-CP, or each plasmid separately as indicated. A plasmid for Renilla luciferase was used as transfection control (data not shown). Mice were imaged before (pretreatment) and 24 h after transfection for expression of firefly luciferase (FLuc with D-luciferin) as well as Renilla luciferase (RLuc with coelenterazine). Images of representative mice are shown. CP; polyomavirus coat protein. (D) Data are plotted for the different plasmid combinations as controlled for transfection efficiencies (FLuc/RLuc; left axis) and as fold-induction (right axis) 24 h after transfection. Data are presented as mean ± range for plasmid combinations (n=2), and without error bars for single plasmids (n=1). Four independent experiments showed similar results. Adapted by permission from the National Academy of Science: Pichler et.al., Proc Nat'l Acad Sci USA, 105, 15932-15937, copyright 2008.
Immunology/Transplant Biology
For immunologists and transplant biologists, BLI has emerged as a valuable tool, not only to longitudinally track spatiotemporal cell fates in vivo, but also to probe signaling pathways involved during a variety of immune responses. BLI has proven especially useful in mouse models of graft versus host disease (GVHD) to non-invasively monitor the location and activity of engrafted T cells, replacing or augmenting techniques that previously relied upon cell labeling and flow cytometry end point assays. Nguyen and colleagues used luciferase positive murine T regulatory cells (Tregs) in an allogeneic bone marrow transplant model and evaluated the in vivo dynamics of Tregs over time; they observed early, robust Treg expansion in secondary lymphoid organs with sequential migration and localization to peripheral tissues, suggesting that T cell priming events occur in secondary lymphoid tissues [18]. Furthermore, by using gene-deficient donor or recipient mice, the effects of cytokines (IL-18, [19]) and their receptors (CD-30, [20]) upon the development and progression of GVHD were evaluated. Nervi and colleagues used retrovirally-transduced human T cells injected retro-orbitally into NOD/SCID-β2m null mice (xenogenic GVHD); BLI allowed them to validate this model and show that lethal GVHD was dependent upon initial retention and early expansion of human T cells in the retro-orbital sinus cavity [21].
Researchers have also utilized bioluminescent islet-specific auto-antigenic T cells (BDCs) to visualize trafficking patterns and proliferation in a model of Type 1 diabetes [22]. Non-CD4 splenocytes cotransferred with the BDCs were found to help induce diabetes by promoting invasive infiltration of BDCs into pancreatic islets without affecting the systemic trafficking patterns of the BDCs within the animals. Furthermore, bioluminescent pancreatic allografts have been used to monitor graft viability and to determine the appropriate timing of antilymphocyte serum therapy, which yielded prolonged graft survival and completely protected from graft loss [23].
BLI has also proven useful for in vivo study of nuclear factor-kappa b (NF-κB) pathway signaling, a key regulator of physiological immunity and pathological inflammation. Recently, a transgenic mouse expressing a luciferase reporter-driven by an NF-κB responsive promoter has been used to examine prostate NF-κB activity in response to acute and chronic cytokine exposure [24], an approach that may be amenable to the in vivo study of pharmacological NF-κB modulators. Ma and colleagues used NF-κB-Luc mice to non-invasively detect NF-κB activation during cardiac allograft rejection and tissue ischemia-reperfusion (IRI) [25]. They showed elevated NF-κB activity in both the cardiac allografts and the IRI cardiac grafts, and demonstrated that CD154 monoclonal antibody therapy inhibited luciferase activity and resulted in long-term graft survival. Using a post-translational reporter strategy, Gross and colleagues demonstrated that an IκBα-FLuc reporter introduced into mouse liver can be used to study in vivo regulation of both ligand-induced Inhibitor of κB Kinase (IKK) activation and the pharmacodynamics of IKK inhibition [26].
Immune cell infiltration and activation at sites of infection or inflammation can also be studied using BLI. Davies and coworkers imaged eosinopoiesis and eosinophil infiltration in a model of schistosome infection that utilized a transgenic mouse expressing luciferase under control of the eosinophil peroxidase promoter [27]. While schistosome egg deposition is the primary stimulus for eosinophil activation (a process that was easily followed using BLI), the sensitivity of BLI allowed the authors to demonstrate that, contrary to expectations, schistosome worms themselves induce significant intestinal eosinophilia and eosinopoesis within the bone marrow at very early stages of infection.
Infectious Disease
Studies of microorganisms in vivo are uniquely suited to BLI. This is evident especially when imaging bacterial infection models, since use of a bacterial lux operon allows for BLI in real time, without requiring administration of exogenous substrate. Disson and colleagues developed a new lux operon-based fetoplacental listeriosis model in gerbils [28], using bioluminescent Listeria monocytogenes, a bacterium associated with pregnancy-related fetal infections. The authors imaged fetoplacental infection in pregnant gerbils in real time and placentas and fetuses ex vivo post-mortem. Using isogenic deletion mutants, they also proved the necessity of Listeria internalin proteins InlA and InlB in the fetoplacental infection process.
Furthermore, traditional in vivo infection models have required host sacrifice and enumeration of microorganisms from individual host organs to determine extent and kinetics of dissemination during infection. BLI provides a unique opportunity to serially monitor infection in a single host over time, often resulting in identification of new sites of replication and persistence within an infected animal. For example, Hwang and colleagues studied in vivo infection by gamma-herpesviruses expressing firefly luciferase [29]. They discovered novel sites of viral replication, including the salivary glands and thymus. Importantly, imaging facilitated this discovery as typical assays involving gamma-herpesvirus replication focused only on lung and spleen. In another study, Sjolinder and colleagues used bioluminescent Neisseria meningitidis to monitor bacterial sepsis in CD46 transgenic mice in real time [30]. The researchers showed three different disease outcomes in CD46 mice: a “sepsis-like” disease, a “meningitis-like” disease, and a mild disease pattern. In addition to the three different manifestations of disease, the mice exhibited waves of replication followed by clearance, followed by re-initiation of infection, making real time BLI an ideal method for longitudinal study of meningococcal disease in vivo.
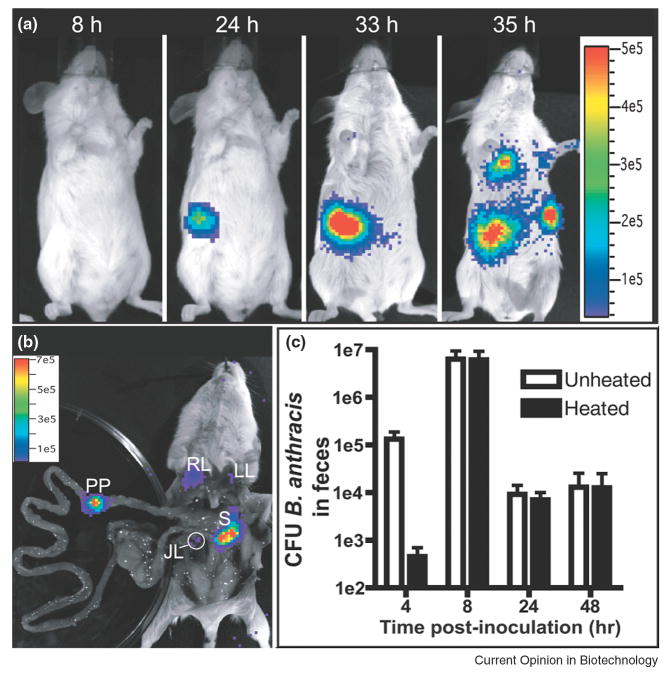
BLI can also be used to visualize noteworthy events during the course of an infection, such as spore germination and parasite stage conversion. Glomski and colleagues used Bacillus anthracis containing the bacterial luxCDABE operon [31]; dormant anthrax bacilli form spores and do not emit bioluminescence, but once the spores germinate in a mouse, bioluminescence will indicate sites of active bacterial infection (Figure 3). This model was used to demonstrate that bacterial spores germinate and replicate at the initial site of infection following both inhalational and cutaneous inoculation, and exhibited localization within Peyer's patches during an intestinal model of anthrax. This contrasts with prevailing dogma that spores travel to the draining lymph nodes before germination. In another study using Toxoplasma gondii, a parasite that exists in two developmental stages in vivo, Saeij and colleagues used firefly luciferase linked to a stage-specific promoter to show stage conversion in vivo for the first time [32]. Furthermore, they also demonstrated a role for stage-specific surface proteins in reactivation of infection.
Figure 3.
Bioluminescence imaging to map germination of B. anthracis in gastrointestinal infection. B. anthracis spores (1×108) were inoculated using a flexible plastic tube. (A) Infection of the lower digestive tract at the indicated times. Image series is representative of 23 mice. (B) Dissection of the mouse in (A) to confirm sources of luminescence. JL, jejunal lymph node; LL, left lung; PP, Peyer's patch; RL, right lung; S, spleen. (C) Total CFU of B. anthracis (unheated) or spores only (heated) were enumerated at the indicated times from the feces of mice inoculated intragastrically (mean ± standard error of the mean, n = 4). Vegetative bacteria were isolated from feces at four hours post-infection, indicating spore germination in the gastrointestinal tract. Adapted by permission from Public Library of Science: Glomski et.al., PLoS Pathogens, 3:0699-0708, copyright 2007.
Imaging also facilitates studies of host response to microbial infection. In a recent study, Hutchens and colleagues used mice with inactive toll-like receptor-4 (TLR4) and bioluminescent vaccinia to demonstrate a protective role of TLR4 in infection by vaccinia, especially following a high initial dose of virus [33]. This paper confirms other recent reports demonstrating a new role for TLR4 in immunity to viral infection, in addition to the traditionally acknowledged role in response to bacterial lipopolysaccharide (LPS).
Finally, both bacteria and viruses have been investigated as potential diagnostic and treatment tools for cancer and use of BLI has often facilitated these studies. Two recent papers employed carrier cells to deliver bioluminescent oncolytic viruses to tumors in vivo. Thorne and colleagues used cytokine-induced killer (CIK) cells loaded with attenuated, luciferase-expressing vaccinia viruses to target and kill tumor cells [34]. Power and colleagues used Renilla luciferase-expressing carrier cells and firefly luciferase-expressing vesicular stomatitis virus in order to image carrier cell dispersion and viral delivery to tumors [35]. In each case, researchers demonstrated by BLI that carrier cells improved viral delivery to tumors and tumor growth was attenuated. In other work by Cheng and colleagues, Escherichia coli expressing bacterial luciferase and prodrug-activating β-glucuronidase were used to target tumors and activate a glucuronide prodrug (9ACG) [36]. BLI was used to determine bacterial replication in the tumors and to demonstrate that drug treatment resulted in delayed tumor growth.
Cardiovascular
BLI has also been widely utilized in cardiovascular research and has provided insights into possible therapies for cardiac ischemia. Firefly luciferase-expressing murine embryonic stem (ES) cells were injected into mouse hearts following left anterior descending (LAD) artery ligation, a common model of myocardial infarction [37]. BLI was used to track surviving ES cells for up to 8 weeks following injection, and increased bioluminescent signal correlated with improved neovascularization and cardiac function as evaluated by histology and echocardiogram, respectively. Further investigation into cell-based therapy was made by comparison of the bioluminescent signal from four different cell types - bone marrow mononuclear cells (MN), mesenchymal stem cells (MSC), skeletal myoblasts (SkMb), and fibroblasts (Fibro) - each expressing firefly luciferase, and injected into mouse hearts following LAD artery ligation [38]. The strongest signal and most favorable cardiac tissue survival was seen in those hearts injected with MN cells. Improvements in both neovascularization and cardiac function post-LAD artery ligation were also observed with shRNA inhibition of the prolyl hydroxylase-2 (PHD2) enzyme [39]. In this system, the source of the bioluminescent signal was a hypoxia response element-incorporated promoter driving firefly luciferase; HIF-1 alpha promotes angiogenesis, so the inhibition of PHD2 indirectly increases neovascularization.
Central Nervous System Disorders
In vivo BLI has also been employed in studies of the central nervous system, including investigation of circadian rhythms mediated by Period1. Measuring the fluctuations in bioluminescent output from the main olfactory bulbs (OBs) of Period1-FLuc transgenic mice whose suprachiasmatic nuclei (SCN) were either intact or lesioned revealed that the OB contains a self-sustained circadian oscillator [40]. Additional insight into SCN intercellular coupling was obtained by crossing circadian clock gene knockout mice with the mPer2-luciferase fusion knock-in reporter line [41]. Through measurement of bioluminescence in tissue explants, the authors concluded that Per1 and Cry1 are required for sustained rhythms in peripheral tissues and cells and SCN-dissociated neurons, but Per1 or Cry1 deficiency can be overcome by oscillator network interactions in the SCN.
Other exciting work in the CNS involves the potential for monitoring neuronal excitation and degeneration via the transforming growth factor-beta (TGF-β) pathway; activation of TGF-β receptors results in the translocation of Smad proteins to the nucleus and activation of the pathway. Using Smad-binding element (SBE)-luciferase transgenic mice, Luo and colleagues were able to localize much of the Smad activity in the CNS to the pyramidal neurons of the hippocampus [42]. Increased bioluminescence, representing increased Smad activity following kainic acid (KA)-induced degeneration, correlated with pathological neurodegeneration. Both bioluminescent signal and pathology were reduced following treatment with a glutamate receptor antagonist (MK-801), indicating that in vivo BLI models can play a role in identifying potential treatments for neurodegenerative diseases.
Conclusions
Animal models of human diseases greatly enhance our knowledge of the mechanisms of pathogenesis by placing target genes and processes in the appropriate physiological setting. Much of our current knowledge has been obtained by monitoring obvious phenotypic changes or by performing destructive analyses at defined end-points. However, many of the obligate steps toward development of disease are dynamic in time and space, and thus, end-point assays are not amenable to gaining a complete understanding of disease pathogenesis. Advances in small animal imaging instrumentation, molecular genetics and reporter gene design have yielded the ability to integrate “imagable” reporters into transgenic murine models of human diseases. Moreover, the successes obtained using BLI strategies to study diseases will continue paving the way for basic, preclinical, and translational research. In the future, we anticipate further incorporation of these non-invasive imaging strategies for use with in vivo high-throughput screening, multiplexed imaging of multiple luciferases simultaneously, and imaging multiple nodes within or across signal transduction pathways.
Acknowledgments
We thank colleagues of the Molecular Imaging Center for helpful discussions and NIH P50 CA94056 for support. BLM is supported in part by a National Science Foundation graduate research fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robin S. Dothager, Email: dothagerr@mir.wustl.edu.
Kelly Flentie, Email: flentiek@mir.wustl.edu.
Britney Moss, Email: blmoss@artsci.wustl.edu.
Mei-Hsiu Pan, Email: mpan@artsci.wustl.edu.
Aparna Kesarwala, Email: kesarwala@gmail.com.
David Piwnica-Worms, Email: piwnica-wormsd@mir.wustl.edu.
References
- 1•.Gross S, Piwnica-Worms D. Spying on cancer: molecular imaging in vivo with genetically encoded reporters. Cancer Cell. 2005;7:5–15. doi: 10.1016/j.ccr.2004.12.011. [DOI] [PubMed] [Google Scholar]; Comprehensive review presenting an introduction to molecular imaging strategies and detailed discussion of the use of genetically-encoded reporters to spatiotemporally resolve transcriptional and post-translational events in vivo.
- 2.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 3.Zhao H, Doyle T, Coquoz O, Kalish F, Rice B, Contag CH. Spectral characterization of firefly, click beetle and Renilla luciferases in mammalian cells and living mice. Mol Imaging. 2004;3:229. [Google Scholar]
- 4.Tannous B, Kim D, Weissleder R. Novel luciferases for in vitro and in vivo imaging. Mol Imaging. 2004;3:227. [Google Scholar]
- 5.Loening A, Wu A, Gambhir S. Red-shifted Renilla reniformis luciferase variants for imaging in living subjects. Nat Methods. 2007;4:641–643. doi: 10.1038/nmeth1070. [DOI] [PubMed] [Google Scholar]
- 6.Pichler A, Prior J, Piwnica-Worms D. Imaging reversal of multidrug resistance in living mice with bioluminescence: MDR1 P-glycoprotein transports coelenterazine. Proc Natl Acad Sci USA. 2004;101:1702–1707. doi: 10.1073/pnas.0304326101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarpey M, White C, Suarez E, Richardson G, Radi R, Freeman B. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 8.Galkin AV, Melnick JS, Kim S, Hood TL, Li N, Li L, Xia G, Steensma R, Chopiuk G, Jiang J, et al. Identification of NVP-TAE684, a potent, selective, and efficacious inhibitor of NPM-ALK. Proceedings of the National Academy of Sciences; 2007. pp. 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Podsypanina K, Du YCN, Jechlinger M, Beverly LJ, Hambardzumyan D, Varmus H. Seeding and propagation of untransformed mouse mammary cells in the lung. Science. 2008;321:1841–1844. doi: 10.1126/science.1161621. [DOI] [PMC free article] [PubMed] [Google Scholar]; In efforts to refine our understanding of cancer metastasis, the authors utilized previously untransformed mammary cells taken from transgenic mice bearing inducible oncogenes. Upon systemic injection into recipient mice, the authors used imaging to demonstrate that mouse mammary cells can bypass transformation at the primary site and develop into metastatic pulmonary lesions upon immediate or delayed oncogene induction.
- 10.Sanz L, Santos-Valle P, Alonso-Camino V, Salas C, Serrano A, Vicario JL, Cuesta AM, Compte M, Sanchez-Martin D, Alvarez-Vallina L. Long-term in vivo imaging of human angiogenesis: critical role of bone marrow-derived mesenchymal stem cells for the generation of durable blood vessels. Microvasc Res. 2008;75:308–314. doi: 10.1016/j.mvr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Xie X, Xia W, Li Z, Kuo HP, Liu Y, Li Z, Ding Q, Zhang S, Spohn B, Yang Y, et al. Targeted expression of BikDD eradicates pancreatic tumors in noninvasive imaging models. Cancer Cell. 2007;12:52–65. doi: 10.1016/j.ccr.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 12•.Naik S, Piwnica-Worms D. Real-time imaging of beta-catenin dynamics in cells and living mice. Proc Natl Acad Sci U S A. 2007;104:17465–17470. doi: 10.1073/pnas.0704465104. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors introduced a novel dual reporter system to monitor real-time β-catenin stabilization and downstream transcription activation simultaneously. This provided a robust platform to investigate potential regulators of Wnt/β-catenin signaling in vitro as well as in vivo.
- 13.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA. 2004;101:12288–12293. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Lee KC, Bhojani MS, Khan AP, Shilman A, Holland EC, Ross BD, Rehemtulla A. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–1119. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- 15.Villalobos V, Naik S, Piwnica-Worms D. Current state of imaging protein-protein interactions in vivo with genetically encoded reporters. Annu Rev Biomed Eng. 2007;9:321–349. doi: 10.1146/annurev.bioeng.9.060906.152044. [DOI] [PubMed] [Google Scholar]
- 16.Li W, Li F, Huang Q, Frederick B, Bao S, Li CY. Noninvasive imaging and quantification of epidermal growth factor receptor kinase activation in vivo. Cancer Res. 2008;68:4990–4997. doi: 10.1158/0008-5472.CAN-07-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pichler A, Prior J, Luker G, Piwnica-Worms D. Generation of a highly-inducible Gal4→Fluc universal reporter mouse for in vivo bioluminescence imaging. Proc Nat'l Acad Sci USA. 2008;105:15932–15937. doi: 10.1073/pnas.0801075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]; In this work, the authors demonstrated the utility of non-invasive BLI in vivo to augment or replace traditional end point assays to study the location and activity of engrafted luciferase-positive T cells in mouse models of graft versus host disease.
- 19.Zeiser R, Zambricki EA, Leveson-Gower D, Kambham N, Beilhack A, Negrin RS. Host-derived interleukin-18 differentially impacts regulatory and conventional T cell expansion during acute graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13:1427–1438. doi: 10.1016/j.bbmt.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeiser R, Nguyen VH, Hou JZ, Beilhack A, Zambricki E, Buess M, Contag CH, Negrin RS. Early CD30 signaling is critical for adoptively transferred CD4+CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood. 2007;109:2225–2233. doi: 10.1182/blood-2006-07-038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, Bonyhadi ML, Berenson RJ, Prior JL, Piwnica-Worms D, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MH, Lee WH, Van Y, Contag CH, Liu CP. Image-guided analyses reveal that non-CD4 splenocytes contribute to CD4+ T cell-mediated inflammation leading to islet destruction by altering their local function and not systemic trafficking patterns. Mol Imaging. 2007;6:369–383. [PubMed] [Google Scholar]
- 23.Chen X, Zhang X, Larson C, Xia G, Kaufman DB. Prolonging islet allograft survival using in vivo bioluminescence imaging to guide timing of antilymphocyte serum treatment of rejection. Transplantation. 2008;85:1246–1252. doi: 10.1097/TP.0b013e31816b66b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vykhovanets EV, Shukla S, MacLennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 2008;68:34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 25.Ma L, Xiang Z, Sherrill TP, Wang L, Blackwell TS, Williams P, Chong A, Chari R, Yin DP. Bioluminescence imaging visualizes activation of nuclear factor-kappaB in mouse cardiac transplantation. Transplantation. 2008;85:903–910. doi: 10.1097/TP.0b013e318166cde1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–614. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 27.Davies SJ, Smith SJ, Lim KC, Zhang H, Purchio AF, McKerrow JH, West DB. In vivo imaging of tissue eosinophilia and eosinopoietic responses to schistosome worms and eggs. Int J Parasitol. 2005;35:851–859. doi: 10.1016/j.ijpara.2005.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, Ragon M, Le Monnier A, Babinet C, Cossart P, et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature. 2008;455:1114–1118. doi: 10.1038/nature07303. [DOI] [PubMed] [Google Scholar]
- 29.Hwang S, Wu TT, Tong LM, Kim KS, Martinez-Guzman D, Colantonio AD, Uittenbogaart CH, Sun R. Persistent gamma-herpesvirus replication and its dynamic interaction with host in vivo. J Virol. 2008 doi: 10.1128/JVI.01152-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjolinder H, Jonsson AB. Imaging of disease dynamics during meningococcal sepsis. PLoS ONE. 2007;2:e241. doi: 10.1371/journal.pone.0000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31••.Glomski IJ, Piris-Gimenez A, Huerre M, Mock Ml, Goossens PL. Primary involvement of pharynx and Peyer's patch in inhalational and intestinal anthrax. PLoS Pathogens. 2007;3:e76. doi: 10.1371/journal.ppat.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]; The investigators utilized bioluminescent Bacillus anthracis for real-time analysis of mouse models of inhalational, cutaneous and gastrointestinal anthrax disease. The authors demonstrated B. anthracis spore germination and establishment of infection at the initial site of inoculation in cutaneous and inhalational anthrax, as well as specific infection of Peyer's patches during the gastrointestinal form of the disease.
- 32.Saeij JP, Arrizabalaga G, Boothroyd JC. A cluster of four surface antigen genes specifically expressed in bradyzoites, SAG2CDXY, plays an important role in Toxoplasma gondii persistence. Infect Immun. 2008;76:2402–2410. doi: 10.1128/IAI.01494-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hutchens MA, Luker KE, Sonstein J, Nunez G, Curtis JL, Luker GD. Protective effect of Toll-like receptor 4 in pulmonary vaccinia infection. PLoS Pathog. 2008;4:e1000153. doi: 10.1371/journal.ppat.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–1784. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 35.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, Stojdl DF, Forsyth PA, Atkins H, Bell JC. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–130. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 36.Cheng CM, Lu YL, Chuang KH, Hung WC, Shiea J, Su YC, Kao CH, Chen BM, Roffler S, Cheng TL. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Ther. 2008;15:393–401. doi: 10.1038/cgt.2008.10. [DOI] [PubMed] [Google Scholar]
- 37.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP, et al. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang M, Chan DA, Jia F, Xie X, Li Z, Hoyt G, Robbins RC, Chen X, Giaccia AJ, Wu JC. Short hairpin RNA interference therapy for ischemic heart disease. Circulation. 2008;118:S226–233. doi: 10.1161/CIRCULATIONAHA.107.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abraham U, Prior JL, Granados-Fuentes D, Piwnica-Worms DR, Herzog ED. Independent circadian oscillations of Period1 in specific brain areas in vivo and in vitro. J Neuroscience. 2005;25:8620–8626. doi: 10.1523/JNEUROSCI.2225-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; To understand the molecular basis of rhythmicity, the authors used BLI to monitor a Per2-FLuc fusion reporter in the context of a variety of murine circadian genetic backgrounds. The authors demonstrated the requirements of clock genes for maintaining cellular rhythmicity as well as intercellular coupling for maintaining the clock system.
- 42.Luo J, Lin AH, Masliah E, Wyss-Coray T. Bioluminescence imaging of Smad signaling in living mice shows correlation with excitotoxic neurodegeneration. Proc Natl Acad Sci U S A. 2006;103:18326–18331. doi: 10.1073/pnas.0605077103. [DOI] [PMC free article] [PubMed] [Google Scholar]