Abstract
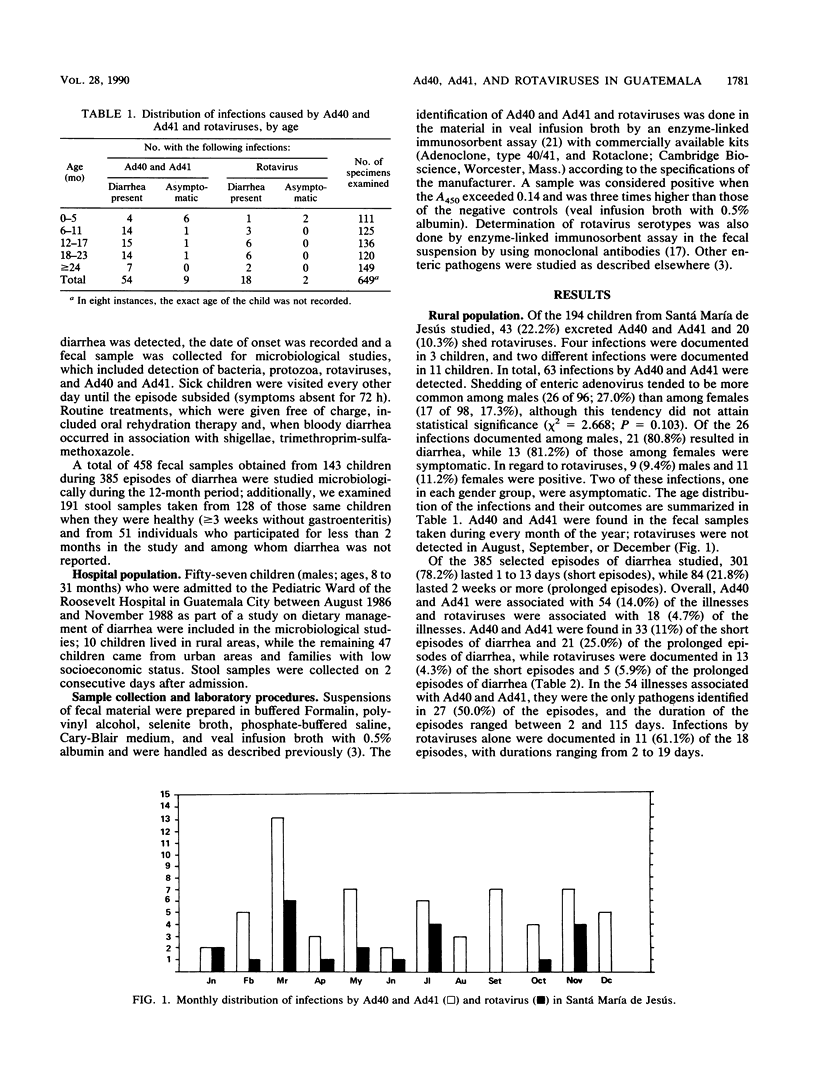
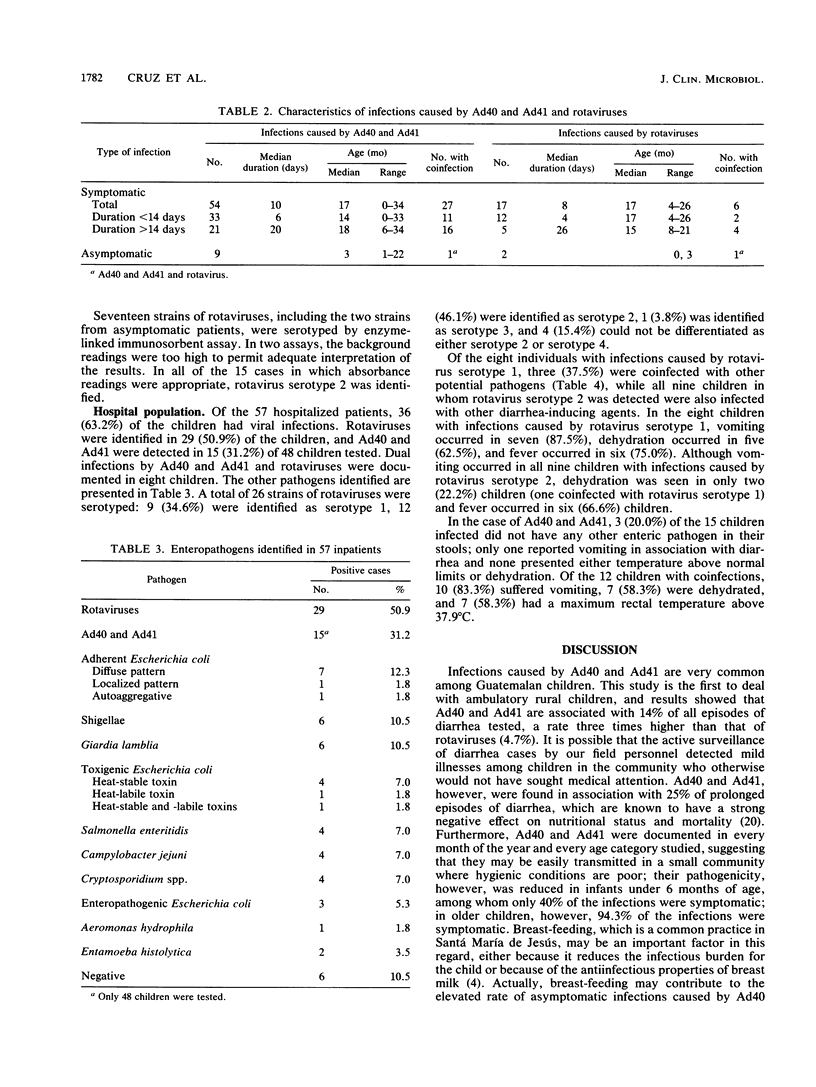
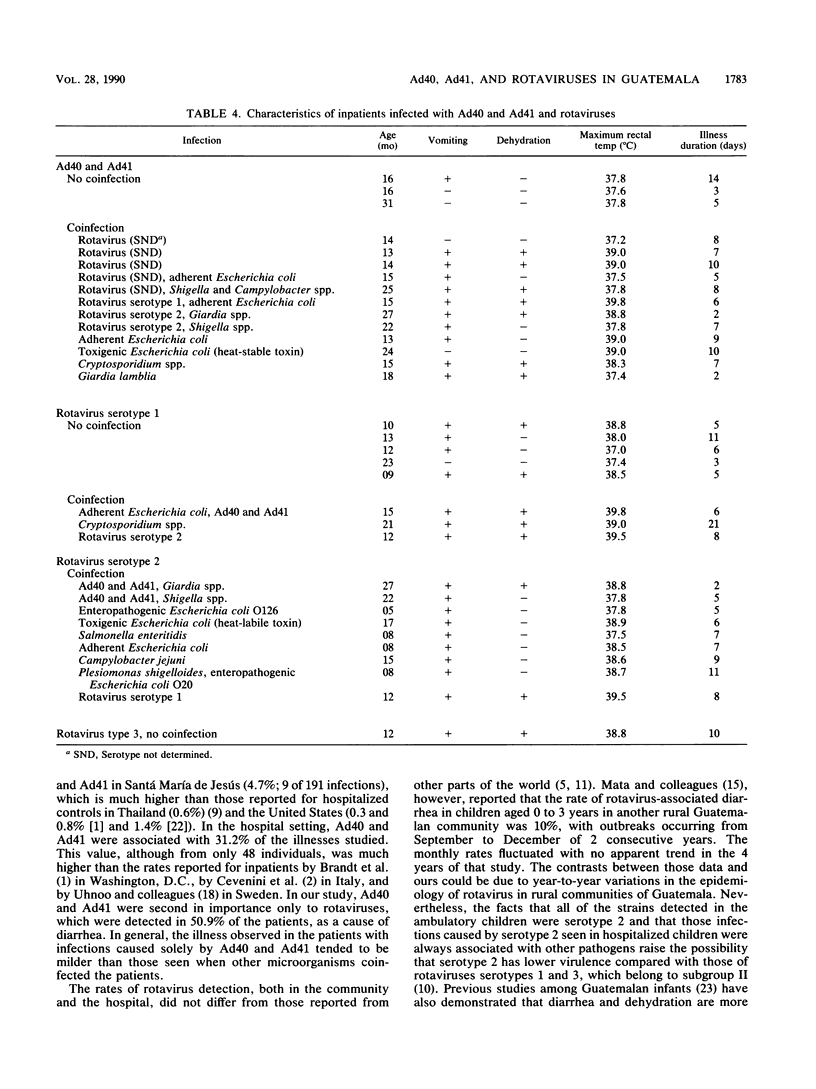
From March 1987 to February 1988, fecal excretion of adenovirus types 40 and 41 and rotavirus serotypes in 194 children (age, 0 to 3 years) from a rural community of Guatemala was monitored. In total, 458 samples taken during 385 episodes of diarrhea and 191 specimens obtained during symptom-free periods were examined by enzyme-linked immunosorbent assay. Fifty-seven children hospitalized because of diarrhea were also studied. Among the rural children, 43 (22.2%) excreted adenovirus types 40 and 41 and 20 (10.3%) shed rotaviruses. Adenovirus types 40 and 41 were associated with 54 (14.0%) illnesses, and rotaviruses were associated with 18 (4.7%) illnesses. Asymptomatic infections with adenovirus types 40 and 41 were documented in nine children and with rotaviruses in two children. Fifteen typeable rotaviruses were identified as serotype 2. In the hospital population, 36 (63.2%) children had viral infections. Rotaviruses were identified in 29 (50.9%) and adenovirus types 40 and 41 were identified in 15 (31.2%) of 48 subjects tested. Dual infections by these viruses were found in eight children. Of 22 typeable strains of rotaviruses, 9 (34.6%) were serotype 1, 12 (46.1%) were serotype 2, and 1 (3.8%) was serotype 3. All the children infected with serotype 2 rotavirus were coinfected with other enteric pathogens, while only three (37.5%) of those infected with rotavirus serotype 1 excreted another pathogen. Adenovirus types 40 and 41 are an important cause of gastroenteritis in both ambulatory and hospitalized Guatemalan children. There seems to be a difference in the pathogenicity among rotavirus serotypes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt C. D., Kim H. W., Yolken R. H., Kapikian A. Z., Arrobio J. O., Rodriguez W. J., Wyatt R. G., Chanock R. M., Parrott R. H. Comparative epidemiology of two rotavirus serotypes and other viral agents associated with pediatric gastroenteritis. Am J Epidemiol. 1979 Sep;110(3):243–254. doi: 10.1093/oxfordjournals.aje.a112809. [DOI] [PubMed] [Google Scholar]
- Cevenini R., Mazzaracchio R., Rumpianesi F., Donati M., Moroni A., Sambri V., La Placa M. Prevalence of enteric adenovirus from acute gastroenteritis: a five year study. Eur J Epidemiol. 1987 Jun;3(2):147–150. doi: 10.1007/BF00239751. [DOI] [PubMed] [Google Scholar]
- Cruz J. R., Cano F., Càceres P., Chew F., Pareja G. Infection and diarrhea caused by Cryptosporidium sp. among Guatemalan infants. J Clin Microbiol. 1988 Jan;26(1):88–91. doi: 10.1128/jcm.26.1.88-91.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukor G., Blacklow N. R. Human viral gastroenteritis. Microbiol Rev. 1984 Jun;48(2):157–179. doi: 10.1128/mr.48.2.157-179.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Taniguchi K., Green K., Perez-Schael I., Garcia D., Sears J., Urasawa S., Kapikian A. Z. Relative frequencies of rotavirus serotypes 1, 2, 3, and 4 in Venezuelan infants with gastroenteritis. J Clin Microbiol. 1988 Oct;26(10):2092–2095. doi: 10.1128/jcm.26.10.2092-2095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Courbot M. C., Beraud A. M., Beards G. M., Campbell A. D., Gonzalez J. P., Georges A. J., Flewett T. H. Subgroups, serotypes, and electrophoretypes of rotavirus isolated from children in Bangui, Central African Republic. J Clin Microbiol. 1988 Apr;26(4):668–671. doi: 10.1128/jcm.26.4.668-671.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. E., Blacklow N. R., Perron-Henry D. M., Clements E., Taylor D. N., Echeverria P. Incidence of enteric adenoviruses among children in Thailand and the significance of these viruses in gastroenteritis. J Clin Microbiol. 1988 Sep;26(9):1783–1786. doi: 10.1128/jcm.26.9.1783-1786.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Kidd A. H., Banatvala J. E., de Jong J. C. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–341. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- Leite J. P., Pereira H. G., Azeredo R. S., Schatzmayr H. G. Adenoviruses in faeces of children with acute gastroenteritis in Rio de Janeiro, Brazil. J Med Virol. 1985 Feb;15(2):203–209. doi: 10.1002/jmv.1890150213. [DOI] [PubMed] [Google Scholar]
- Madeley C. R. The emerging role of adenoviruses as inducers of gastroenteritis. Pediatr Infect Dis. 1986 Jan-Feb;5(1 Suppl):S63–S74. doi: 10.1097/00006454-198601001-00012. [DOI] [PubMed] [Google Scholar]
- Mata L., Simhon A., Urrutia J. J., Kronmal R. A., Fernández R., García B. Epidemiology of rotaviruses in a cohort of 45 Guatamalan Mayan Indian children observed from birth to the age of three years. J Infect Dis. 1983 Sep;148(3):452–461. doi: 10.1093/infdis/148.3.452. [DOI] [PubMed] [Google Scholar]
- Shinozaki T., Araki K., Ushijima H., Fujii R. Antibody response to enteric adenovirus types 40 and 41 in sera from people in various age groups. J Clin Microbiol. 1987 Sep;25(9):1679–1682. doi: 10.1128/jcm.25.9.1679-1682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele A. D., Bos P., Alexander J. J. Clinical features of acute infantile gastroenteritis associated with human rotavirus subgroups I and II. J Clin Microbiol. 1988 Dec;26(12):2647–2649. doi: 10.1128/jcm.26.12.2647-2649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Uhnoo I., Wadell G., Svensson L., Johansson M. E. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984 Sep;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L., Perez I., Perez M., Urbina G., Greenberg H., Kapikian A., Flores J. Relative frequency of rotavirus subgroups 1 and 2 in Venezuelan children with gastroenteritis as assayed with monoclonal antibodies. J Clin Microbiol. 1984 Apr;19(4):516–520. doi: 10.1128/jcm.19.4.516-520.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Kim H. W., Clem T., Wyatt R. G., Kalica A. R., Chanock R. M., Kapikian A. Z. Enzyme-linked immunosorbent assay (ELISA) for detection of human reovirus-like agent of infantile gastroenteritis. Lancet. 1977 Aug 6;2(8032):263–267. doi: 10.1016/s0140-6736(77)90951-5. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Lawrence F., Leister F., Takiff H. E., Strauss S. E. Gastroenteritis associated with enteric type adenovirus in hospitalized infants. J Pediatr. 1982 Jul;101(1):21–26. doi: 10.1016/s0022-3476(82)80173-x. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Zissis G., Brandt C. D., Rodriguez W. J., Kim H. W., Parrott R. H., Urrutia J. J., Mata L., Greenberg H. B. Epidemiology of human rotavirus Types 1 and 2 as studied by enzyme-linked immunosorbent assay. N Engl J Med. 1978 Nov 23;299(21):1156–1161. doi: 10.1056/NEJM197811232992103. [DOI] [PubMed] [Google Scholar]


