Abstract
Purpose
To determine the age-specific prevalence of refractive errors in White and African-American preschool children.
Design
The Baltimore Pediatric Eye Disease Study is a population-based evaluation of the prevalence of ocular disorders in children aged six through 71 months in Baltimore, Maryland, United States.
Participants
Among 4,132 children identified, 3,990 eligible children (97%) were enrolled and 2,546 children (62%) were examined.
Methods
Cycloplegic autorefraction was attempted on all children using a Nikon Retinomax K-Plus 2. If a reliable autorefraction could not be obtained after three attempts, cycloplegic streak retinoscopy was performed.
Main Outcome Measures
Mean spherical equivalent (SE) refractive error, astigmatism, and prevalence of higher refractive errors among African American and White children.
Results
The mean spherical equivalent (SE) of right eyes was +1.49 diopter (D) (standard deviation (SD) =1.23) in White and +0.71D (SD=1.35) in African-American children (mean difference of 0.78D, 95% CI: 0.67, 0.89). Mean SE refractive error did not decline with age in either group. The prevalence of myopia of 1.00 D or more in the eye with the lesser refractive error was 0.7% in White and 5.5% in African-American children (RR: 8.01 95% confidence interval (CI): 3.70, 17.35). The prevalence of hyperopia of +3D or more in the eye with the lesser refractive error was 8.9% in White and 4.4% in African-American children (relative risk (RR): 0.49, 95% CI: 0.35, 0.68). The prevalence of emmetropia (less than −1.00 D to less than +1.00 D) was 35.6% in Whites and 58.0 % in African-Americans (RR: 1.64, 95% CI: 1.49, 1.80). Based on published prescribing guidelines 5.1% of the children would have benefited from spectacle correction. However, only 1.3% had been previously prescribed correction.
Conclusions
Significant refractive errors are uncommon in this population of urban preschool children. There was no evidence for a myopic shift over this age range in this cross-sectional study. A small proportion of preschool children would likely benefit from refractive correction, but few have had this prescribed.
Keywords: refractive error, children, prevalence, risk factors
Introduction
While many studies have assessed the refractive status of school-aged children1–7, few population-based studies have been conducted looking at the refractive status among preschool children8, and none have been conducted in the United States. Significant refractive errors left uncorrected in younger children places them at risk for amblyopia9 and strabismus.10
We conducted a population-based study of the visual system of urban African-American and White children age six through 71 months. In this report we provide the distribution of refractive errors. These data will aid in the estimation of the need for spectacles and provide preliminary information on how much of that need is currently being met.
Methods
The Baltimore Pediatric Eye Disease Study (BPEDS) is a population-based study of African-American and non-Hispanic White children designed to estimate the prevalence and risk factors for pediatric ocular disease.11 The study included children age six through 71 months who resided in contiguous portions of northeastern and eastern Baltimore City and eastern Baltimore County. All study procedures were approved by the institutional review boards of the Johns Hopkins Bloomberg School of Public Health, the Maryland Department of Health and Mental Hygiene, and the Battelle Memorial Institute.
The details of the methodology of BPEDS are reported elsewhere.11 In brief, all children aged six through 71 months who resided in selected contiguous census tracts of Baltimore City and County were eligible to participate. The BPEDS eye examination included assessments of ocular adnexae, anterior and posterior segments, ocular alignment, extraocular muscle motility, stereoacuity, visual acuity, ocular biometry, and refractive error under cycloplegia.
Examinations were conducted from December 2003 through March 2007 in a centrally located, free-standing clinic set up specifically for this study in the community. Children who failed to present to the clinic were offered an in-home examination. Cycloplegia and refraction were conducted in the home exactly as they were in the clinic setting.
Determination of Refractive Error
Refractive error was measured after cycloplegia using a single drop of proparacaine 0.5% followed by two drops of cyclopentolate. The cyclopentolate concentration was determined by age: 1% for children one year of age or older and 0.5% for children six months to 11 months of age. Five minutes elapsed between instillation of each cyclopentolate drop. Streak retinoscopy was performed a minimum of 30 minutes following instillation of the second drop of cyclopentolate. If fluctuation of the retinoscopic reflex was observed, an additional drop of cyclopentolate was administered and the refraction performed 30 minutes later.
Cycloplegic autorefraction was attempted on all children using a handheld Nikon Retinomax K-Plus 2 (Nikon Corporation, Tokyo, Japan). Calibration of the autorefractor was performed daily using a model eye. An instrument-generated confidence level of 8 or greater was required for the measurement to be considered reliable. A maximum of three autorefraction attempts were performed. In the event that a reliable autorefraction measure could not be obtained, a study-certified optometrist or ophthalmologist performed cycloplegic streak retinoscopy in a dimly illuminated room using a Welch Allyn retinoscope (Welch Allyn Medical Products, Skaneateles Falls, NY) with the child fixating at distance. For children with strabismus, the fellow eye was occluded while performing retinoscopy to prevent off-axis measurements. Children whose parent(s)/guardian(s) refused cycloplegia were not included in the refractive error analyses.
Guidelines based on clinical consensus for the prescription of spectacles for infants through age three years have been published in a Preferred Practice Pattern by the American Academy of Ophthalmology.12 We extended those prescribing guidelines for the treatment of myopia, hyperopia, and astigmatism through 47 months of age. We also included children age 48 to 71 months by adding isoametropic myopia and hypermetropia thresholds for prescribing spectacles to −1.50 diopters (D) and +3.50 D, respectively. We then applied the guidelines to our cohort to determine how many children should have had correction prescribed. In addition we evaluated the effect of decreasing the threshold for astigmatism correction to > +1.50 D from +2.00 D.
During our intake interview we determined which patients had been previously prescribed glasses. We evaluated the refractive error findings of these children against the prescribing guidelines to determine what proportion should have received glasses.12 For this analysis we stipulated that a patient with esotropia or a history of esotropia, even if not meeting the refractive error thresholds, would be considered appropriately treated with correction.
Statistical Analysis and Data Management
Confidence intervals for prevalence estimates were calculated using the normal approximation or Poisson distribution where appropriate. Emmetropia was defined as spherical equivalent (SE) refractive error of > −1.00 D and < +1.00 D. Myopia was defined as SE refractive error less than or equal to −1.00 D and hyperopia was defined as SE refractive error greater than or equal to +1.00 D. Astigmatism was defined as cylinder power of 1.5 D or greater and anisometropia was defined as an interocular difference of 1.0D or more in SE. A multiple linear regression model was used to assess the association of age, gender and ethnicity with refractive errors, and a comparable model with normal distribution, identity link, and exchangeable correlation was fit using Generalized Estimating Equations when both right and left eyes were included. SAS version 9.1.3 (Cary, NC) was used for data analyses.
Results
BPEDS enrolled either through door-to-door contact or on the phone 4132 children and 2546 (62%) were examined. Of these, 248 were excluded from these analyses because they were not categorized as African-American or White. Thus, 1268 African-Americans and 1030 Whites (Table 1) are the subjects of this report. The groups of children examined and not examined were similar, except that children examined were more likely to have a caregiver not working outside of the home and a caregiver more likely to have 16 or more years of education.11 Of the 2298 who were examined, the parents of 107 children refused cycloplegia and their refractive errors were based on the non-cyclopleged evaluation. Thirteen children (0.5%) were unable to be refracted (and were excluded from the analysis), of whom 3 had refused drops and the cycloplegia status of the other 10 was not determined.
Table 1.
Demographic Characteristics of Participants
| Age | White (n=1030) | African American (n=1268) | Total (n=2298) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| 6–11 months | 84 (8.2%) | 83 (6.6%) | 167 (7.3%) |
| 12–23 months | 175 (17.0%) | 191 (15.1%) | 366 (15.9%) |
| 24–35 months | 189 (18.4%) | 248 (19.6%) | 437 (19.0%) |
| 36–47 months | 210 (20.4%) | 240 (18.9%) | 450 (19.6%) |
| 48–59 months | 201 (19.5%) | 261 (20.6%) | 462 (20.1%) |
| 60–72 months | 171 (16.6%) | 245 (19.3%) | 416 (18.1%) |
| Gender | |||
| Male | 467 (45.3%) | 627 (49.4%) | 1094 (47.6%) |
| Female | 563 (54.7%) | 641 (50.6%) | 1204 (52.4%) |
Spherical Equivalent Refractive Error
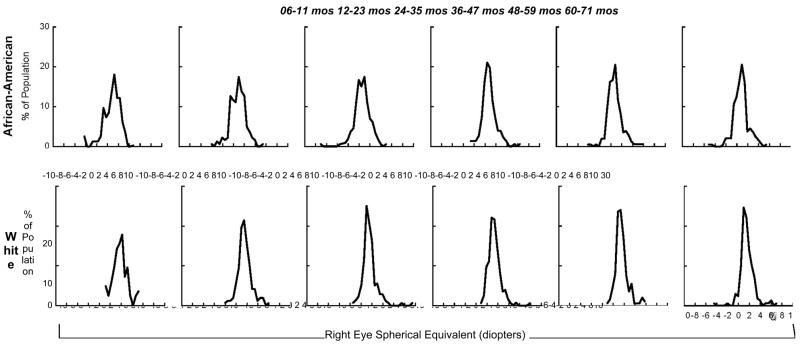
Refractive error was determined by cycloplegic autorefraction in 1805 subjects in both eyes (70.9%), while cycloplegic streak retinoscopy was required in either eye of 480 subjects (18.9%). The mean SE refractive error for African-American children was +0.71 D (standard deviation (SD) 1.35) and +0.73 D (SD 1.34) in right and left eyes respectively (Table 2). Mean SE error for White children was +1.49 D for right (SD 1.23) and left eyes (SD 1.28). The range for SE was −5.00 to +9.75 for whites and −8.50 to +6.50 for blacks. The difference between White and African American children was 0.78 D (95% CI: 0.67, 0.89) in the right eye, and 0.77 D (95% CI: 0.66, 0.88) in the left eye. The SE refractive error in both African-Americans and Whites did not change significantly between six through 71 months (p=0.99 and p=0.15, respectively) (Figure 1). Using both eyes in a multivariate regression model, the difference in refractive error between White and African American children was 0.77 D (95% CI: 0.66, 0.88), adjusted for age and sex. The sex difference in this model was 0.13 D (95% CI: 0.02, 0.24), and there was a non-significant decline in refractive error by 0.0011 D (95% CI: −0.0041, 0.0018) for each increasing month of age
Table 2.
Mean Refractive Error by Age and Race
| Age (mo), n=(AA, White) | Sphere (D), (SD) | Cylinder (D), (SD) | Spherical Equivalent (D), (SD) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right Eye | Left Eye | Right Eye | Left Eye | Right Eye | Left Eye | |||||||
| AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | |
| 6–11 (83, 84) | 0.45 (1.65) | 1.46 (1.44) | 0.53 (1.55) | 1.43 (1.40) | 0.61 (0.62) | 0.82 (0.83) | 0.62 (0.59) | 0.75 (0.74) | 0.75 (1.56) | 1.88 (1.37) | 0.84 (1.47) | 1.79 (1.36) |
| 12–23 (191, 175) | 0.52 (1.44) | 1.27 (1.25) | 0.52 (1.43) | 1.27 (1.21) | 0.54 (0.54) | 0.49 (0.52) | 0.53 (0.50) | 0.50 (0.53) | 0.80 (1.40) | 1.52 (1.25) | 0.78 (1.39) | 1.52 (1.24) |
| 24–35 (248, 189) | 0.32 (1.45) | 1.21 (1.26) | 0.30 (1.40) | 1.30 (1.36) | 0.56 (0.61) | 0.54 (0.57) | 0.58 (0.59) | 0.50 (0.54) | 0.60 (1.37) | 1.48 (1.28) | 0.59 (1.32) | 1.55 (1.45) |
| 36–47 (240, 210) | 0.38 (1.27) | 1.03 (1.15) | 0.40 (1.24) | 1.01 (1.09) | 0.57 (0.59) | 0.49 (0.54) | 0.57 (0.55) | 0.49 (0.60) | 0.67 (1.25) | 1.28 (1.11) | 0.68 (1.23) | 1.25 (1.09) |
| 48–59 (261, 201) | 0.44 (1.40) | 1.30 (1.14) | 0.49 (1.43) | 1.29 (1.19) | 0.57 (0.72) | 0.43 (0.47) | 0.59 (0.68) | 0.43 (0.53) | 0.73 (1.31) | 1.51 (1.16) | 0.79 (1.39) | 1.50 (1.22) |
| 60–72 (245, 171) | 0.46 (1.47) | 1.28 (1.27) | 0.46 (1.44) | 1.33 (1.30) | 0.63 (0.74) | 0.51 (0.58) | 0.59 (0.74) | 0.42 (0.60) | 0.78 (1.37) | 1.53 (1.28) | 0.76 (1.35) | 1.54 (1.37) |
| TOTAL (1268, 1030)* | 0.42 (1.42) | 1.23 (1.23) | 0.44 (1.40) | 1.25 (1.25) | 0.58 (0.65) | 0.52 (0.57) | 0.58 (0.62) | 0.49 (0.58) | 0.71 (1.35) | 1.49 (1.23) | 0.73 (1.34) | 1.49 (1.28) |
AA - African American
W – White
D – Diopters
SD – Standard deviation
5 African Americans missing sphere and cylinder measures in both eyes (2 in age group 12–23, 1 in age group 36–47, 1 in age group 48–59 and 1 in age group 60–71)
8 Whites missing sphere and cylinder measures in the right eye and 9 are missing these in the left eye (1 in age group 6–11 missing left eye only, 2 in age group 12–23, 4 in age group 24–35, 1 in age group 36–47, 1 in age group 48–59 missing both eyes)
Figure 1.
Distribution of Spherical Equivalent Refractive Error in the Right Eye by Age and Ethnicity.
Considering the eye with the greater SE refractive error, African-Americans had a higher prevalence of emmetropia than Whites (47.0% versus 25.1%, relative prevalence= 1.87 (95% CI: 1.60, 2.11, Table 3). Conversely, Whites had a higher prevalence of hypermetropia (relative prevalence = 1.62 (95% CI: 1.51, 1.74). Similar results were found when assessing the eye with the lesser SE refractive error (Table 4, Table 1 (available at http://aaojournal.org).).
Table 3.
Prevalence of Spherical Equivalent Refractive Error in the Eye with Greater Refractive Error among African Americans and Whites
| Age (mo), n= (AA, W) |
Myopic SE, n (%) | Emmetropic SE, n (%) |
Hyperopic SE, n (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −4 D or less |
−3 D or less | −2 D or less | −1 D or less |
> −1 D to < +1 D |
+1 D or greater |
+2 D or greater |
+3 D or greater |
+4 D or greater |
+5 D or greater |
|||||||||||
| AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | |
| 6–11 (83, 84) | 2 (2.4) | 0 (0.0) | 3 (3.6) | 0 (0.0) | 5 (6.0) | 0 (0.0) | 8 (9.6) | 0 (0.0) | 29 (34.9) | 16 (19.1) | 46 (55.4) | 68 (81.0) | 20 (24.1) | 43 (51.2) | 4 (4.8) | 20 (23.8) | 1 1.2) | 6 (7.1) | 0 (0.0) | 3 (3.6) |
| 12–23 (181, 175) | 1 (0.5) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 4 (2.1) | 1 (0.6) | 14 (7.5) | 4 (2.3) | 75 (39.7) | 35 (20.2) | 100 (53.2) | 134 (77.5) | 40 (21.2) | 57 (33.0) | 14 (7.4) | 21 (12.1) | 4 (2.1) | 10 (5.8) | 1 (0.5) | 2 (1.2) |
| 24–35 (248, 189) | 1 (0.4) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 8 (3.2) | 0 (0.0) | 26 (10.5) | 2 (1.1) | 111 (44.8) | 47 (25.4) | 111 (44.8) | 136 (73.5) | 39 (15.7) | 56 (30.3) | 13 (6.3) | 24 (13.0) | 3 (1.2) | 10 (5.4) | 2 (0.8) | 5 (2.7) |
| 36–47 (240, 210) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | 6 (2.5) | 0 (0.0) | 14 (5.9) | 0 (0.0) | 133 (55.7) | 73 (34.9) | 92 (38.5) | 136 (65.1) | 37 (15.5) | 54 (25.8) | 15 (6.3) | 20 (9.6) | 3 (1.3) | 6 (2.9) | 2 (0.8) | 3 (1.4) |
| 48–59 (261, 201) | 1 (0.4) | 0 (0.0) | 3 (1.2) | 0 (0.0) | 5 (1.9) | 0 (0.0) | 16 (6.2) | 3 (1.5) | 129 (49.6) | 50 (25.0) | 115 (44.2) | 147 (73.5) | 42 (16.2) | 55 (27.5) | 22 (8.5) | 28 (14.0) | 9 (3.5) | 12 (6.0) | 4 (1.5) | 7 (3.5) |
| 60–72 (245, 171) | 2 (0.8) | 1 (0.6) | 2 (0.8) | 1 (0.6) | 4 (1.6) | 1 (0.6) | 16 (6.6) | 2 (1.2) | 117 (48.0) | 36 (31.1) | 111 (45.5) | 133 (77.8) | 42917.2) | 57 (33.3) | 19 (7.8) | 22 (12.9) | 5 (2.1) | 9 (5.3) | 4 (1.6) | 5 (2.9) |
| TOTAL (1268, 1030)* | 7 (0.6) | 1 (0.1) | 12 (1.0) | 1 (0.1) | 32 (2.5) | 2 (0.2) | 94 (7.4) | 11 (1.1) | 594 (47.0) | 257 (25.1) | 575 (45.5) | 754 (73.8) | 220 (17.4) | 322 (31.5) | 87 (6.9) | 135 (13.2) | 25 (2.0) | 53 (5.2) | 13 (1.0) | 25 (2.4) |
AA – African American
W - White
SE – spherical equivalent
D - Diopters
5 African Americans missing sphere and cylinder measures in both eyes (2 in age group 12–23, 1 in age group 36–47, 1 in age group 48–59 and 1 in age group 60–71)
8 Whites missing sphere and cylinder measures in the right eye and 9 are missing these in the left eye (1 in age group 6–11 missing left eye only, 2 in age group 12–23, 4 in age group 24–35, 1 in age group 36–47, 1 in age group 48–59 missing both eyes)
17 children (3 White, 14 African American) with spherical equivalent of equal but opposite magnitude. All were within the emmetropic range.
Table 4.
Prevalence of Spherical Equivalent Refractive Error in the Eye with Lesser Refractive Error among African Americans and Whites
| Age (mo), n= (AA, W) |
Myopic SE, n (%) | Emmetropic SE, n (%) |
Hyperopic SE, n (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −4 D or less |
−3 D or less | −2 D or less | −1 D or less | > −1 D to < +1 D |
+1 D or greater |
+2 D or greater |
+3 D or greater |
+4 D or greater |
+5 D or greater |
|||||||||||
| AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | AA | W | |
| 6–11 (83, 84) | 1 (1.2) | 0 (0.0) | 1 (1.2) | 0 (0.0) | 3 (3.6) | 0 (0.0) | 5 (6.0) | 0 (0.0) | 35 (42.2) | 22 (26.2) | 43 (51.8) | 62 (73.8) | 18 (21.7) | 34 (40.5) | 3 (3.6) | 16 (19.1) | 1 (1.2) | 5 (6.0) | 0 (0.0) | 1 (1.2) |
| 12–23 (191, 175) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 4 (2.1) | 1 (0.6) | 12 (6.4) | 4 (2.3) | 91 (48.2) | 56 (32.4) | 86 (45.5) | 113 (65.3) | 32 (16.9) | 49 (28.3) | 10 (5.3) | 19 (11.0) | 4 (2.1) | 8 (4.6) | 1 (0.5) | 2 (1.2) |
| 24–35 (248, 189) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 5 (2.0) | 0 (0.0) | 19 (7.7) | 0 (0.0) | 146 (58.9) | 68 (36.8) | 83 (33.5) | 117 (63.2) | 29 (11.7) | 40 (21.6) | 8 (3.2) | 14 (7.6) | 1 (0.4) | 8 (4.3) | 1 (0.4) | 2 (1.1) |
| 36–47 (240, 210) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.3) | 0 (0.0) | 11 (4.6) | 0 (0.0) | 156 (65.3) | 94 (45.0) | 72 (30.1) | 115 (55.0) | 29 (12.1) | 35 (16.8) | (62.5) | 12 (5.7) | 3 (1.3) | 2 (1.0) | 2 (0.8) | 1 (0.5) |
| 48–59 (261, 201) | 1 (0.4) | 0 (0. 0) | 2 (0.8) | 0 (0.0) | 3 (1.2) | 0 (0.0) | 10 (3.9) | 1 (0.5) | 165 (63.5) | 69 (34.5) | 85 (32.7) | 130 (65.0) | 29 (11.2) | 40 (20.0) | 14 (5.4) | 17 (8.5) | 4 (1.5) | 7 (3.5) | 2 (0.8) | 3 (1.5) |
| 60–72 (245, 171) | 2 (0.8) | 1 (0.6) | 2 (0.8) | 1 (0.6) | 3 (1.2) | 1 (0.6) | 12 (4.9) | 2 (1.2) | 140 (57.4) | 55 (32.2) | 92 (37.7) | 114 (66.7) | 30 (12.3) | 41 (24.4) | 14 (5.7) | 13 (7.6) | 4 (1.6) | 6 (3.5) | 0 (0.0) | 4 (2.3) |
| TOTAL (1268, 1030)* | 4 (0.3) | 1 (0.1) | 8 (0.6) | 1 (0.1) | 21 (2.3) | 2 (0.2) | 69 (5.5) | 7 (0.7) | 733 (58.0) | 364 (35.6) | 461 (36.5) | 651 (63.7) | 167 (13.2) | 239 (23.4) | 55 (4.4) | 91 (8.9) | 17 (1.3) | 36 (3.5) | 6 (0.5) | 13 (1.3) |
AA – African American
W - White
SE – spherical equivalent
D - Diopters
5 African Americans missing sphere and cylinder measures in both eyes (2 in age group 12–23, 1 in age group 36–47, 1 in age group 48–59 and 1 in age group 60–71)
8 Whites missing sphere and cylinder measures in the right eye and 9 are missing these in the left eye (1 in age group 6–11 missing left eye only, 2 in age group 12–23, 4 in age group 24–35, 1 in age group 36–47, 1 in age group 48–59 missing both eyes)
17 children (3 White, 14 African American) with spherical equivalent of equal but opposite magnitude. All were within the emmetropic range.
Large SE refractive errors were uncommon. In the eye with the greater SE refractive error 78 (3.4%) children had hyperopia of +4 D or more, and 13 (0.6%) had myopia of −3 D or more. SE refractive error analyses did not differ by gender. The SE findings by race for the eye with the greatest refractive error, the least refractive error, the left and the right eye are presented in Appendix 1 (see online supplemental material).
Astigmatic Refractive Error
Mean astigmatism for African-American children was +0.58 D (SD 0.65) for right eyes and +0.58 D (SD 0.62) for left eyes (Table 2). Mean astigmatism for White children was +0.52 D (SD 0.57) for right eyes and +0.49 D (SD 0.58) for left eyes. The mean astigmatic error remained stable in African-American children across the age range studied from six through 71 months (p=0.26), while for Whites there was a statistically significant, although small, decrease of 0.04 D (95% CI: 0.02, 0.06, p=0.0005) of astigmatism per year of age. The decline was most evident among White children younger than 24 months.
There was a low prevalence of astigmatism of 3.00 D or more (Table 5), with-the-rule (WTR) in 1.2%, oblique in 0.5%, and no subjects with against-the-rule (ATR) astigmatism. There were no significant ethnic differences in the prevalence or direction of astigmatism.
Table 5.
Prevalence of Astigmatism by Severity and Age in the Eye with Greater Astigmatism among African Americans and Whites
| Age (mo), N=(AA, White) | Cylinder Power (D) | Worse Eye, n (%) | |||||
|---|---|---|---|---|---|---|---|
| 75 to 105 degrees (WTR) | 165 to 195 degrees (ATR) | Oblique | |||||
| AA | White | AA | White | AA | White | ||
| 6–11 (81, 83) | 1.5 or greater | 6 (7.4) | 11(13.3) | 3 (3.7) | 2 (2.4) | 4 (4.9) | 8 (9.6) |
| 3.0 or greater | 0 (0.0) | 2 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | |
| 12–23 (186, 171) | 1.5 or greater | 12 (6.5) | 9 (5.3) | 3 (1.6) | 3 (1.8) | 5 (2.7) | 6 (3.5) |
| 3.0 or greater | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | |
| 24–35 (246, 183) | 1.5 or greater | 18 (7.3) | 17 (9.3) | 2 (0.8) | 1 (0.5) | 14 (5.7) | 1 (0.5) |
| 3.0 or greater | 2 (0.8) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| 36–47 (237, 205) | 1.5 or greater | 27 (11.4) | 13 (6.3) | 1 (0.4) | 1 (0.5) | 5 (2.1) | 7 (3.4) |
| 3.0 or greater | 2 (0.8) | 4 (2.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 48–59 (258, 199) | 1.5 or greater | 26 (10.1) | 16 (8.0) | 0 (0.0) | 0 (0.0) | 5 (1.9) | 1 (0.5) |
| 3.0 or greater | 3 (1.2) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 0 (0.0) | |
| 60–72 (243, 171) | 1.5 or greater | 23 (9.5) | 18 (10.5) | 3 (1.2) | 0 (0.0) | 6 (2.5) | 1 (0.6) |
| 3.0 or greater | 8 (3.3) | 3 (1.8) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 0 (0.0) | |
| TOTAL (1251, 1012)* | 1.5 or greater | 112 (9.0) | 84 (8.3) | 12 (1.0) | 7 (0.7) | 39 (3.1) | 24 (2.4) |
| 3.0 or greater | 15 (1.2) | 12 (1.2) | 0 (0.0) | 0 (0.0) | 4 (0.3) | 2 (0.2) | |
AA – African American
D - Diopters
Oblique axis astigmatism includes all orientations not with-the-rule (WTR) or against-the-rule (ATR).
In children with equal power astigmatism where one eye has an oblique axis and the other eye has WTR or ATR astigmatism, the eye with oblique axis is defined as the worse eye.
There were 7 children with equal power astigmatism in each eye, with one eye axis WTR and the other ATR, but the cylinder measure was <1.5 D for all of them.
5 African Americans missing sphere and cylinder measures in both eyes (2 in age group 12–23, 1 in age group 36–47, 1 in age group 48–59 and 1 in age group 60–71). An additional 12 African Americans missing axis measures in both eyes (2 in age group 6–11, 3 in age group 12–23, 2 in age group 24–35, 2 in age group 36–47, 2 in age group 48–59, 1 in age group 60–71).
8 Whites missing sphere and cylinder measures in the right eye and 9 are missing these in the left eye (1 in age group 6–11 missing left eye only, 2 in age group 12–23, 4 in age group 24–35, 1 in age group 36–47, 1 in age group 48–59 missing both eyes).
An additional 10 Whites missing axis measures in both eyes (1 in age group 6–11, 2 in age group 12–23, 2 in age group 24–35, 4 in age group 36–47, 1 in age group 48–59).
Anisometropia
Clinically important anisometropia was uncommon. The prevalence of 2.00 D or more of anisometropia was 1.0% among African-American and 1.5% among White children. (Table 6) Anisometropia of 3.00 D or more was present in 0.2% of African-Americans and 0.7% of Whites (RR:0.35, 95% CI: 0.09, 1.34).
Table 6.
Prevalence and Types of Anisometropia in African Americans and Whites
| Age (mo), n=(AA, White) | Difference in Power (D) | Hyperopic SE | Myopic SE | SE Antimetropic (mixed) | Astigmatic | Total SE Anisometropia | Total Anisometropia | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | White | AA | White | AA | White | AA | White | AA | White | AA | White | ||
| 6–11 (83, 84) | 1.0 or > | 2 (2.4) | 5 (6.0) | 2 (2.4) | 0 (0.0) | 2 (2.4) | 1 (1.2) | 4 (4.8) | 5 (6.0) | 6 (7.2) | 6 (7.1) | 10 (12.0) | 11 (13.1) |
| 2.0 or > | 0 (0.0) | 3 (3.6) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 3 (3.6) | 1 (1.2) | 3 (3.6) | |
| 3.0 or > | 0 (0.0) | 1 (1.2) | 1 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 1 (1.2) | 1 (1.2) | 1 (1.2) | |
| 12–23 (191, 175) | 1.0 or > | 3 (1.6) | 5 (2.9) | 0 (0.0) | 0 (0.0) | 3 (1.6) | 2 (1.1) | 6 (3.1) | 6 (3.4) | 6 (3.1) | 7 (4.0) | 12 (6.3) | 13 (7.4) |
| 2.0 or > | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.6) | 1 (0.5) | 1 (0.6) | |
| 3.0 or > | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 24–35 (248, 189) | 1.0 or > | 5 (2.0) | 7 (3.7) | 2 (0.8) | 0 (0.0) | 3 (1.2) | 3 (1.6) | 13 (5.2) | 9 (4.8) | 10 (4.0) | 10 (5.3) | 23 (9.3) | 19 (10.1) |
| 2.0 or > | 1 (0.4) | 2 (1.1) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.5) | 2 (0.8) | 2 (1.1) | 3 (1.2) | 3 (1.6) | |
| 3.0 or > | 1 (0.4) | 2 (1.1) | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.8) | 2 (1.1) | 2 (0.8) | 2 (1.1) | |
| 36–47 (240, 210) | 1.0 or > | 3 (1.3) | 6 (2.9) | 0 (0.0) | 0 (0.0) | 4 (1.7) | 1 (0.5) | 12 (5.0) | 11 (5.2) | 7 (2.9) | 7 (3.3) | 19 (7.9) | 18 (8.6) |
| 2.0 or > | 1 (0.4) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.5) | 2 (1.0) | 1 (0.4) | 1 (0.5) | 1 (0.4) | 3 (1.4) | |
| 3.0 or > | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.5) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.0) | |
| 48–59 (261, 201) | 1.0 or > | 10 (3.8) | 11 (5.5) | 0 (0.0) | 0 (0.0) | 5 (1.9) | 1 (0.5) | 14 (5.4) | 5 (2.5) | 15 (5.7) | 12 (6.0) | 29 (11.1) | 17 (8.5) |
| 2.0 or > | 2 (0.8) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.4) | 1 (0.5) | 2 (0.8) | 0 (0.0) | 3 (1.1) | 3 (1.5) | 5 (1.9) | 3 (1.5) | |
| 3.0 or > | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| 60–72 (245, 171) | 1.0 or > | 8 (3.3) | 6 (3.5) | 2 (0.8) | 0 (0.0) | 1 (0.4) | 3 (1.8) | 15(6.1) | 8 (4.7) | 11 (4.5) | 9 (5.3) | 26 (10.6) | 17 (9.9) |
| 2.0 or > | 0 (0.6) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 2 (0.8) | 2 (1.2) | |
| 3.0 or > | 0 (0.6) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.2) | 0 (0.0) | 2 (1.2) | |
| TOTAL(1268, 1030)* | 1.0 or > | 31 (2.4) | 40 (3.9) | 6 (0.5) | 0 (0.0) | 18 (1.4) | 11 (1.1) | 64 (5.0) | 44 (4.3) | 55 (4.3) | 51 (5.0) | 119 (9.4) | 95 (9.2) |
| 2.0 or > | 4 (0.3) | 9 (0.9) | 2 (0.2) | 0 (0.0) | 2 (0.2) | 3 (0.3) | 5 (0.4) | 3 (0.3) | 8 (0.6) | 12 (1.2) | 13 (1.0) | 15 (1.5) | |
| 3.0 or > | 1 (0.1) | 4 (0.4) | 2 (0.2) | 0 (0.0) | 0 (0.00) | 1 (0.1) | 0 (0.0) | 2 (0.2) | 3 (0.2) | 5 (0.5) | 3 (0.2) | 7 (0.7) | |
AA – African American
SE – spherical equivalent
D - Diopters
5 AA missing spherical equivalent in both eyes.
9 Whites missing spherical equivalent in at least one eye (8 in both eyes).
Spectacle Use and Need
Based on consensus guidelines for spectacle correction modified to include the age range of BPEDS, 116 of 2,298 (5.05%; 95%CI: 4.14, 5.96%) participants would benefit from spectacle correction for ametropia (Table 7). If we applied a criterion of astigmatism of > 1.50D of astigmatism, the overall proportion increased to 7.0%.
Table 7.
Spectacle Need Based on Prescribing Guidelines*
| Age in months (n) | ||||
|---|---|---|---|---|
| 6–11mo (167) | 12–23mo (366) | 24–47mo (887)† | 48–71mo (878)†† | |
| Type of Ammetropia | n (%) [recommendation] | n (%) [recommendation] | n (%) [recommendation] | n (%) [recommendation] |
| Isoametropia | ||||
| Myopia | 1 (0.60) [>= −4 D] | 0 (0.00) [>= −4 D] | 2 (0.23) [>= −3 D] | 16 (1.82) [>= −1.5 D] |
| Hyperopia no esotropia | 1 (0.60) [>= +6 D] | 3 (0.82) [>= +5 D] | 7 (0.79) [>= +4.50 D] | 26 (2.96) [>= +3.5 D] |
| Hyperopia with esotropia | 1 (0.60) [> +2 D] | 1 (0.27) [> +2 D] | 4 (0.45) [> +1.50] | 3 (0.34) [> +1.50] |
| Astigmatism | 2 (1.20) [>= 3 D] | 0 (0.00) [>= 2.50 D] | 11 (1.24) [> 2 D] | 20** (2.28) [> 2 D] |
| Anisometropia | ||||
| Myopia | 1 (0.60) [>= −2.50 D] | 0 (0.00) [>= −2.50 D] | 1 (0.11) [>= −2 D] | 1 (0.11) [>= −2 D] |
| Hyperopia | 0 (0.00) [>= +2.50 D] | 0 (0.00) [>= +2 D] | 9 (1.01) [>= +1.50 D] | 12 (1.37) [>=+1.50 D] |
| Astigmatism | 0 (0.00) [>= 2.50 D] | 0 (0.00) [>= 2 D] | 4 (0.45) [>= 2 D] | 4 (0.46) [>=2 D] |
Guidelines from American Academy Ophthalmology (AAO), Preferred Practice Patterns (PPP) 2007.
Guidelines extended from 36 months through 47 months.
Not part of AAO PPP.
If astigmatism in 48–72 mo age is changed to >1.5D, then 67 need glasses due to this criterion and a total of 160 need glasses. Of these 16 presented with glasses.
Only 29 participants (1.26%) were wearing glasses at the time of their study evaluation. Twelve (41%) had esotropia or a history of esotropia. Of these 12, six (21%) had no need for correction based on refractive error, but may have been prescribed spectacles for management of the strabismus or for other unknown reasons. Of the 17 non-strabismic children, 3 had astigmatism, 1 anisometropia, 2 hypermetropia and 1 myopia meeting prescribing guidelines (Table 7).12 Ten children (35%) did not meet refractive error correction guidelines or have a strabismus.
Discussion
We have conducted a population-based cross-sectional prevalence study of ocular disease among children six through 71 months living in Baltimore City and the adjacent areas of Baltimore County. Significant refractive errors were uncommon. Hyperopia was the most common refractive error among both African-American and White children, but African-Americans were on average about +0.75D less hyperopic than Whites. The difference in refractive errors was consistent across the age range of the study population. In addition, while others have reported that refractive error is hypermetropic in infants and shifts towards emmetropia during the first few years of life13–17, we did not find this trend toward emmetropia in our study for either racial group when analyzing the entire age range. We did find a small decline in hypermetropia among White children between age 6–11 months and age 12–23 months.
One study of the refractive error of 298 infants residing in Contra Costa, California17 (ethnicity was not mentioned) between 3 and 36 months of age found the mean SE to decrease significantly between 3 and 9 months of age. However, they found no significant change after 9 months of age similar to our observation. Another study used cycloplegic retinoscopy of 113 infants in the United Kingdom reported a 0.76 D myopic shift during the first year of life.15 In a study of 254 predominately White children, also from the United Kingdom, subjects were examined at age nine and 20 months of age.16 A significant decrease in mean SE occurred between these two refractions. Possible explanations for the difference between these previously published reports and our study may include sampling error in previously published clinic-based reports, real differences in the refractive status of the populations studied, methods of refraction, and use of cycloplegia.
Risk factors for the development of amblyopia in children include anisometropia, high ametropia, and astigmatism. Anisometropia of 2.00 D or more was uncommon in our study population, affecting 1.0% of African-Americans and 1.5% of Whites studied. Astigmatism of 3.00 D or more was uncommon in both groups, though about 11% had astigmatism of 1.50 D or more. The magnitude of astigmatism did not change with increasing age among African-American children, but decreased with increasing age among White children. A decrease in astigmatism has been previously reported.17–20 The observed reduction in astigmatism could be a testing artifact due to more accurate visual axis measurement of older and presumably more cooperative children.
Previous reports differ on the most common form of astigmatism among children, with some finding ATR most prevalent18,21,22 while others found WTR.23,24 Cross-sectional studies show an increase in prevalence of WTR and decrease in prevalence of ATR with increasing age.25,26 Similarly, the axis of astigmatism in our study population was nearly always WTR, with little change with age and no differences by race.
Requirement of Refractive Correction
The need to correct ametropia in preschool children is a recognized public health intervention to prevent the development of amblyopia and strabismus. Debate exists regarding the type and magnitude of refractive errors that warrant correction and at what age.12,27–30 The 2007 Preferred Practice Pattern from the American Academy of Ophthalmology includes prescribing guidelines for refractive error for children from infancy to 3 years of age.12 Extending these guidelines through age 71 months (with modification for myopia and hypermetropia in 4–5 year-olds – see Methods) yields a need for prescribed refractive correction of 5.1% of our urban population (Table 7). If we were to apply a more inclusive criterion of astigmatism of > 1.50D of astigmatism as suggested by Donahue26 for children age 4–5 years old, the overall proportion increases to 7.0%. Of the 29 children who had been prescribed correction prior to their BPEDS examination, no indication for use could be found for 10 (34%). These observations of an unmet need in our study population for refractive correction plus the prescription of unnecessary glasses to some children suggests the need to improve the quality of eye care being provided to children in this urban area.
There are a number of strengths to our study. The subjects participated in a population-based study with prospectively developed procedures including an objective measure of refractive error with complete cycloplegia. The large number of children studied allows for fairly precise estimates of the prevalence of refractive errors for each of the age ranges.
There are limitations to the findings. First, participation in the study was incomplete and there may have been sampling bias if more or fewer children with refractive errors or visual symptoms attended the clinic than were present in the larger population. If there was a bias associated with caregiver characteristics, we are uncertain in which direction it would have driven our findings. Second, children who had health problems at birth were more likely to be examined which may have increased the estimated prevalence of refractive error. However, no differences in guardian-reported history of ocular disease or visual symptoms existed between those who were examined and those who were not. Lastly, African American subjects were more myopic than Whites. In designing the study we were concerned that African-American children were more likely to have inadequate cycloplegia, thus biasing the African-American patients to less measured hypermetropia. In order to limit this problem, the protocol required that the study refractionists perform dynamic retinoscopy to determine if complete cycloplegia had been achieved after 30 minutes, and administer an additional dose of cyclopentolate if residual accommodation was observed. However, it is still possible that the disparity in mean SE refractive error was due in part to systematic inadequate cycloplegia among African-Americans.
Conclusion
This population-based study of urban African-American and White children aged six through 71 months of age documented low rates of clinically significant spherical equivalent refractive error, anisometropia and astigmatism. African-Americans were more myopic and had slightly more astigmatism than Whites. Spectacle use was rare in this population, even among those children with clinically important refractive errors.
Acknowledgments
Support: Supported by the National Eye Institute, National Institutes of Health, Bethesda, MD (EY14483)
We thank the members of the Data Monitoring and Oversight Committee, Drs. Eileen Birch, Karen Cruickshanks, Jonathan Holmes (chair), Natalie Kurinij (NEI ex-officio), Maureen Maguire, Joseph Miller, Graham Quinn, and Karla Zadnik, for their help in completing this study. These individuals met with the study investigators both by phone and in person prior to initiating the study and throughout the study. They assisted in the design of the research and the analysis of the data. They provided feedback on the conduct of the study as well as on this specific manuscript.
Footnotes
Conflict of Interest: The authors have no conflict of interest
Online Materials: This article contains additional online-only material. The following should appear online-only: Table 4 and Appendix 1.
References
- 1.Negrel AD, Maul E, Pokharel GP, Zhao J, Ellwein LB. Refractive error study in children: sampling and measurement methods for a multi-country survey. Am J Ophthalmol. 2000;129:421–6. doi: 10.1016/s0002-9394(99)00455-9. [DOI] [PubMed] [Google Scholar]
- 2.Zaho J, Pan X, Sui R, et al. Refractive error study in children: results from Shunyi District, China. Am J Ophthalmol. 2000;129:427–435. doi: 10.1016/s0002-9394(99)00452-3. [DOI] [PubMed] [Google Scholar]
- 3.Pokharel GP, Negrel AD, Munoz SR, Ellwein LB. Refractive error study in children: results from Mechi Zone, Nepal. Am J Ophthalmol. 2000;129:436–444. doi: 10.1016/s0002-9394(99)00453-5. [DOI] [PubMed] [Google Scholar]
- 4.Maul E, Barroso S, Munoz SR, et al. Refractive error study in children: results from La Florida, Chile. Am J Ophthalmol. 2000;129:445–454. doi: 10.1016/s0002-9394(99)00454-7. [DOI] [PubMed] [Google Scholar]
- 5.Murthy GVS, Gupta SK, Ellwein LB, et al. Refractive error in children in an urban population in New Delhi. Inves Ophthalmol Vis Sci. 2002;43:623–631. [PubMed] [Google Scholar]
- 6.Naidoo KS, Raghunandan A, Mashige KP, et al. Refractive error and visual impairment in African children in South Africa. Inves Ophthalmol Vis Sci. 2003;44:3764–3770. doi: 10.1167/iovs.03-0283. [DOI] [PubMed] [Google Scholar]
- 7.He M, Zeng J, Liu Y, et al. Refractive error and visual impairment in urban children in Southern China. Inves Ophthalmol Vis Sci. 2004;45:793–9. doi: 10.1167/iovs.03-1051. [DOI] [PubMed] [Google Scholar]
- 8.Dandona R, Dandona L, Naduvilath TJ, et al. Refractive errors in an urban population in southern India: the Andhra Pradesh eye disease study. Inves Ophthalmol Vis Sci. 1999;40:2810–8. [PubMed] [Google Scholar]
- 9.Weakley DR. The association between anisometropia, amblyopia, and binocularity in the absence of strabismus. Trans Am Ophthalmol Soc. 1999;97:987–1021. [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson J, Braddick O, Bobier B, et al. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo- and videorefractive screening. Eye. 1996;10:189–198. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DS, Repka MX, Katz J, et al. Prevalence of Decreased Vision among Preschool Children 30 to 71 months of age in an American Urban Population. The Baltimore Pediatric Eye Disease Study. In Press. [Google Scholar]
- 12.American Academy of Ophthalmology. Preferred Practice Pattern. San Francisco: American Academy of Ophthalmology; 2007. Pediatric Eye Evaluations, I. Screening, II. Comprehensive Ophthalmic Evaluation. [Google Scholar]
- 13.Mayer DL, Hansen RM, Bruce DM, et al. Cycloplegic refractions in healthy children aged 1 through 48 months. Arch Ophthalmol. 2006:1625–8. doi: 10.1001/archopht.119.11.1625. [DOI] [PubMed] [Google Scholar]
- 14.Ingram RM, Gill LE, Lambert TW. Emmetropisation in normal and strabismic children and the associated changes in anisometropia. Strabismus. 2003;11:71–84. doi: 10.1076/stra.11.2.71.15104. [DOI] [PubMed] [Google Scholar]
- 15.Wood IC, Hodi S, Morgan L. Longitudinal change of refractive error in infants during the first year of life. Eye. 1995;9:551–7. doi: 10.1038/eye.1995.138. [DOI] [PubMed] [Google Scholar]
- 16.Erlich DL, Braddick OJ, Atkinson J, et al. Infant emmetropization: longitudinal changes in refraction components from nine to twenty months of age. Optom Vis Sci. 1997;74:822–843. doi: 10.1097/00006324-199710000-00022. [DOI] [PubMed] [Google Scholar]
- 17.Mutti DO, Mitchell GL, Jones LA, et al. Refractive astigmatism and the toricity of ocular components in human infants. Optom Vis Sci. 2004;81:753–761. doi: 10.1097/00006324-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamsson M, Fabian G, Sjostrand J. Changes in astigmatism between ages of 1 and 4 years: a longitudinal study. Br J Ophthalmol. 1988;72:145–9. doi: 10.1136/bjo.72.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atkinson J, Braddick O, French J. Infant astigmatism: it’s disappearance with age. Vis Research. 1980;20:891–3. doi: 10.1016/0042-6989(80)90070-x. [DOI] [PubMed] [Google Scholar]
- 20.Gwiazda J, Grice K, Held R, et al. Astigmatism and the development of myopia in children. Vis Res. 2000;40:1019–1026. doi: 10.1016/s0042-6989(99)00237-0. [DOI] [PubMed] [Google Scholar]
- 21.Abrahamsson M, Fabian G, Anderson AK, Sjostrand J. A longitudinal study of a population based sample of astigmatic children I. Refraction and amblyopia. Acta Ophthalmol. 1990;68:428–434. doi: 10.1111/j.1755-3768.1990.tb01671.x. [DOI] [PubMed] [Google Scholar]
- 22.Howland HC, Sayles N. Photorefractive measurements of astigmatism in infants and young children. Invest Ophthalmol Vis Sci. 1984;25:93–102. [PubMed] [Google Scholar]
- 23.Dobson V, Miller JM, Harvey EM. Corneal and refractive astigmatism in a sample of 3 to 5 year old children with a high prevalence of astigmatism. Optom Vis Sci. 1999;76:855–860. doi: 10.1097/00006324-199912000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Fan DSP, Rao SK, Cheung EYY, et al. Astigmatism in Chinese preschool children: prevalence, change, and effect on refractive development. Br J Ophthalmol. 2004;88:938–941. doi: 10.1136/bjo.2003.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwiazda J, Scheiman M, Mohindra I, Held R. Astigmatism in children: changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci. 1984;25:88–92. [PubMed] [Google Scholar]
- 26.Dobson V, Fulton AB, Sebris SL. Cycloplegic refractions of infants and young children: the axis of astigmatism. Invest Ophthalmol. 1984;25:83–7. [PubMed] [Google Scholar]
- 27.Miller JM, Harvey EM. Spectacle prescribing recommendations of AAPOS members. J Pediatr Ophthalmol Strabismus. 1998;35:51–2. doi: 10.3928/0191-3913-19980101-17. [DOI] [PubMed] [Google Scholar]
- 28.Donahue SP. Prescribing spectacles in children: a pediatric ophthalmologist’s approach. Optom Vis Sci. 2007;84:110–4. doi: 10.1097/OPX.0b013e318031b09b. [DOI] [PubMed] [Google Scholar]
- 29.Lyons SA, Jones LA, Walline JJ, et al. A survey of clinical prescribing philosophies for hyperopia. Optom Vis Sci. 2004;81:233–7. doi: 10.1097/00006324-200404000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Harvey EM, Miller JM, Dobson V, Clifford CE. Prescribing eyeglass correction for astigmatism in infancy and early childhood: a survey of AAPOS members. J AAPOS. 2005;9:189–191. doi: 10.1016/j.jaapos.2004.12.001. [DOI] [PubMed] [Google Scholar]