SUMMARY
Background
Poor levels of medication adherence for patients with coronary heart disease (CHD) have been documented but it is unclear whether adherence has improved over time.
Methods
We assembled a retrospective cohort of lower-income Medicare beneficiaries who were discharged from the hospital after their first acute myocardial infarction (MI) between 1 January 1995 and 31 December 2003. For patients prescribed a statin, ACEI/ARB, beta-blocker, and all 3 of these medications after the hospital discharge, we evaluated medication adherence by determining the proportion of days covered (PDC) for each medication in the subsequent year.
Results
Our cohort consisted of a total of 33 646 patients. Adherence rates for statins and beta-blockers, but not ACEI/ARB, increased significantly over time but remained suboptimal. For example, among those patients that received a statin after discharge, 38.6% were fully adherent with therapy in 1995 in contrast to 56.2% in 2003 (p value for trend <0.001). Of patients prescribed all 3 of statin, beta-blocker, and ACEI/ARB, 29.1% and 46.4% were fully adherent in 1995 and 2003, respectively (p value for trend <0.001).
Conclusions
Our analysis demonstrates statistically significant but modest improvements in medication adherence for statins and beta-blockers, but not ACEI/ARBs, among patients discharged from hospital after acute MI. Despite these improvements, rates of non-adherence to these highly effective therapies remain extremely high. Given the health and economic consequences of non-adherence, the development of cost-effective strategies to improve medication adherence should be a clear priority.
Keywords: adherence, myocardial infarction, preventice therapy, drug utilization
INTRODUCTION
Coronary heart disease (CHD) remains the leading cause of death in the US and other developed countries;1 more than 1 million Americans have an acute myocardial infarction (MI) every year.2 In 2006, CHD is estimated to have accounted for more than $140 billion in direct and indirect health care costs.2
Highly effective medications for the treatment and prevention of CHD-related events have been identified and evaluated,3-8 and practice guidelines now recommend that post-MI patients receive treatment with a beta-blocker, a lipid lowering agent, an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), and aspirin,9 unless a contraindication exists. Aided by large-scale efforts, such as the American Heart Association’s Get With the Guidelines program10 and the American College of Cardiology’s Guidelines Applied in Practice (GAP) initiative,11 more MI patients are prescribed effective pharmacotherapy when being discharged from hospital.12
Patients must adhere to their prescribed treatment regimens in order for these medications to maximally reduce the burden of CHD.13 Poor adherence to post-MI medications has been documented.14 For example, only 45% of patients are fully adherent with beta-blockers in the 1 year after an acute MI,15 and only 50% of patients are adherent with their prescribed statin.16 Less than 20% of acute MI patients use all four of the recommended agents.17 Non-adherence is of particular relevance to the elderly, as the prevalence of CHD and the risk of death after MI increases as a function of age.18,19
Interventions to improve medication adherence have been developed and evaluated.20 However, it is unclear if these interventions and the growing attention to non-adherence more generally have resulted in improvements in medication-taking behavior for elderly patients with CHD and other common chronic conditions. Accordingly, we used a large population-based cohort of Medicare beneficiaries discharged from hospital after acute MI to evaluate trends in adherence to commonly prescribed secondary prevention medications.
METHODS
Setting and design
We assembled a retrospective cohort of Medicare patients who were discharged from hospital after their first acute MI between 1 January 1995 and 31 December 2003 by linking Medicare files to data from the Pennsylvania Pharmaceutical Assistance Contract for the Elderly (PACE)18 and the New Jersey Pharmaceutical Assistance to the Aged and Disabled (PAAD)19 programs. Both PACE and PAAD provide prescription drug benefits to individuals of age ≥65 whose yearly earnings are above the threshold to qualify them for Medicaid. Participants pay co-payments between $5 and $10 per prescription without any deductibles and are supplied a maximum of 30 days of medication with each prescription. The programs cover all medications that require a prescription and do not restrict which medications can be prescribed (i.e., the programs do not use formularies, preferred drug lists, or prior authorization programs).
We combined filled prescription data from PACE and PAAD with complete paid claims data from Medicare Parts A and B describing all clinical encounters for these individuals. The data were assembled into a relational database consisting of data for all filled prescriptions, procedures, inpatient and outpatient physician encounters, hospitalizations, long-term care admissions, and deaths for the patients in our cohort. These data sources have been used extensively to study population-based health outcomes.20-22 All traceable person-specific identifying factors were transformed into anonymous, coded study numbers to protect subjects’ privacy.
The date of hospital discharge after acute MI was considered as the index date for our analysis. In order to create a cohort of patients who experienced their first MI during the study period, we excluded patients who had an MI during the year prior to their index admission and considered only the first MI discharge for patients who had more than one eligible event during the follow-up time. We also excluded patients who were not active users of Medicare and either drug benefit program during the 1-year period prior to and after their MI hospitalization so as to have complete ascertainment of co-morbidities and outcomes. If patients died during follow-up, they were included in our cohort but were censored on the date of death.
The institutional review board of Brigham and Women’s Hospital approved this study.
Medication use and adherence assessment
We identified whether the patients in our cohort were prescribed a statin, ACEI/ARB, beta-blocker, and all 3 of these medications within 90 days after the hospital discharge. For patients who received one or more of the drugs of interest, we evaluated medication adherence by determining the proportion of days covered (PDC) for each medication, a valid and widely used adherence metric.21 PDC is calculated by dividing the number of days of medication supplied (numerator) by the number of days (denominator) in a given interval.
For our analysis, the numerator was the sum of number of days of medication supplied on each prescription after hospital discharge to 1 year after the index date or death, whichever came first. For example, a patient who was discharged from hospital on 31 December 2003 was followed until 31 December 2004. The denominator was the number of days between the first prescription date and 1 year after the index date or death, whichever came first. For each drug, we considered all prescriptions written for an agent in the class, regardless of the specific agent. For prescriptions written near the end of the observation window that had more days supplied than were left in the observation window, we counted the days supplied as only the number of days between that prescription date and the end of the observation window. We subtracted any days patients spent in hospital or a nursing home after the index date from the denominator. To assess simultaneous adherence to all three medication classes, we calculated the average PDC. On the basis of their PDCs, we classified patients into 1 of 3 groups for each medication using standard thresholds: ≥80% (‘fully adherent’), 40–79% (‘partially adherent’), and <40% (‘non-adherent’).22
Covariates
We determined patient co-morbidities by searching all data from ambulatory care visits and hospitalizations for relevant diagnostic codes in the 1 year prior to the index date. In this manner, we identified the following characteristics: age at index date, gender, race, hypertension, diabetes, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic kidney disease, and chronic obstructive pulmonary disease. We also assessed use of the following medications in the year prior the index hospitalization: statins, ACEI/ARB, beta-blockers, calcium channel blockers, diuretics, nitrates, digoxin, and clopidogrel. Finally, we evaluated whether patients underwent angioplasty and coronary stent insertion when they were hospitalized for their index event.
Statistical analysis
We categorized patients based on the year they experienced their MI and then plotted the annual proportion of patients who received each and all three of the of the study medications (statins, ACEI/ARB, and beta-blocker) within 90 days of hospital discharge and who were fully adherent to these drugs. We assessed whether the proportion of patients who were fully adherent to therapy (i.e., PDC ≥80%) changed over time using bivariate logistic regression models. We then used multivariable logistic models to adjust for differences in patient co-morbidity and demographics and their use of cardiovascular procedures and medications between the annual cohorts and to identify other predictors of changes in adherence. In a secondary analysis, we assessed whether trends in adherence differed for patients who were using statins, ACEI/ARB, beta-blockers, and all 3 of these agents prior to their MI (i.e., ‘prevalent’ users) and those who were newly prescribed medications after their MI (i.e., ‘new users’), defined as not having filled a prescription for a given medication in the 12 months before their first prescription date. Finally, we repeated the analyses using multivariable linear models to assess PDC as a continuous, rather than categorical, variable. The statistical significance of regression coefficients in all our models was assessed using two-sided t tests. All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
RESULTS
Patient population
Our cohort consisted of a total of 33 646 patients discharged from hospital after their first MI. Patients had a mean age of 81 years, three-quarters were female and 90% were white (Table 1). Patients in the later years of the study were more likely than patients in the early study years to have had cardiac risk factors, co-morbidities, and to use cardiovascular medications before being initially hospitalized. The frequency with which percutaneous interventions were used also increased over time.
Table 1.
Baseline characteristics of study cohort
| Characteristic | Cohort year |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1995 (n=3873) |
1996 (n=4684) |
1997 (n=4097) |
1998 (n=4040) |
1999 (n=3973) |
2000 (n=3738) |
2001 (n=3064) |
2002 (n=3117) |
2003 (n=3060) |
Total (n=33 646) |
|
| Demographics | ||||||||||
| Age, mean years | 79 | 80 | 80 | 80 | 81 | 81 | 82 | 82 | 82 | 81 |
| Female, % | 73.8 | 73.2 | 73.8 | 73.4 | 74.4 | 75.4 | 77.2 | 75.3 | 75.3 | 74.5 |
| White, % | 90.1 | 89.9 | 88.7 | 88.3 | 86.9 | 87.5 | 91.8 | 92.3 | 92.6 | 89.6 |
| Nursing home residence*, % | 7.3 | 9.3 | 10.1 | 11.7 | 14.1 | 14.7 | 14.5 | 12.7 | 12.4 | 11.5 |
| Co-morbid conditions, % | ||||||||||
| Congestive heart failure | 68.0 | 68.9 | 70.4 | 72.8 | 71.9 | 73.3 | 72.0 | 73.3 | 72.5 | 71.3 |
| Cerebrovascular disease | 31.6 | 33.6 | 34.8 | 36.7 | 37.9 | 42.3 | 41.3 | 40.2 | 41.0 | 37.5 |
| Peripheral vascular disease | 25.5 | 26.3 | 28.9 | 29.0 | 31.3 | 32.1 | 32.1 | 29.8 | 31.8 | 29.5 |
| Hypertension | 65.6 | 68.5 | 71.5 | 72.7 | 73.8 | 77.2 | 79.7 | 81.2 | 83.4 | 74.2 |
| Diabetes | 46.0 | 44.3 | 47.0 | 46.7 | 47.6 | 48.0 | 48.2 | 48.4 | 48.6 | 47.0 |
| Chronic obstructive pulmonary disease |
36.4 | 37.0 | 38.3 | 40.5 | 43.1 | 42.2 | 40.2 | 44.8 | 44.1 | 40.5 |
| Chronic kidney disease | 17.0 | 17.2 | 20.2 | 20.9 | 21.3 | 25.2 | 25.1 | 26.4 | 29.5 | 22.1 |
| Prior medication use, % | ||||||||||
| Statin | 10.1 | 11.0 | 15.7 | 19.4 | 21.5 | 23.1 | 28.4 | 33.4 | 35.6 | 21.0 |
| ACEI or ARB | 34.0 | 33.4 | 35.4 | 37.4 | 38.7 | 40.7 | 43.0 | 42.9 | 43.4 | 38.3 |
| Clopidogrel | 0.0 | 0.0 | 0.0 | 0.9 | 5.6 | 8.5 | 10.0 | 13.0 | 14.1 | 5.2 |
| Beta-blocker | 23.1 | 24.1 | 26.6 | 27.8 | 30.6 | 33.8 | 36.7 | 40.7 | 42.7 | 31.0 |
| Calcium-channel blocker | 53.2 | 50.6 | 48.5 | 47.2 | 45.7 | 43.6 | 43.3 | 40.9 | 40.2 | 46.3 |
| Diuretics | 24.4 | 26.8 | 31.1 | 34.3 | 36.5 | 38.6 | 39.8 | 42.2 | 41.3 | 34.4 |
| Nitrates | 49.1 | 46.6 | 45.9 | 44.4 | 44.0 | 40.5 | 39.5 | 39.2 | 35.6 | 43.2 |
| Procedures on index hospitalization |
||||||||||
| PCI | 11.6 | 12.8 | 13.3 | 15.2 | 16.0 | 15.8 | 18.0 | 19.5 | 21.1 | 15.6 |
| CABG | 7.0 | 8.4 | 8.6 | 8.0 | 8.1 | 8.4 | 7.7 | 7.9 | 7.1 | 7.9 |
During the year prior to the index MI.
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; PCI, percutaneous intervention; CABG, coronary artery bypass.
Post-MI medication use and adherence
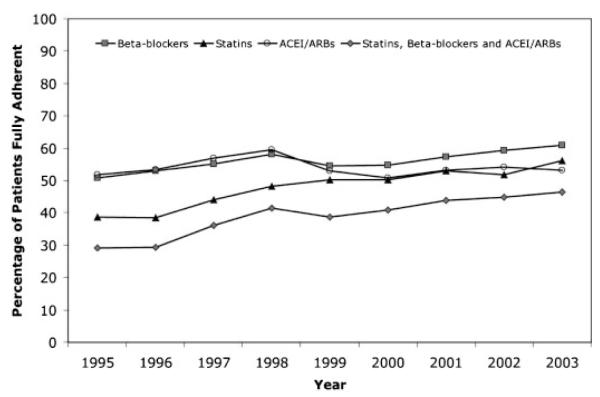
After acute MI discharge, the proportion of patients that filled prescriptions for statins, beta-blockers, and all 3 of statins, beta-blockers and ACEI/ARB within 90 days of hospital discharge increased significantly over time. For example, 9.2% of patients filled a statin within 90 days of hospital discharge in 1995 as compared to 43.1% of patients in 2003 (p value for trend <0.001). In contrast, use of ACEI or ARB after hospital discharge remained at approximately 45%.
Adherence rates for statins and beta-blockers, but not ACEI/ARB, also increased significantly over time although the magnitude of these changes was smaller than those observed for improvements in prescription rates (Figure 1). For example, among those patients that received a statin after discharge, 38.6% were fully adherent with therapy in 1995 in contrast to 56.2% in 2003 (p value for trend <0.001). Of patients prescribed all 3 of statin, beta-blocker, and ACEI/ARB, 29.1 and 46.4% were fully adherent in 1995 and 2003, respectively (p value for trend <0.001). Adherence to ACEI/ARB therapy remained unchanged through the study period (p=0.33).
Figure 1.
Trends in 1 year adherence to post-myocardial infarction medications by year of hospital discharge
In our secondary analysis, we found that while rates of adherence among prevalent users were significantly higher than those among patients newly prescribed the study medications after their MI, the trends in adherence for new and prevalent users did not differ for ACEI/ARB, beta-blockers, or all 3 of the study medications. In contrast, patients newly prescribed statins after their MI had greater improvements over time than for prevalent statin users (p<0.001).
Repeating our analysis using PDC as a continuous variable yielded identical results to those presented above.
Predictors of adherence
Trends in adherence persisted after adjusting for patient demographics, co-morbidity, medication, and procedure use (Table 2). Other consistent predictors of increased adherence included white race and a diagnosis of hypertension. In contrast, patients with chronic obstructive pulmonary disease were less likely to adhere to post-MI medications. Women and nursing home residents were more likely to adhere to beta-blockers and ACEI/ARBs but not statins. Patients who had undergone CABG were less likely to adhere to statins and ACEI/ARB but not beta-blockers.
Table 2.
Multivariable predictors of the odds of being fully adherent
| Predictor | Statin | Beta—blocker | ACEI /ARB | All of statin, beta—blocker, and ACEI/ARB |
|---|---|---|---|---|
| Year of discharge (per year) | 1.08 (1.06–1.10) | 1.03 (1.02–1.05) | 0.99 (0.98–1.01) | 1.09 (1.06–1.12) |
| Demographics | ||||
| Age (per year) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (1.00–1.01) | 1.00 (0.99–1.01) |
| Male | 1.07 (0.97–1.17) | 0.88 (0.82–0.94) | 0.93 (0.86–1.00) | 0.85 (0.74–0.98) |
| White race | 1.79 (1.56–2.05) | 1.54 (1.40–1.70) | 1.46 (1.32–1.61) | 1.74 (1.43–2.11) |
| Nursing home residence* | 1.05 (0.86–1.27) | 1.22 (1.08–1.38) | 1.30 (1.15–1.47) | 1.41 (1.06–1.87) |
| Co—morbid conditions | ||||
| Congestive heart failure | 0.99 (0.91–1.08) | 1.00 (0.94–1.07) | 1.25 (1.16–1.34) | 1.02 (0.90–1.16) |
| Cerebrovascular disease | 1.04 (0.95–1.13) | 0.97 (0.97–1.04) | 1.02 (0.96–1.09) | 0.99 (0.87–1.12) |
| Peripheral vascular disease | 1.06 (0.97–1.17) | 1.04 (0.97–1.11) | 0.98 (0.92–1.06) | 1.05 (0.92–1.19) |
| Hypertension | 1.14 (1.03–1.26) | 1.15 (1.07–1.24) | 1.08 (1.00–1.17) | 1.20 (1.02–1.40) |
| Diabetes | 1.00 (0.92–1.08) | 0.97 (0.92–1.03) | 1.05 (0.98–1.12) | 1.05 (0.93–1.19) |
| Chronic obstructive pulmonary disease | 0.82 (0.75–0.89) | 0.83 (0.91–1.04) | 0.87 (0.81–0.93) | 0.76 (0.67–0.86) |
| Chronic kidney disease | 1.13 (1.02–1.25) | 1.05 (0.98–1.14) | 0.80 (0.73–0.86) | 0.89 (0.76–1.03) |
| Procedures on index hospitalization | ||||
| Angioplasty or stent insertion | 1.03 (0.94–1.13) | 1.09 (1.01–1.17) | 0.89 (0.82–0.97) | 1.05 (0.92–1.19) |
| CABG | 0.79 (0.69–0.90) | 0.99 (0.89–1.10) | 0.84 (0.75–0.94) | 0.84 (0.69–1.03) |
| Health system use | ||||
| Concurrent drugs (per drug) | 1.01 (1.00–1.02) | 1.01 (1.00–1.01) | 1.01 (1.00–1.01) | 1.00 (0.99–1.01) |
| Hospitalizations in prior year (per day) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) |
| Number of outpatient visits in prior year | 1.00 (0.99–1.00) | 0.99 (0.99–1.00) | 0.99 (0.99–1.00) | 1.00 (0.99–1.01) |
During the year prior to the index MI.
DISCUSSION
Our analysis of elderly Medicare beneficiaries demonstrates statistically significant but modest improvements in medication adherence for statins and beta-blockers, but not ACEI/ARBs, among patients discharged from hospital after acute MI. However, these improvements represented only a small proportion of the shortfall in medication adherence; rates of non-adherence to these highly effective therapies remain extremely high. For example, among patients prescribed a statin, a beta-blocker, and an ACEI/ARB, as currently recommended by practice guidelines for the care of post-MI patients,9 50% of patients were adherent with these medications 1 year after their hospital discharge.
Poor adherence is associated with poor health outcomes. Patients who adhere to a statin after MI have a relative risk of recurrent MI which is 81% lower than that of non-adherent patients.24 Post-MI patients who discontinue their prescribed aspirin, statin, and beta-blocker are more than 3 times more likely to die than patients who remain adherent.25 Such disparities are unexplained by the ‘healthy user effect’; that is, the observation that healthier patients are more likely to adhere to therapy (rather than adherence leading to better health).22 Health care costs are also lower among adherent patients and may more than offset their greater medication costs.26,27 Thus, our results highlight the impact which non-adherence has on health care quality and expenditures.
There are many reasons for medication non-adherence. Medication cost, complexity of treatment regimens, treatment side-effects, cognitive impairment, poor understanding of the benefits of treatment, poor provider-patient relationships, and difficulties accessing physicians or pharmacies have all been identified as relevant factors.28 Adherence may be particularly challenging for some cardiovascular medications since patients may not immediately appreciate improvements in symptoms from taking them.
The small magnitude of improvement in adherence that we observed for beta-blockers and statins and the absence of improvement for ACEI/ARBs likely reflects the complex reasons for non-adherence and, consequently, the modest effectiveness of most interventions that have been thus far developed to address this problem.20,29 In addition, many of the most successful adherence interventions are extremely labor intensive and multi-faceted and involve strategies such as individual counseling, medication education, reducing medication frequency, simplified pill distribution dispensing systems, and reminders for missed refills and appointments.20 Accordingly, the cost-effectiveness of these efforts in real world community-based settings such as those that we studied may make their practical applicability less useful.
High rates of medication non-adherence have been demonstrated by previous investigators and the rates of adherence we observed in our study mirror these.16,30 For example, Newby et al. 31 assessed medication adherence among patients who had undergone cardiac procedures at the Duke University Medical Center using annual surveys in which patients listed the medications they were taking. They found that in 2002 approximately 40% of subjects reported use of combination lipid-lowering agents, beta-blockers, and aspirin and that 35% of patients prescribed these drugs were classified as ‘consistent’ users.31
Our study builds upon these and other results by applying valid measures of adherence21 to study longitudinal trends among a state-wide sample of elderly patients discharged from hospital after an acute MI who face little cost-relating for their post-MI medications. Our analysis also identifies other predictors of adherence such as race, which has been demonstrated as a potent predictor of adherence in other studies14 and co-morbid conditions, such as COPD, which may in some cases distract physicians and patients from the importance of preventive medications like those prescribed after acute MI.32
Our results should be interpreted in context of several limitations. First, we conducted our analysis using data from two state-wide pharmacy assistance programs that provide prescription drug benefits to lower middle income individuals of age ≥65. As a result, our cohort consisted predominantly of female patients with a high prevalence of co-morbid medical conditions. Therefore, while adherence to post-MI medications may be especially important in these high-risk populations, the results of our analysis may not be generalizable to other populations with different demographic and clinical characteristics. Second, the administrative data used do not contain detailed clinical information such as cholesterol levels or ejection fraction. Therefore, it is possible that patients appeared to be non-adherent with their prescribed therapy when in fact they had been discontinued for clinically appropriate reasons, such as hyperkalemia in patients taking ACEI/ARBs or bronchospasm in patients taking beta-blockers. Third, we are unable to assess adherence to aspirin in our cohort as this medication is primarily purchased over-the-counter and accordingly we cannot comment on whether there have been meaningful improvements in adherence to this important medication.
Nevertheless, our results have important implications for elderly patients with CHD and their physicians by highlighting the magnitude of the problem of medication non-adherence. While strategies to improve adherence to these essential medications may have generated modest gains, the majority of the problems remain. Given the health and economic consequences of non-adherence, the development of cost-effective strategies to improve medication adherence should be a clear priority. In fact, the cost of some strategies, such as reducing cost-sharing for essential cardiovascular medications, may be more than offset by the cost-savings from improved health that result from them, as we have recently demonstrated for post-MI Medicare beneficiaries enrolled in the Part D drug benefit.33 Thus, initial efforts to improve adherence may be targeted at those interventions that will improve adherence and health outcomes without increasing overall health care costs.
KEY POINTS
While prescribing rates for post-myocardial infarction (MI) secondary prevention medications have improved, it is unclear whether longer-term adherence rates have also increased.
In our analysis of post-NI Medicare beneficiaries we found that adherence rates for statins and beta-blockers, but not ACEI/ARB, have increased significantly over time but remain suboptimal.
Given the health and economic consequences of non-adherence, the development of cost-effective strategies to improve medication adherence should be a clear priority.
ACKNOWLEDGEMENTS
The authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
No conflict of interest was declared.
REFERENCES
- 1.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 2.American Heart Association . Heart Disease and Stroke Statistics—2006 Uptake. Dallas: [Google Scholar]
- 3.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. Br Med J. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease. The task force on ACE-inhibitors of the European Society of Cardiology. Eur Heart J. 2004;25:1454–1470. doi: 10.1016/j.ehj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Lopez-Sendon J, Swedberg K, McMurray J, et al. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J. 2004;25:1341–1362. doi: 10.1016/j.ehj.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Lau J, Antman EM, Jimenez-Silva J, Kupelnick B, Mosteller F, Chalmers TC. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–254. doi: 10.1056/NEJM199207233270406. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 9.Antman EM, Hand M, Armstrong PW, et al. Focused Update of the ACC/AHA 2004 Guidelines for the Management of patients with ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of patients with ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. 2007. [DOI] [PubMed] [Google Scholar]
- 10.American Heart Association [Accessed [10 July 2007]];Get with the guidelines. http://www.americanheart.org/presenter.jhtml?identifier=3045578.
- 11.Rogers AM, Ramanath VS, Grzybowski M, et al. The association between guideline-based treatment instructions at the point of discharge and lower 1 year mortality in Medicare patients after acute myocardial infarction: The American College of Cardiology’s Guidelines Applied in Practice (GAP) initiative in Michigan. Am Heart J. 2007;154:461–469. doi: 10.1016/j.ahj.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Lee TH. Eulogy for a quality measure. N Engl J Med. 2007;357:1175–1177. doi: 10.1056/NEJMp078102. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry NK, Winkelmayer WC. Medication adherence after myocardial infarction: a long way left to go. J Gen Intern Med. 2008;23:216–218. doi: 10.1007/s11606-007-0478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. Jama. 2002;288:455–461. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 15.Kramer JM, Hammill B, Anstrom KJ, et al. National evaluation of adherence to beta-blocker therapy for 1 year after acute myocardial infarction in patients with commercial health insurance. Am Heart J. 2006;152:454–e1-8. doi: 10.1016/j.ahj.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 16.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. J Am Med Assoc. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay SE, Whincup PH, Lawlor DA, et al. Secondary prevention of coronary heart disease in older patients after the national service framework: population based study. British Medical Journal. 2006;332:144–145. doi: 10.1136/bmj.38704.770127.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 Update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Cohen M, Bernink PJLM, et al. The TIMI risk score for unstable Angina/Non-ST Elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 20.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Inter Med. 2007;167:540–549. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 22.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. Jama. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 23.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction) J Am Coll Cardiol. 2004;44:E1–E211. doi: 10.1016/j.jacc.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Wei L, Wang J, Thompson P, Wong S, Struthers AD, MacDonald TM. Adherence to statin treatment and readmission of patients after myocardial infarction: a six year follow up study. Heart. 2002;88:229–233. doi: 10.1136/heart.88.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho PM, Spertus JA, Masoudi FA, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166:1842–1847. doi: 10.1001/archinte.166.17.1842. [DOI] [PubMed] [Google Scholar]
- 26.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 27.Choudhry NK, Avorn J, Antman EM, Schneeweiss S, Shrank WH. Should patients receive secondary prevention medications for free after a myocardial infarction? An economic analysis. Health Aff (Millwood) 2007;26:186–194. doi: 10.1377/hlthaff.26.1.186. [DOI] [PubMed] [Google Scholar]
- 28.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 29.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. Jama. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 30.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. J Am Med Assoc. 1998;279:1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 31.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–212. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]
- 32.Redelmeier DA, Tan SH, Booth GL. The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med. 1998;338:1516–1520. doi: 10.1056/NEJM199805213382106. [DOI] [PubMed] [Google Scholar]
- 33.Choudhry NK, Patrick AR, Antman EM, Avorn J, Shrank WH. Cost-effectiveness of providing full drug coverage to increase medication adherence in post-myocardial infarction Medicare beneficiaries. Circulation. 2008;117:1261–1268. doi: 10.1161/CIRCULATIONAHA.107.735605. [DOI] [PMC free article] [PubMed] [Google Scholar]