Abstract
The neurorestorative effects of exogenous neurturin (NTN) delivered directly into the putamen via multiport catheters were studied in 10 MPTP-lesioned rhesus monkeys expressing stable parkinsonism. The parkinsonian animals were blindly assigned to receive coded solutions containing either vehicle (n =5) or NTN (n =5, 30 µg/day). Both solutions were coinfused with heparin using convection-enhanced delivery for 3 months. The NTN recipients showed a significant and sustained behavioral improvement in their parkinsonian features during the treatment period, an effect not seen in the vehicle-treated animals. At study termination, locomotor activity levels were increased by 50% in the NTN versus vehicle recipients. Also, DOPAC levels were significantly increased by 150% ipsilateral (right) to NTN infusion in the globus pallidus, while HVA levels were elevated bilaterally in the NTN-treated animals by 10% on the left and 67% on the right hemisphere. No significant changes in DA function were seen in the putamen. Volumetric analysis of putamenal NTN labeling showed between-subject variation, with tissue distribution ranging from 214 to 744 mm3, approximately equivalent to 27–93% of area coverage. Our results support the concept that intraparenchymal delivery of NTN protein may be effective for the treatment of PD. More studies are needed to determine strategies that would enhance tissue distribution of exogenous NTN protein, which could contribute to optimize its trophic effects in the parkinsonian brain.
Keywords: Parkinson’s disease, MPTP, Neurturin, Putamen, Neuroregeneration
INTRODUCTION
Neurotrophic factors are proteins with enormous therapeutic potential in the treatment of neurodegenerative diseases such as Parkinson’s disease (PD). Neurotrophic factors may not only slow or halt the degeneration of substantia nigra dopamine (DA) neurons due to their neuroprotective properties, but may also enhance the function of residual DA neurons or even repair and restore function to injured DA neurons. Over the last decade, we and others have focused on the effects of glial cell line-derived neurotrophic factor (GDNF) on midbrain DA neurons (3,13). Using computer-controlled infusion pumps, our group has demonstrated that chronic administration of GDNF into the lateral ventricle, putamen, or substantia nigra promotes restoration of the nigrostriatal DA system and significantly improves motor functions in rhesus monkeys with neural deficits modeling PD (4,7).
Based on the promising studies of the chronic effects of intraparenchymal GDNF in nonhuman primate models of PD, a series of studies was conducted in PD patients. However, translational studies from the laboratory to the clinic for the treatment of advanced PD have been difficult. On one hand, two independent open-label phase I studies have reported marked functional improvements in a total of 15 advanced PD patients receiving intraputamenal infusion of GDNF for several months (5,17,23,28). On the other hand, a randomized, blinded multicenter phase II trial conducted in 34 advanced PD patients, of which 17 received intraputamenal GDNF infusion for 6 months, did not achieve its primary study endpoint (i.e., 25% change from baseline in off-medication motor scores) (16,20). There were major technical differences between these clinical trials in catheter design and infusion protocols (27), which may have resulted in variable distribution of GDNF in the brain parenchyma and likely contributed to the different study outcomes (25). Although there is no evidence of serious GDNF-induced adverse effects in patients at this time, all trials testing for human recombinant GDNF were stopped due to safety concerns, including but not limited to the presence of neutralizing antibodies to GDNF in some patients (16,28).
As an alternative to GDNF, other members of the GDNF family ligands have been tested for their therapeutic potential, such as neurturin (NTN), which shares about 42% similarity with mature GDNF (15). NTN protein has been shown to promote the survival and morphological differentiation of several different neuronal subtypes in vitro, including midbrain DA neurons (11, 32). Along the same lines, intrastriatal injections of NTN protein in rodent models of PD have demonstrated neuroprotective and neuroregenerative effects on DA nigrostriatal function (21,24). In nonhuman primates, the intracranial delivery of adeno-associated type 2 viral vector encoding human NTN (AAV2-NTN) has been reported to prevent toxin-induced motor disability following injection into the striatum (14). While gene delivery allows for bilateral administration of the trophic factor into multiple sites and long-term transgene expression with no need for chronically implanted hardware, the development of techniques for determining and controlling dosing and timing remain difficult. In addition, the direct delivery of NTN protein into the brain has not previously been tested in a nonhuman primate model of PD, and whether this approach could be used as an alter-native to viral vector delivery of NTN is unknown.
Here we report the results of an experiment designed to blindly assess the restorative effects of exogenous NTN protein chronically delivered to the nonhuman primate nigrostriatal DA system. To promote tissue distribution, NTN was coadministered with heparin similar to Hamilton et al. (9) and infused using convection-enhanced delivery via computer-controlled pumps and indwelling catheters. While targeting of multiple sites is difficult, programmable pumps can dispense drugs in a variety of ways. The rate and timing of delivery (e.g., continuous or timed infusion) are noninvasively programmed through an external computer, allowing a greater range of flexibility and control in administrating trophic factors at a desired dose into specific brain sites. Locomotor activity was measured using automated video tracking software, and movement dysfunctions characteristic of PD were rated using a nonhuman primate clinical rating scale. Following 3 months of infusion treatment, the animals were euthanized and their brains recovered for quantitative post mortem analyses, including neurochemical measurements of DA function and unbiased stereological cell counting. Our results support the concept that intraparenchymal delivery of NTN protein may be effective for the treatment of PD.
MATERIALS AND METHODS
Animals
Ten female rhesus monkeys (Macaca mulatta) ranging in age from 14 to 19 years old and weighing between 6 and 10 kg were used for this study. The animals were maintained on a 12-h light/12-h dark cycle and single-housed with individual access to an adjacent activity module. The diet consisted of certified nonhuman primate Harlan Teklad chow, supplemented daily in mid-afternoon with fresh fruit or vegetables. Water was available ad libitum. All procedures were approved by the University of Kentucky Animal Care and Use Committee and were conducted in the Laboratory Animal Facilities of the University of Kentucky, which are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Veterinarians skilled in the health care and maintenance of nonhuman primates supervised all animal care.
Surgical Procedures
MPTP Administration
The animals received right intracarotid artery infusions of 1-methyl-4-phenyl-1,2, 3,6-tetrahydropyridine (MPTP; 0.8–0.9 mg per animal) to induce continuously expressed parkinsonian features as per procedures previously described (22). Briefly, following ligation of the external carotid artery, a volume of 4–4.5 ml of MPTP solution (0.2 mg/ml) was delivered at a rate of 1 ml/min using a 25-gauge butterfly needle bent at the tip to a slight angle and inserted in retrograde direction to the blood flow through the common carotid artery. The incision was then closed as per normal surgical procedures. Following MPTP administration, all animals developed stable parkinsonian features as assessed using a clinical rating scale adapted for rhesus monkeys (22).
Pump and Catheter Placement
NTN or its vehicle was chronically delivered via intraparenchymal multiport catheters and programmable infusion pumps (Medtronic Inc., Minneapolis, MN). The anesthetized animals (1–3% isoflurane) were placed in a stereotaxic head frame in a ventral-lateral position. Using sterile procedures, an incision was made through the scalp and the skin and muscles overlying the skull were reflected. Then a small hole was drilled in the skull directly over the right putamen as per MRI-guided stereotaxic procedures and the overlying meninges were punctured to expose the surface of the brain for insertion of the catheter. The catheter (1 mm O.D., Model 8770IP24A) is designed so that six laser holes are placed radially on a longitudinal distance of 3 mm over each 90° of the catheter’s circumference, for a total of 24 laser holes (0.0375 mm in diameter). Once positioned, the catheter was then connected via flexible polyurethane tubing to a Synchro Med II programmable infusion pump (model 8637-20) subcutaneously implanted in the lateral abdominal region (6). After completion of the procedures, the incisions were sutured over the exposed areas per normal procedures. Each animal was given buprenorphine (0.01 mg/kg, IM) and monitored until it was ambulatory.
Drug Treatment
The animals were rank ordered into two groups of five animals as per their parkinsonian features and blindly assigned to receive either vehicle (10 mM NaAcetate, pH 5.5, 4% Mannitol) or NTN (0.2 mg/ml). Coded solutions containing NTN or its vehicle were provided by Rinat Neuroscience, Inc. (South San Fransico, CA). To enhance tissue distribution, the pumps were programmed to deliver brief pulses of 25 µl over short 2-min periods (12.5 µl/min), four times a day. In addition, the minimum basal infusion rate, which is necessary to keep the pumps operating properly between each bolus, was set at 0.033 µl/min (2 µl/h) for a total infusion volume of 150 µl per day. This represents a dose of 30 µg NTN delivered daily for 3 months. To reduce the binding of NTN to heparin binding sites in the extracellular matrix and promote tissue distribution, NTN was coinfused with heparin (9) as per the following ratio: 0.6 units of heparin per 1 µg of NTN. Each pump was refilled monthly using aseptic procedures.
Behavioral Measurements
Rating Scale
The animals were videotaped using standardized procedures (22,30). Changes in motor functions were assessed using our previously published nonhuman primate parkinsonian scale, patterned after the human Unified Parkinson’s Disease Rating Scale (22). Behavioral parameters associated with motor function were scored from coded videotapes from 0 (normal) to 3 (severe disability) in the following categories: rigidity, bradykinesia, posture, balance, tremor, and hand dexterity. Rigidity was defined as a decrease in limb extension and/or use. Motor dysfunctions were rated in half-point increments by a single experienced rater blinded to the treatment administered to the animals. In addition, the tapes were analyzed to determine if the animals displayed side effects from the treatment, including vomiting, stereotypies, and dyskinesia.
EthoVision
As described elsewhere (30), distance traveled (cm) was quantified from the same videotapes using a commercially available video tracking system (EthoVision Pro 2.3, Noldus Technology, Asheville, NC). This automated method relies on determining the position of the center of mass of the animal in the cage using a gray scale detection method by calibrating the software to distinguish the dark-colored animal from the cage background, which is then defined as all other pixels. The resultant x–y coordinates extracted as a function of time are used for calculating the movement pattern during the observation period. These coordinates were subsequently related to actual spatial measures by calibrating the software to the cage width, so that the distance traveled by the animal was calculated in centimeters instead of pixels.
Post Mortem Analyses
Tissue Biopsies
At the end of the study, each animal was deeply anesthetized with sodium pentobarbital (~15 mg/kg, IV) and transcardially perfused with 4–6 L of heparinized ice-cold saline. Then the brain was removed quickly and sectioned into 4-mm coronal slabs, rostral to the midbrain, using an ice-cold rhesus monkey brain mold. The coronal slabs were then placed at −20°C, so that multiple tissue punches could be taken bilaterally from frozen 4-mm thick coronal tissue sections using a 14-gauge biopsy needle in the putamen (n =15 punches per side) and globus pallidus (n =5 punches per side). All punches were rapidly transferred to pre-weighed storage tubes, weighed, and stored at −80?C until assayed for DA, homovanillic acid (HVA), and 3,4-dihydroxy-phenylacetic acid (DOPAC) measurements by HPLC with electrochemical detection (2,7).
Immunohistochemistry and Quantitative Morphology
In parallel, the midbrain was taken out as a block and postfixed in 4% paraformaldehyde in phosphate buffer (pH 7.4) along with all 4-mm coronal slabs rostral to the midbrain for quantitative immunocytochemistry of substantia nigra DA neurons and volume of NTN distribution in the brain. As detailed elsewhere (4), 40-µm-thick coronal sections were cut on a frozen sliding microtome through the substantia nigra and processed for immunohistochemical staining for tyrosine hydroxylase (TH, monoclonal antibody, 1:1000; Chemicon International Inc., Temecula, CA). The number of TH+ mid-brain dopaminergic neurons was estimated bilaterally using an optical fractionator method for unbiased stereological cell counting (1,4). In addition, a series of 40-µm-thick coronal sections was cut on a frozen sliding microtome through the striatum and immunostained for NTN (1:500 goat anti-human neurturin antibody; AF387, R&D). The volume of distribution of NTN was quantified using three-dimensional reconstruction software (Bioquant, Nashville, TN) on every 12th section through the brain (4,25). Quantitative morphology and volumetric analysis was performed by a single investigator blinded to the experimental conditions.
Statistics
The cumulative parkinsonian scores of the vehicle and NTN recipients were analyzed using a nonparametric Friedman test followed with a post hoc analysis using a Dunn’s Multiple Comparison Test, when appropriate. Between-group comparisons were conducted using a nonparametric Mann-Whitney Test (one-tailed). Loco-motor activity levels were analyzed using a two-factor repeated-measures analysis of variance (ANOVA) with treatment as between-subjects factor and week of treatment as within-subjects factor, followed by Newman-Keuls post hoc comparisons. Pre-MPTP locomotor activity levels are shown for comparison, but were not included in the analysis. For each hemisphere, independent sample t-tests (one-tailed) were used to estimate differences in DA, DOPAC, or HVA levels and nigral cell counts between animals in the control and NTN treatment groups. A value of p <0.05 was considered significant in all analyses.
RESULTS
Adverse Effects
No animals were observed exhibiting nausea (vomiting), stereotypic movements, dyskinesias, self-mutilation (e.g., hair loss, bite marks), or other psychotic behavior (e.g., apparently following imaginary objects or not demonstrating awareness of humans in the room) throughout the study. Also, no significant changes in body weight were measured during the study in either the vehicle- or NTN-treated animals. However, one of the NTN recipients (#P680) unexpectedly died over-night, 8 weeks posttreatment, and was excluded from the analysis for this reason. An autopsy revealed that this animal died from a massive brain hemorrhage around the catheter implantation site of undetermined cause.
Motor Functions
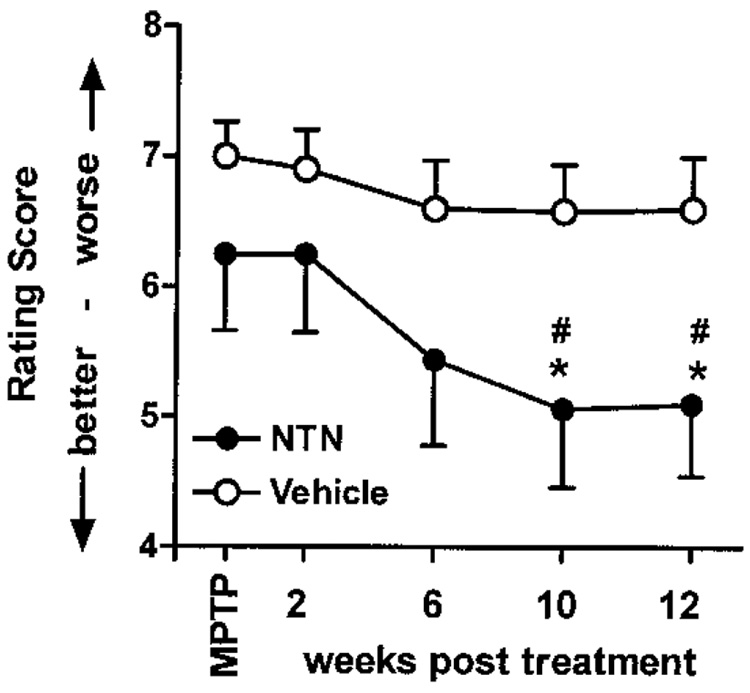
As described above, standardized videotaping procedures were conducted pre- and posttreatment to evaluate changes in motor functions from coded videotapes. Prior to chronic infusion into the right putamen, both vehicle-and NTN-treated animals were given comparable cumulative parkinsonian scores of 7.0 ± 0.26 and 6.25 ± 0.59 points, respectively, as assessed using our rating scale adapted to monkeys (Fig. 1). No significant changes in parkinsonian features were seen in the vehicle-treated animals over the 12-week infusion period (Fig. 1, open circles). In contrast, the parkinsonian scores progressively improved in the NTN recipients over the course of the study, reaching a significant 20% improvement in motor scores by the 10th week of NTN infusion (Fig. 1, filled circles). This level of improvement was maintained through the 12th week of treatment.
Figure 1.
Cumulative PD scores. Behavioral response to daily infusions of NTN or vehicle. Only the NTN recipients showed a significant and sustained behavioral improvement in their parkinsonian features during the treatment period. *p < 0.05, MPTP versus NTN treatment; #p < 0.05, vehicle versus NTN—same time point.
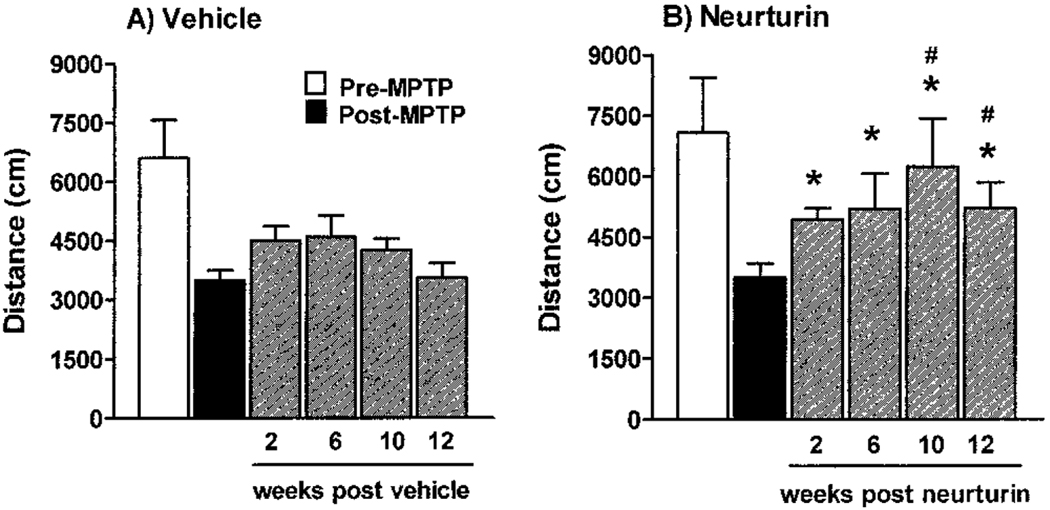
Locomotor activity levels were quantified from the same videotapes over 60-min-long observation periods using the automated video-tracking system, EthoVision. As seen in Figure 2, distance traveled (cm) measured pre-MPTP administration was comparable between the two treatment groups. Following the MPTP administration, locomotor activity levels significantly decreased by ~50% in both treatment groups compared to pre-MPTP activity levels (one-tailed, paired t-tests). No significant changes in locomotor activity levels were seen in the vehicle-treated animals over the course of the 3-month study (Fig. 2A). A slight increase in distance traveled was noted up to 6 weeks postoperatively, an effect possibly due to catheter placement into the brain parenchyma that eventually subsided to return to prevehicle treatment levels by the end of the study. In contrast, locomotor activity levels were progressively and significantly increased in the NTN recipients by up to 78% at week 10 posttreatment compared to post-MPTP baseline levels. By the end of the study, locomotor activity levels in the NTN-treated animals were still 50% higher versus post-MPTP baseline levels (Fig. 2B).
Figure 2.
Locomotor activity in vehicle- and NTN-treated animals. No significant changes in loco-motor activity levels were seen in the vehicle-treated animals over the course of the study (A). At study termination, locomotor activity levels were increased by 50% in the NTN recipients versus post-MPTP baseline levels (B). *p < 0.05, MPTP versus NTN treatment; #p < 0.05, vehicle versus NTN—same time point.
Neurochemistry
At the end of the 3-month infusion study, multiple tissue punches were taken bilaterally in the putamen (n =15 punches per side) and globus pallidus (n =5 punches per side). All punches were assayed for changes in DA, HVA, and DOPAC between animals in the control and NTN treatment groups. MPTP administration caused a marked decrease in DA and DA metabolite levels. In the vehicle-only recipients at the termination of the study, tissue levels of DA, DOPAC, and HVA in the right putamen were significantly decreased by >99%, >99% and 92%, respectively, versus the levels on the left side of the brain (Table 1). No significant differences were seen in DA, DOPAC, or HVA putamenal tissue levels between vehicle- and NTN-treated animals, either contralateral (left) or ipsilateral (right) to NTN infusion (Table 1).
Table 1.
Putamen Tissue Levels of Dopamine (DA), Homovanillic Acid (HVA), and 3,4-Dihydroxyphenylacetic Acid (DOPAC) in MPTP-Lesioned Monkeys Chronically Infused With Either Vehicle or Neurturin (NTN) Into the Right Putamen for 12 Weeks
| Treatment | Hemisphere | DA (ng/g) | DOPAC (ng/g) | HVA (ng/g) |
|---|---|---|---|---|
| Vehicle | left | 8,357 ± 468 | 2,264 ± 243 | 20,713 ± 1,155 |
| NTN | left | 8,208 ± 345 | 1,881 ± 166 | 19,812 ± 849* |
| Vehicle | right | 36 ± 9* | 22 ± 5* | 1,577 ± 308* |
| NTN | right | 42 ± 1* | 25 ± 10* | 2,214 ± 239* |
Values shown are mean ± SEM.
p < 0.01, left versus right side (same treatment group), one-tailed paired t-tests.
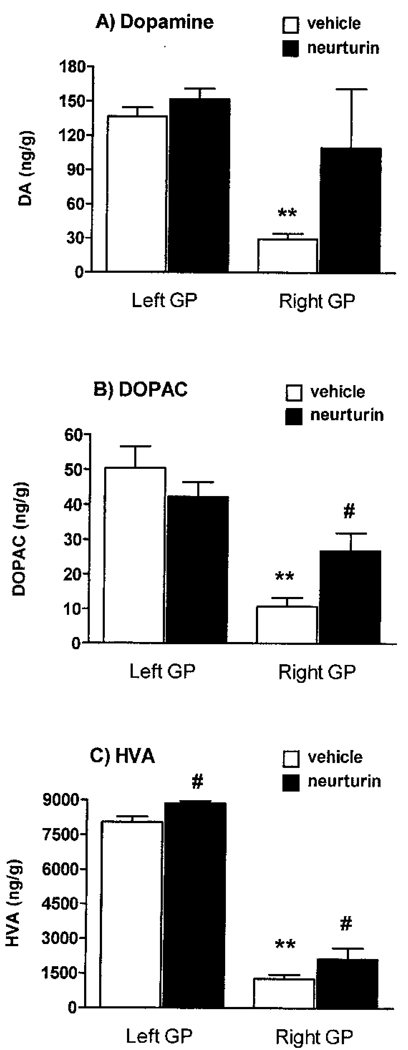
MPTP also decreased pallidal levels of DA and related metabolites in the vehicle-treated animals. When compared to tissue levels in the left pallidum, DA (137 ± 8 vs. 30 ± 5), DOPAC (50 ± 6 vs. 11 ± 3), and HVA (8,049 ± 237 vs. 1,277 ± 172) were significantly decreased by 78%, 78%, and 84%, respectively, in the right pallidum of the vehicle recipients (Fig. 3). In contrast to the putamen, significant increases in DOPAC (150%) and HVA (67%) tissue levels were measured in the globus pallidus ipsilateral (right) to NTN infusion compared to vehicle treatment (Fig. 3). A nonsignificant trend was seen for DA due to variability within the NTN-treated animals (p =0.06). Additionally, a more marginal but significant increase in HVA levels (10%) was also measured in the globus pallidus contralateral (left) to NTN infusion.
Figure 3.
Globus pallidus tissue levels of DA, HVA, and DOPAC. As seen in the vehicle recipients, MPTP administration markedly reduced the levels of DA, HVA, and DOPAC. In contrast, DOPAC levels were significantly increased 150% ipsilateral (right) to NTN infusion (B). Also, HVA levels were elevated bilaterally in the NTN-treated animals by 10% on the left and 67% on the right hemisphere (C). Values are expressed as ng/g wet weight of tissue. **p < 0.01, left versus right brain—vehicle treatment (paired t-tests); #p< 0.05, vehicle versus NTN—same side (unpaired t-tests).
Quantitative Morphology and Volume of Distribution
Consistent with decreased DA function in the putamen and globus pallidus (Table 1, and Fig. 3), the number of TH+ neurons in the right substantia nigra of vehicle recipients was significantly reduced to 13% of that found in the contralateral (left) hemisphere (Table 2). However, quantitative morphology showed no significant differences in the number of dopaminergic neurons expressing TH between NTN and vehicle recipients in the substantia nigra (Table 2).
Table 2.
Number of Substantia Nigra (SN) Cells Expressing Tyrosine Hydroxylase in MPTP-Lesioned Monkeys Chronically Infused With Either Vehicle or Neurturin (NTN) Into the Right Putamen for 12 Weeks
| Animal | Left SN Cell Number | Right SN Cell Number |
|---|---|---|
| Vehicle | ||
| H812 | 195,143 | 29,028 |
| H745 | 185,234 | 22,770 |
| 916U | 210,077 | 24,638 |
| 080I | 181,378 | 26,320 |
| P758 | 220,777 | 25,609 |
| 198,522 ± 7,452 | 25,673 ± 1,029* | |
| NTN | ||
| P429 | 198,450 | 30,970 |
| H820 | 175,338 | 21,765 |
| 89N177 | 214,510 | 25,423 |
| P685 | 157,366 | 28,566 |
| 186,416 ± 12,585 | 26,681 ± 1,994* |
p < 0.01, left versus right side (same treatment group), one-tailed paired t-tests.
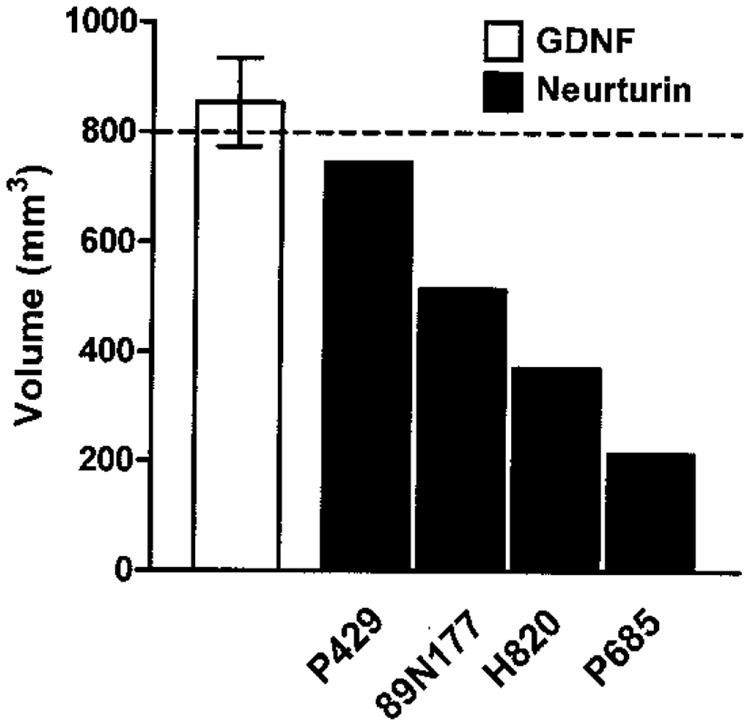
Tissue distribution of NTN in the brain was quantified from immunocytochemically stained sections using three-dimensional reconstruction software. As seen in Figure 4, the volume of distribution (Vd) of NTN in the putamen varied over threefold between animals ranging from a low 214 mm3 in one animal to 744 mm3 in the monkey with the highest volume of distribution (mean Vd ± SEM =461 ± 112 mm3), roughly equivalent to 27–93% area coverage (mean % ± SEM =58 ± 14%) based on an estimated putamenal volume of 800 mm3. In prior studies (4), the volume of distribution quantified by immunocytochemistry for chronically infused GDNF using similar multiport catheters and delivery method was found to be twofold greater than that achieved with NTN, ranging from 647 to 990 mm3 (mean Vd ± SEM =854 ± 81 mm3).
Figure 4.
Volume of tissue distribution in the putamen of four MPTP-lesioned monkeys receiving NTN via multiport catheter for 12 weeks. Volumetric analysis of putamenal NTN labeling showed between-subject variation, with tissue distribution ranging from 214 to 744 mm3. The mean volume of distribution achieved with GDNF delivered in the rhesus monkey putamen using similar multiport catheters is shown for comparison (4). The dotted line indicates the estimated volume of the entire putamen (800 mm3).
DISCUSSION
The 10 animals in this study were long-term stable hemiparkinsonian monkeys. The animals were implanted with a multiport catheter into the right putamen, which was connected to a computer-controlled pump to chronically deliver the trophic factor NTN for 3 months at a dose of 30 µg per day. One neurturin-treated animal unexpectedly died from a brain hemorrhage of undetermined cause after 8 weeks of treatment. Chronic infusion of heparin may have been a contributing factor. This is the first case of death by brain hemorrhage reported in all our studies using over 50 indwelling intraventricular or intraparenchymal catheters for the chronic infusion of a trophic factor (4,7,8,18).
Nonetheless, the results from our study indicate that exogenous NTN exerts some restorative effects in MPTP-lesioned rhesus monkeys modeling PD and extend prior reports demonstrating that the delivery of AAV2-NTN to the nigrostriatal system can restore motor function for up to 10 months and prevent nigral cell loss in parkinsonian monkeys (14). In aged monkeys, AAV2-NTN resulted in increased striatal [18F]fluorodopa uptake and enhanced TH+ fibers in the striatum (10). Consistent with a gradual increase in general locomotor activity over the course of the study described here, the parkinsonian scores were significantly improved in the NTN recipients. In accordance with our prior studies with GDNF (7), HVA (bilaterally) and DOPAC tissue levels were increased in the globus pallidus between vehicleand NTN-treated animals, whereas no significant differences were seen in DA or DA metabolite tissue levels in the putamen. These data support the hypothesis that behavioral improvements are not always associated with changes in striatal DA levels (3). The effects of NTN on pallidal DA function are of importance considering that the globus pallidus receives dopaminergic input from the substantia nigra and is involved in regulating motor functions by sending outputs to the motor cortex via the thalamus (29). Similar to GDNF in rodents (12), NTN has been shown to be transported anterogradely from the striatum to the globus pallidus in nonhuman primates (10,14). In rodent models of PD, intrastriatal administration of NTN protein promoted axonal sprouting in the globus pallidus (24). It may be that changes in extrastriatal DA function and metabolism such as those seen here in the globus pallidus are more important than striatal tissue levels of DA for promoting improvements in motor function. In contrast to our prior studies on GDNF (1,7), quantitative morphology showed no significant differences in the number of dopaminergic neurons expressing TH between NTN and vehicle recipients in the substantia nigra. Similarly, no significant differences in the survival of nigral TH+ neurons were found between vehicle controls and NTN-treated parkinsonian rats following injection of NTN into the striatum 12 weeks after 6-hydroxydopamine administration (21). While no increase in nigral cell number was seen in the NTN-treated animals herein, it is possible that NTN may be acting by upregulating DA uptake and/or release in the substantia nigra as seen before in rhesus monkeys with GDNF (8). Additional studies would be needed to determine if NTN can upregulate DA uptake and release in the nigrostriatal system as a mechanism to promote improvement in motor function.
Although statistically significant, the effects of NTN on parkinsonian features (20% reduction) reported here following single-site, unilateral delivery into the putamen were more modest compared to those obtained with GDNF in our prior studies. To put that in perspective, we have previously reported that bilateral, chronic intraputamenal infusion of 22.5 µg per day of GDNF promotes a 40% improvement in parkinsonian scores in MPTP-lesioned hemiparkinsonian rhesus monkeys using a similar delivery method (7). Also, in a recent study (14), AAV2-NTN-treated monkeys showed a mean 88% reduction in the parkinsonian features at 10 months posttreatment into multiple sites: caudate (n =2 injections), putamen (n =3 injections), and substantia nigra (n =1 injection). One possibility to explain these differences in the percentage change in antiparkinsonian benefit is that multiple site delivery of trophic factor may promote greater improvement in motor function than single site delivery. Along the same lines, dosing of exogenous NTN (30 µg/day) may not have been optimal in our study considering that NTN binds preferentially to GFR-α2 receptors of which very few are found in the nigrostriatal system (26,31). Like GDNF, NTN also binds to GFR-α1 receptors through which it may exert its action in the striatum but to a lesser extent than GDNF. Additional dose–response studies would be needed to address this question.
Tissue distribution of trophic factor is a critical variable to achieve optimal effects on DA function and promote behavioral improvement in rhesus monkeys modeling PD. In a prior study, the volume of distribution of GDNF in the trophic factor recipients significantly correlated with motor function improvements (4). Similarly, Kordower and colleagues (14) reported the case of one animal in their study with incomplete functional recovery, which had the least NTN expression within the nigrostriatal system following viral vector delivery. Thus, another possibility to explain the different effects on motor behavior noted between GDNF and NTN infusion treatments is that tissue distribution may not have been optimal with NTN. In the current study, the average tissue bioavailability of NTN achieved in the brain was limited to approximately 58 ± 14% of the rhesus putamen. This is in contrast to the much greater tissue distribution achieved with GDNF in other studies (4,18). In these studies, the volume of distribution of GDNF achieved using multiport catheters implanted into the putamen and connected to programmable pumps was twofold greater than that achieved in the current study with NTN coinfused with heparin.
Overall, the effects of exogenous NTN on motor and dopaminergic function in the globus pallidus suggest its potential therapeutic use for the treatment of PD and age-associated movement dysfunctions. However, chronic infusion of heparin may pose safety concerns, and other methods for binding heparin sites to increase volume of distribution are needed if exogenous delivery of NTN is to be pursed in the clinic. Alternative delivery methods for NTN such as viral vector-mediated delivery are currently being clinically tested and show great promise for the treatment of PD (19).
ACKNOWLEDGMENTS
This work was supported by NIH Grant NS039787. The authors wish to thank Amy Branham, Naomi Carter, Michael Dunlap, and Eric Forman for technical assistance with the study as well as Dr. Anders Andersen for his help with statistical analyses. Medtronic Inc. (Minneapolis, MN) provided the pump/catheter system and Rinat Neuro-science, Inc. (South San Fransico, CA) provided neurturin and vehicle solutions.
REFERENCES
- 1.Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, Gash DM. Intraputamenal infusion of GDNF in aged Rhesus monkeys: Distribution and dopaminergic effects. J. Comp. Neurol. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- 2.Cass WA. GDNF selectively protects dopamine neurons over serotonin neurons against the neurotoxic effects of methamphetamine. J. Neurosci. 1996;16:8132–8139. doi: 10.1523/JNEUROSCI.16-24-08132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gash DM, Zhang Z, Ovadia A, Cass WA, Ai Y, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 4.Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. GDNF Distribution predicts dopaminergic restoration in parkinsonian monkeys. Ann. Neurol. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- 5.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. Direct brain infusion of glial cell linederived neurotrophic factor in Parkinson disease. Nat. Med. 2003;59:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 6.Grondin R, Zhang Z, Elsberry D, Gerhardt GA, Gash DM. Chronic intracerebral delivery of trophic factors via a programmable pump as a treatment for parkinsonism. In: Mouradian MM, editor. Parkinson’s disease-methods & protocols. Clifton, NJ: Humana Press; 2001. pp. 257–267. [DOI] [PubMed] [Google Scholar]
- 7.Grondin R, Zhang Z, Cass WA, Ai Y, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 8.Grondin R, Cass WA, Zhang Z, Stanford JA, Gerhardt GA, Gash DM. GDNF increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, Oldfield E, Bankiewicz KS. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Exp. Neurol. 2001;168:155–161. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- 10.Herzog CD, Dass B, Holden JE, Stansell J, 3rd, Gasmi M, Tuszynski MH, Bartus R, Kordower JH. Striatal delivery of CRE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Mov. Disord. 2007;22:1124–1132. doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- 11.Horger BA, Nishimura MC, Armanini MP, Wang L, Poulsen KT, Rosenblad C, Kirik D, Moffat B, Simmons L, Johnson EM, Jr, Milbrandt J, Rosenthal A, Bjorklund A, Vandlen RA, Hynes MA, Phillips HS. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. J. Neurosci. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: Intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J. Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen E-Y, Palfi S, Roitberg B, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 14.Kordower JH, Herzog CD, Dass B, Backay RAE, Stansell J, 3rd, Gasmi M, Bartus RT. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann. Neurol. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- 15.Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, Milbrandt J. Neurturin, a relative of glial cell line-derived neuro-trophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- 16.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten FVG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson’s disease. Ann. Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 17.Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- 18.Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, Gerhardt GA. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor in aged rhesus monkeys. Neurobiol. Aging. 2002;23:881–889. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 19.Marks W, Verhagen L, Metman P, Starr P, Larson P, Bakay R, Taylor R, Lee D, Bartus R, Ostrem J. Neurturin gene transfer for Parkinson’s disease: Motor outcomes from the initial CERE-120 clinical trial. Mov. Disord. 2006;21 Suppl. 15:P1012. [Google Scholar]
- 20.Nutt JG, Burchiel KJ, Comella CL, Jankovic A, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Oiwa Y, Yoshimura R, Nakai K, Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson’s disease. Brain Res. 2002;947:271–283. doi: 10.1016/s0006-8993(02)02934-7. [DOI] [PubMed] [Google Scholar]
- 22.Ovadia A, Zhang Z, Gash DM. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiol. Aging. 1995;16:931–937. doi: 10.1016/0197-4580(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 23.Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: A two-year outcome study. Ann. Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 24.Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. Eur. J. Neurosci. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 25.Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin R, Zhang Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp. Neurol. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Sariola H, Saarma M. Novel functions and signaling pathways for GDNF. J. Cell Sci. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- 27.Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson’s disease. Mov. Disord. 2006;21:136–141. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- 28.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young AB. Unilateral intraputamenal GDNF improves bilateral motor functions in patients with Parkinson’s disease. J. Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 29.Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23 Suppl.:S28–S33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 30.Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol. Aging. 2006;27:1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Widenfalk J, Nosrat C, Tomac A, Westphal H, Hoffer B, Olson L. Neurturin and glial cell line-derived neurotrophic factor receptor-beta (GDNFR-beta), novel proteins related to GDNF and GDNFR-alpha with specific cellular patterns of expression suggesting roles in the developing and adult nervous system and in peripheral organs. J. Neurosci. 1997;17:8506–8519. doi: 10.1523/JNEUROSCI.17-21-08506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhihlmann KB, Ducray AD, Schaller B, Huber AW, Krebs SH, Andres RH, Seiler RW, Meyer M, Widmer HR. The GDNF family members neurturin, artemin and persephin promote the morphological differentiation of cultured ventral mesencephalic dopaminergic neurons. Brain Res. Bull. 2005:42–53. doi: 10.1016/j.brainresbull.2004.10.012. [DOI] [PubMed] [Google Scholar]