Abstract
Precis
Complex hyperplasia regression is common with and without progestin therapy, and the likelihood of atypical hyperplasia regression is greater with progestin therapy than without.
Objective
To assess likelihood of histologic persistence/progression of complex hyperplasia and atypical hyperplasia among women treated with progestin compared to those not treated, with attention to type, dose and duration.
Methods
This was a cohort study of women at an integrated health plan, ages 18-85 years, with complex or atypical hyperplasia on independent pathology review with a second endometrial specimen in the 2-6 months following the index diagnosis. Progestin therapy between index diagnosis and follow-up biopsy was determined from the pharmacy database. Medical record abstraction was performed. Relative risks (RR), adjusted for age and body mass index, were calculated.
Results
Among 185 women, average age 55.9 years, follow-up 16.1 weeks, 115 women had complex and 70 had atypical hyperplasia. Among women with complex hyperplasia 28.4% of women treated with progestin and 30.0% of those not treated had persistence/progression (RR 1.20, 95% confidence interval (CI) 0.53-2.72). Among women with atypical hyperplasia, 26.9% of those treated with progestin and 66.7% of those not treated had persistence/progression (RR 0.39, 95% CI 0.21-0.70); there was a suggestion that use of at least a medium dose, or a duration of at least 3 months, was associated with a particularly low probability of persistence/progression.
Conclusion
While progestin treatment of women with atypical hyperplasia was associated with a substantial increase in the likelihood of regression of the lesion during the ensuing 2-6 months, persistence/progression was nonetheless present in more than one-quarter of treated women. Regression of complex hyperplasia without atypia was common whether progestin had or had not been used.
Introduction
Endometrial hyperplasia is defined as abnormal proliferation of the uterine endometrial glands. Women with endometrial hyperplasia have an elevated risk of endometrial carcinoma, and like endometrial carcinoma, the incidence of endometrial hyperplasia is related to endogenous and exogenous estrogen exposure. (1, 2) The efficacy of hormonal treatment of endometrial hyperplasia has not been well evaluated, and hormonal management of women with this condition has been largely based on descriptions of case series.
Endometrial hyperplasia is most typically classified as simple or complex, without or with cytological atypia. (3) Simple hyperplasia often spontaneously regresses and rarely progresses to endometrial cancer. (4, 5) Complex hyperplasia and atypical hyperplasia, in particular, are more likely to progress to cancer and are therefore commonly treated with a progestin or hysterectomy. (6) Generally, treatment guidelines recommend that women with complex hyperplasia be treated with progestins and women with atypical hyperplasia be treated with hysterectomy. (6-8) However, there is still debate regarding the merits of hysterectomy versus progestin therapy for women with complex and atypical hyperplasia. (1, 6, 7)
Regression of endometrial hyperplasia following the use of a progestin was described as early as 1959 by Kistner. (9) Since that time, additional reports indicate that even endometrial carcinoma may respond to progestin therapy. (10-15) Prior studies report a wide range of risks for persistence or progression of endometrial hyperplasia in women treated with progestin, from 0 to 60% for complex hyperplasia, and from 10 to 100% for atypical hyperplasia. (1, 5, 15-19) Not only does treatment failure vary widely in these reports, but only one study reported results of outcomes comparing treatment with progestin to no progestin therapy. (17) An important challenge in endometrial tissue studies is diagnostic reproducibility. (20-24) The lack of standardized pathology review by research pathologists in most studies may contribute to the observed variability in outcomes. In addition, very few data exist to suggest the duration, dose and type of progestin that is most effective, and the characteristics of women that predict successful progestin therapy are also largely unknown. As recently as 2001, in a review of the management of endometrial hyperplasia, it was concluded that, “the optimal dosage of progestins has not been investigated and the regimens advocated are essentially arbitrary”. (25)
Our primary aim was to assess the likelihood of histologic persistence or progression of these two types of endometrial hyperplasia over 2 to 6 months among women treated with progestin compared to those not treated with progestin, with specific attention to duration, dose and the type of progestin used.
Materials and Methods
Following approval from the Fred Hutching Cancer Research Center Institutional Review Board, we performed a cohort study of 185 women with documented complex or atypical hyperplasia; all biopsies were subjected to centralized pathology review.
Study Population
The study was conducted at Group Health (GH), a mixed-model integrated health plan with over 500,000 enrollees in Washington State. Automated pathology, enrollment, pharmacy, inpatient and outpatient databases were linked for data on all women over age 18, diagnosed with complex and atypical endometrial hyperplasia, between January 1, 1985 and April 1, 2005. Follow-up continued for up to 6 months from the time of the index diagnosis of hyperplasia GH automated pharmacy databases contain data on all prescription medications dispensed to enrollees through GH pharmacies. The data include the specific drug and drug class, date and amount dispensed, and dosing instructions. Surveys among women aged 50-80 years have shown that 97% of all estrogen and progestin prescriptions are filled at GH pharmacies. (26)
Women were excluded if within 8 weeks of their index diagnosis they had a hysterectomy, were diagnosed with endometrial cancer, or disenrolled. Women were included if they had at least one additional endometrial pathology specimen (biopsy or hysterectomy), taken 8 weeks to 6 months following the index diagnosis. This interval was chosen because the majority of clinicians attempt hormonal therapy for endometrial hyperplasia for 8 weeks to 6 months, and then reassess pathology with a second biopsy. (7, 8) Among women with 2 biopsy specimens, there were 563 whose first biopsy was interpreted as complex or possible complex hyperplasia (e.g. “cannot rule-out complex hyperplasia”) and 146 women whose first biopsy showed atypia or possible atypia (e.g. “cannot rule-out atypia”, “cannot rule-out carcinoma”). Text searches indicating possible diagnoses of complex or atypical hyperplasia were included to maximize our opportunity to identify women with the conditions of interest.
Case Verification and Outcome Ascertainment
Histology slides and tissue blocks were obtained for histopathologic review and characterization. All eligible cases were reviewed independently, and in random order, by two University of Washington pathologists (RG, KA), using standard International Society of Gynecological Pathologists and World Health Organization criteria. (3, 27) If the two pathologists did not agree on the diagnosis (38.1% of the time), a third pathologist (DJ) reviewed the case. If all three pathologists disagreed, the diagnosis was assigned by the senior pathologist (RG) (6.8% of the time). The overall unweighted kappa value for agreement on endometrial diagnoses made by the two primary pathologists on a review of 2,531 specimens was 0.54 (weighted 0.71). (28)The pathologists were blinded to the original diagnosis and slide batches contained a mixture of index and follow-up specimens originally diagnosed as normal endometrium, simple, complex, or atypical hyperplasia, and endometrial cancer. Only the 222 women with complex (N=139) and atypical (N=83) endometrial hyperplasia confirmed in this review were considered for inclusion.
Exposure Ascertainment and Outcome Definitions
Progestin prescriptions were ascertained from the GH automated pharmacy database. All dispensings for any progestin from one week before the index biopsy up to 6 months after the index biopsy were included. Days of use were calculated from the time of the index biopsy to the follow-up biopsy. Women were classified as progestin “users” if they were dispensed over 14 days of progestin and as “never users” if they had no progestin dispensed. Progestins were categorized by type - megesterol acetate (MEGA), medroxyprogesterone acetate (MPA), and norethindrone acetate (NETA)- in women whose dispensings indicated that that progestin accounted for at least 80% of the progestin received. Progestin type was categorized as “mixed” if a woman was dispensed several types of progestin and the predominant progestin was dispensed for less than 80% of the days or if the woman was dispensed a combined oral contraceptive. Low, medium and high doses were assigned. “Low dose” included MPA <10mg per day, and NETA < 1mg per day. (29-32) No doses of MEGA were considered low. A “high dose” was defined as MEGA ≥ 40 mg per day. (31) All other doses were designated as “medium” and included MPA ≥ 10 mg, MEGA < 40mg, and NETA ≥ 1 mg daily. Duration of exposure was defined as < 3 months and ≥ 3 months.
Ascertainment of any hormone use during the 6 months prior to the index biopsy occurred in a similar fashion and included unopposed estrogen (ET), combined postmenopausal estrogen and progestin therapy (EPT), unopposed progestin (PT) and combined oral contraceptives (OC). These covariables were considered for inclusion in multivariable analyses.
Outcome was determined by comparing the diagnosis of the follow-up biopsy to the index diagnosis and was dichotomized into “regression” and “persistence/progression”. Follow-up diagnoses of no hyperplasia or simple hyperplasia were classified as “regression” for those with an index diagnosis of complex hyperplasia without atypia; diagnoses of complex, atypical hyperplasia or carcinoma were classified as “persist/progress”. Follow-up diagnoses of no hyperplasia, simple hyperplasia or complex hyperplasia were classified as “regression” for those women with an index diagnosis of atypical hyperplasia; whereas follow-up diagnoses atypical hyperplasia or carcinoma were classified as “persist/progress”. An outcome of persist/progress versus regress was chosen because failure to regress after 3-6 months of progestin therapy is commonly an indication for hysterectomy. (6-8)
Data Collection
Additional information regarding medical and family history, and demographic, reproductive, and physical characteristics, including height and weight at the time of the index biopsy were collected from the GH medical record, a single document containing all records from outpatient visits, test reports, and records of hospitalizations and consultations. Three trained abstractors performed the medical record reviews, utilizing archived paper charts and the more recent electronic medical record (EMR). Menstrual characteristics and bleeding patterns preceding the biopsy, ultrasound findings, age at menopause, race, parity, personal history of breast, colon, or ovarian cancers, diabetes, hypertension and smoking status were ascertained. Indications for hysterectomy and endometrial biopsies were recorded.
Statistical Analyses
Analyses were performed separately for women with complex hyperplasia and for women with atypical hyperplasia. We computed the proportion of women with each type of hyperplasia (complex; atypical) who persisted or progressed in relation to treatment with a progestin. We computed Mantel-Haenszel adjusted relative risks using the “cs” procedure in STATA 9.2 (STATA Corporation, College Station, Texas).We considered and evaluated potential confounding factors including histologic features (e.g. “mild” complex hyperplasia as determined by the original pathologist) and adjusted for variables that influenced the risk estimates associated with progestin use by more than 10%, specifically, age (<50, ≥50 years) and body mass index (BMI) (<30, ≥ 30 kg/m2). Exposure to progestin for greater than 3 months was assessed only in women who had their follow-up biopsy 3-6 months after the index biopsy.
Results
Of the 139 women whose index biopsies were diagnosed as complex hyperplasia without atypia and of the 83 others diagnosed as atypical hyperplasia, 37 were excluded. Reasons included: follow-up specimen was not available for review (1 atypical); non-diagnostic follow-up specimens (1 complex; 3 atypical); more than 14 days of estrogen dispensed (19 complex; 6 atypical) and only 1-14 days of progestin dispensed (4 complex; 3 atypical) between index and follow-up biopsies. A total of 115 women with complex and 70 women with atypical hyperplasia were included in our analyses (N=185). Time between index and follow-up biopsy ranged from 8 to 26 weeks (mean = 16.1 weeks; 16.4 weeks among women who received progestin and 14.9 weeks among women who did not receive progestin).
The average age of women in the cohort was 55.9 years and 33.5% were less than 50 years of age, 88.5% were white, 11.5% were smokers, 21.4% were nulliparous, and 50% had a BMI ≥ 30 kg/m2. Eleven women (5.9%), evenly distributed between progestin users and nonusers, had sparse medical records; all were missing data on ethnicity, smoking status, diabetes, parity and BMI. In the 6 months prior to the index diagnosis, 8.1% had been dispensed EPT, 11.3% ET, 2.2% PT, and 1.1% OCs. Menstrual characteristics were not related to progestin exposure or risk of persistence/progression of hyperplasia (data not shown). The indication for the index endometrial biopsy was abnormal bleeding in 85.9% of the women, abnormal ultrasound in an additional 4.3%, unopposed estrogen use in 3.7% and other indications in 6.1%. Older women (at least 50 years of age) were slightly more likely to not have progestin therapy dispensed after index biopsy as compared to younger women (p= 0.05), but other baseline characteristics bore no relation as to whether progestin was dispensed between index and follow-up biopsies (Table 1).
Table 1.
Characteristics of 185 women with complex hyperplasia with and without atypia who did not receive progestin or received progestin for over 14 days
| Complex N=115 | Atypia N=70 | |||
|---|---|---|---|---|
| No Progestin N=20 | Progestin > 14 d N=95 | No Progestin N=18 | Progestin > 14 d N=52 | |
| Age (years) | ||||
| <39 | 2(10.0) | 9(9.5) | 0(0) | 5(9.6) |
| 40-49 | 3(15.0) | 32(33.6) | 2(11.1)) | 9(17.3) |
| 50-59 | 3(15.0) | 26(27.4) | 7(38.9) | 19(36.5) |
| 60-69 | 7(35.0) | 19(20.0) | 4(22.2) | 10(19.2) |
| ≥70 | 5(25.0) | 9(9.5) | 5(27.8) | 9(17.3) |
| Caucasian1 | 18(90.0) | 80(84.2) | 18(100.0) | 38(82.6) |
| Diabetes1 | 2(10.0) | 8(8.4) | 4(22.2) | 6(13.0) |
| Breast/Colon Cancer1 | 1(5.0) | 2(2.1) | 2(11.1) | 3(5.8) |
| Current Smoker1 | 2(10.0) | 12(12.6) | 0(0) | 5(10.9) |
| BMI (kg/m2)1 | ||||
| <25 | 5(25.0) | 22(23.3) | 5(27.8) | 10(22.2) |
| 25 - 29.9 | 7(35.0) | 20(21.1) | 3(16.7) | 14(31.1) |
| ≥30 | 7(35.0) | 48(50.5) | 10(55.6) | 21(46.7) |
| Nulliparous1 | 4(20.0) | 17(17.9) | 3(16.7) | 13(28.3) |
| Oral contraceptive2 | 0(0) | 2(2.1) | 0(0) | 0(0) |
| HT2,3 | 0(0) | 9(9.5) | 0(0) | 6(11.5) |
| Unopposed Estrogen2,4 | 4(20.0) | 10(10.5) | 1(5.6) | 6(11.5) |
| Progestin only2,5 | 0 (14.3) | 4(2.1) | 1(5.6) | 0(0) |
| Index biopsy year | ||||
| 1985 - 1989 | 6(30.0) | 13(13.7) | 2(11.1) | 4(7.7) |
| 1990 - 1994 | 5(25.0) | 27(28.4) | 3(16.7) | 16(30.8) |
| 1995 - 1999 | 2(10.0) | 29(30.5) | 7(38.9) | 22(42.3) |
| 2000 - 2005 | 7(35.0) | 26(27.4) | 6(33.3) | 10(19.2) |
missing data on: race and diabetes - 11; smoking - 20; BMI - 13; parity and history of breast/colon cancer - 12.
BMI = body mass index.
Dispensed in the six months preceding diagnosis of endometrial hyperplasia.
HT = postmenopausal hormone therapy dispensed in the six months preceding diagnosis of endometrial hyperplasia (estrogen plus progestin for 2 months or more and the progestin was dispensed for at least 1/3 of the time that estrogen was dispensed).
Unopposed estrogen = postmenopausal estrogen therapy dispensed in the six months preceding diagnosis of endometrial hyperplasia (estrogen alone or estrogen plus progestin for 2 months or more and the progestin was dispensed less than1/3 of the time that estrogen was dispensed).
Dispensed for at least 2 months.
Of the 185 women, only 38 (20.5%) received no progestin therapy in the 6 months following their diagnosis of complex or atypical hyperplasia. The other 147 women were dispensed at least 14 days of progestin therapy (mean 61.3 days) including: megesterol acetate (33.0%), medroxyprogesterone acetate (35.7%), and norethindrone acetate (5.9%). Among the 147 women dispensed progestin, 84.2% received only one type, 11.5 % received more than one type but the predominant formulation was for > 80% of the days, and only 4.3 % received several progestins without a predominant type, one of whom received a combined OC.
Among women with complex hyperplasia, 28.4% who received progestin therapy experienced persistence or progression of their hyperplasia, as compared to 30.0% persistence or progression in women who did not receive progestin. Treatment with progestin was not associated with a decreased likelihood of persistence or progression of complex hyperplasia, (RR 1.20, 95% CI 0.53-2.72) (Table 2).
Table 2.
Risk of persistence/progression of complex hyperplasia and atypical hyperplasia in relation to progestin dispensed following the diagnosis of hyperplasia
| Complex | Atypia | |||||
|---|---|---|---|---|---|---|
| Regress N=82 | Persist/progress N=33 | Adjusted risk ratio1 | Regress N=44 | Persist/progress N=26 | Adjusted risk Ratio1 | |
| No progestin | 14 (70.0) | 6 (30.0) | 1.0 | 6 (33.3) | 12 (66.7) | 1.0 |
| Any progestin | 68 (71.6) | 27(28.4) | 1.20 (0.53-2.72) | 38 (73.1) | 14 (26.9) | 0.39 (0.21-0.70) |
| DURATION | ||||||
| No progestin | 14 (70.0) | 6 (30.0) | 1.0 | 6 (33.3) | 12 (66.7) | 1.0 |
| <3 mo any progestin | 41 (69.5) | 18 (30.5) | 1.28 (0.55-2.98) | 18 (62.1) | 11 (37.9) | 0.58 (0.32-1.02) |
| No progestin, among those with ≥ 3 mo follow-up | 8 (72.7) | 3 (27.3) | 1.0 | 3 (33.3) | 6 (66.7) | 1.0 |
| ≥3mo any progestin, among those with ≥3 mo follow-up | 27 (75.0) | 9 (25.0) | 1.51 (0.36-6.26) | 20 (87.0) | 3 (13.0) | 0.33 (0.12-0.89) |
| DOSE | ||||||
| No progestin | 14 (70.0) | 6 (30.0) | 1.0 | 6 (33.3) | 12 (66.7) | 1.0 |
| Low dose | 3 (50.0) | 3 (50.0) | 1.75 (0.58-5.26) | 2 (50.0) | 2 (50.0) | 0.57 (0.09-3.77) |
| Med/high dose | 47 (72.3) | 18 (27.7) | 1.18 (0.50-2.79) | 27 (77.1) | 8 (22.9) | 0.34 (0.16-0.72) |
| Maximum dose | 17 (73.9) | 6 (26.1) | 0.95 (0.34-2.70) | 9 (69.2) | 4 (30.8) | 0.45 (0.18-1.09) |
| TYPE | ||||||
| No progestin | 14 (70.0) | 6 (30.0) | 1.0 | 6 (33.3) | 12 (66.7) | 1.0 |
| MEGA | 26 (72.2) | 10 (27.8) | 0.98 (0.38-2.51) | 19 (76.0) | 6 (24.0) | 0.31 (0.13-0.73) |
| MPA | 33 (71.7) | 13 (28.3) | 1.24 (0.51-3.03) | 14 (70.0) | 6 (30.0) | 0.51 (0.22-1.18) |
| NETA | 6 (85.7) | 1 (14.3) | 0.85 (0.20-3.62) | 2 (50.0) | 2 (50.0) | 0.61 (0.21-1.83) |
Progestin dispensed for at least 14 days
MEGA= megesterol acetate, MPA= medroxyprogesterone acetate, NETA= norethindrone acetate
Adjusted for age (<50, ≥50 years); body mass index (<30, ≥30 kg/m2); Note: 7 women with atypical hyperplasia and 5 with complex who received progestin and 1 complex who never received progestin were excluded from all models because body mass index was missing.
For women with atypical hyperplasia, 26.9% who received progestin experienced persistence or progression of their hyperplasia, compared to 66.7% of women who did not receive progestin (RR 0.39, 95% CI 0.21-0.70). Among women with atypia treated with progestin for at least 3 months, the risk of persistence/progression was decreased 72% (RR 0.33, 95% CI 0.12-0.89) whereas that risk was decreased only 42%, (RR 0.58, 95% CI 0.32-1.02) for those treated for less than 3 months, as compared to women not treated with progestin. Women with atypical hyperplasia treated with megesterol acetate had a decreased risk for persistence/progression (RR 0.31, 95% CI 0.13-0.73) as compared to women not treated with progestin. Among women treated with medroxyprogesterone acetate, the risk was also decreased, but was not statistically significant (RR 0.51, 95% CI 0.22-1.18). Too few women were treated with norethindrone acetate to make meaningful comparisons (Table 2).
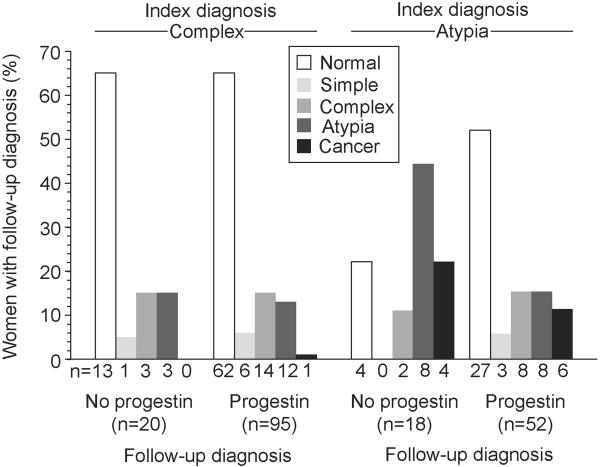
Follow-up endometrial diagnoses are shown in Figure 1; 15.1 % of specimens were hysterectomies. Eleven women (5.9%) were diagnosed with endometrial carcinoma (all FIGO grade 1) at follow-up, and of those 9 had hysterectomies; only one had an index diagnosis of complex hyperplasia. No women developed a high grade endometrial carcinoma.
Figure 1.
Proportion of women with complex hyperplasia and atypical hyperplasia with follow-up diagnoses of normal endometrium, simple, complex and atypical hyperplasia, and endometrial carcinoma, by progestin treatment
Discussion
In our study, cases of endometrial hyperplasia were all those diagnosed within a defined population who went on to have a second endometrial biopsy between 2-6 months after the initial diagnosis. Ultimately we included only those documented as having complex or atypical hyperplasia by research pathologists who reviewed all of the specimens and were blinded as to treatment status. Approximately 70% of women with complex hyperplasia without atypia had regression of their lesion, whether or not they were given a progestin. Untreated women with atypical endometrial hyperplasia had a lower likelihood of regression (33.3%), compared to women with atypical hyperplasia who had a progestin dispensed (73.1%). Risk of endometrial carcinoma at follow-up was low overall (11/185; 5.9%); and was greatest among women with atypical hyperplasia (10/70; 14.3%).
One prior study (17) also examined the likelihood of regression of endometrial hyperplasia in relation to receipt of progestin therapy. In 208 women with complex hyperplasia without atypia treated with progestin for 3-5 months in whom a second biopsy was obtained, regression was evident in 128 (61.5%). In our study, the corresponding percentage in progestin-treated women was 75.0%. In the earlier study, (17) only 37 of 182 women (23%) who did not receive progestin had regression of complex hyperplasia without atypia, a proportion smaller than we observed in untreated women with this abnormality (70.0%). The discrepancy between the results of these 2 studies evaluating the likelihood of persistence/progression of complex hyperplasia in relation to progestin therapy - based almost entirely on the difference in frequency of regression among untreated women - is difficult to explain. In addition, the earlier study (17) found not a single instance of regression in the 105 women with atypical hyperplasia not treated with progestin (whereas one in three of such women in our study experienced regression). There were but seven women with atypical hyperplasia in the earlier study (17) who received progestin. None of their lesions regressed, but it would be hazardous to infer from this tiny sample that progestin treatment of atypical hyperplasia has no efficacy. Several additional differences between the two studies should be noted. In the earlier study, (17) the progestins used were NETA 5 mg daily (premenopausal women only) and MPA 10-20 mg daily. No women received megesterol acetate, as compared to the 42% of women given progestin in our study who were dispensed megesterol acetate. In the earlier study, case ascertainment was “confirmed” by a second reviewer who reread just the 560 cases originally diagnosed as either complex or atypical hyperplasia.
Seven other studies, (1, 5, 15, 16, 18, 19, 33) published after the endometrial hyperplasia WHO classification system was proposed in 1975, reported the outcomes of treatment of complex endometrial hyperplasia and atypical hyperplasia, although numbers of women in general were small, particularly for atypia (2-20 women) and the studies lacked comparison groups. From these seven studies, very little information is available regarding the possible influence of duration, dose, or type of progestin therapy on the likelihood of regression of complex and atypical endometrial hyperplasia.
We found that as compared to women who did not receive any progestin, women with atypical hyperplasia dispensed at least 3 months of progestin had a 67% decreased risk of persistence/progression (95% CI 0.12-0.89), and those dispensed less than 3 months of progestin had a 42% decreased risk of persistence progression (95% CI 0.32-1.02). However, because the number of women in each category was relatively small, conclusions regarding the merits of 3-6 months duration therapy versus less than 3 months cannot be made with any certainty. We are not aware of any other studies that have attempted to compare shorter to longer progestin durations.
We were unable to adequately assess the effect of dose on risk of persistence/progression of complex or atypical hyperplasia, as only 6 women with complex and 4 women with atypical hyperplasia received “low dose” progestin. The point estimates for persistence/progression were higher than those for “medium” (65 complex; 35 atypia) and “high” doses (23 complex; 13 atypia), but confidence intervals overlapped. In the Postmenopausal Estrogen/Progestin Intervention (PEPI) trial, 10 women with atypia were treated with medroxyprogesterone acetate 10 mg/day, a dose we classified as “medium”; 8 showed regression (80%). (1) In contrast, another study described regression in only 5 out of 20 women (25%) with atypical hyperplasia treated with slightly higher doses of medroxyprogesterone acetate, but also classified as “medium” in our study.(16) Very high progestin doses resulted in regression of atypical endometrial hyperplasia in 9/10 women treated with medroxyprogesterone acetate 500 mg given intramuscularly twice weekly, (19) and in 10/11 women treated with megesterol acetate 40-160 mg daily. (15) None of these studies (1, 15, 16, 19) included women who did not receive progestin therapy. Although progestin dosing studies suggest extremely high doses of progestins may not be needed to treat complex or atypical hyperplasia, (29-32) the correct dose for treatment of endometrial hyperplasia is yet to be determined.
It is unclear whether the type of progestin affects outcomes. As compared to women who did not receive any progestin, women in our study with atypical hyperplasia dispensed megesterol acetate had a 69% decreased risk of persistence/progression (95% CI 0.13-0.73) and those dispensed medroxyprogesterone acetate had a 49% decreased persistence/progression risk (95% CI 0.22-1.18). No other studies exist to compare the effectiveness of various types of progestin (Search: PubMed; terms: endom* hyperplasia, progest*; Language: English; Dates: January 1985-October 2008). Results, when available from GOG 224 (34), a randomized, controlled Phase II trial of megestrol (different doses and duration) for treatment of atypical endometrial hyperplasia, should add to our understanding of this question.
There are limitations of our study. Most importantly, this was not a randomized trial and we did not have statistical power to assess differences among women with complex hyperplasia. The challenges with standardization of diagnostics in endometrial tissues are well established and acknowledged. (28) The majority of pre-operative assessments in our study were performed by Pipelle endometrial biopsy. In a prior study, the sensitivities of the Pipelle and curettage among 360 women who subsequently underwent hysterectomy and had a diagnosis of endometrial cancer were shown to be 94% and 97%, respectively. (35) We could not control for the method of endometrial sampling, but one would not expect therapy choice to be associated with biopsy technique. A possible explanation for finding endometrial carcinoma at follow-up is that endometrial hyperplasia progressed rapidly over an average of 16.1 weeks. Alternatively, and we believe more likely, endometrial carcinoma was present but not detected at the time of the index biopsy, a risk estimated to be as high as 43% for women with atypical hyperplasia in a previous study (23) but potentially lower. (35) Regardless of whether the carcinoma was present at index biopsy or not in our study, there was no evidence of a worrisome outcome for any of these women, none of them developed a high grade endometrial carcinoma. The number of women included in our study limited our ability to fully assess the possible impact of progestin duration, dose and type on the likelihood of persistence or progression. Finally, we were unable to assess compliance, nor to determine reasons for women not receiving progestin therapy. And although we controlled for age, BMI and prior HT exposure, we were unable to control for potential confounding variables that were unmeasured.
Nonetheless, results from our study may assist women with atypical hyperplasia trying to make a decision as to whether to proceed with progestin therapy or hysterectomy. Our results suggest a 3-month trial of at least medium dose progestin (medroxyprogesterone acetate 10 mg, megesterol acetate 20 mg or norethindrone acetate 1mg), followed by adequate sampling is not an unreasonable approach if a woman desires uterine conservation, rather than proceeding immediately to hysterectomy. This conservative approach should only be adopted in the setting of careful follow-up. Complex hyperplasia has a high frequency of spontaneous regression, and a much larger study is needed to adequately evaluate the efficacy of progestin therapy for this lesion.
Acknowledgments
Supported by the National Institutes of Health (PI Reed; NICHD 5 R01 HD44813-02). The authors thank Dr. Walter Clinton, Mr. Kevin Beverly, Ms. Kay Byron, and Ms. Kelly Ehrlich for data management, and Ms. Kay Hager, Kathy Plant, and Sarah Nielson for assistance with data collection and data entry.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.The Writing Group for the PEPI Trial Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The Writing Group for the PEPI Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 2.Epplein M, Reed SD, Voigt LF, Newton KW, Holt VL, Weiss NS. Endometrial hyperplasia risk in relation to characteristics and exposures that influence endogenous hormone levels. Am J Epidemiol. 2008 [Google Scholar]
- 3.Tavassoli FA. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of the Breast and Female Genital Organs. IARC Press; Lyon: 2003. [Google Scholar]
- 4.Figueroa-Casas PR, Ettinger B, Delgado E, Javkin A, Vieder C. Reversal by medical treatment of endometrial hyperplasia caused by estrogen replacement therapy. Menopause. 2001;8:420–3. doi: 10.1097/00042192-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56:403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Hammond R, Johnson J. Endometrial hyperplasia. Current Obstetrics and Gynaecology. 2004;40:99–103. [Google Scholar]
- 7.American College of Obstetricians and Gynecologists ACOG practice bulletin, clinical management guidelines for obstetrician-gynecologists, number 65, August 2005: management of endometrial cancer. Obstet Gynecol. 2005;106:413–425. doi: 10.1097/00006250-200508000-00050. [DOI] [PubMed] [Google Scholar]
- 8.Zacur HA, Giuntoli RL, Jurema M. Endometrial Hyperplasia. 2008 Up to Date. http://www uptodateonline com/ 16.1[Last literature review version 16.1: January 2008] 5-12-2008. http://www.uptodateonline.com/
- 9.Kistner RW. Histological effects of progestins on hyperplasia and carcinoma in situ of the endometrium. Cancer. 1959;12:1106–10. doi: 10.1002/1097-0142(195911/12)12:6<1106::aid-cncr2820120607>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Gotlieb WH, Beiner ME, Shalmon B, et al. Outcome of fertility-sparing treatment with progestins in young patients with endometrial cancer. Obstet Gynecol. 2003;102:718–725. doi: 10.1016/s0029-7844(03)00667-7. [DOI] [PubMed] [Google Scholar]
- 11.Kaku T, Yoshikawa H, Tsuda H, et al. Conservative therapy for adenocarcinoma and atypical endometrial hyperplasia of the endometrium in young women: central pathologic review and treatment outcome. Cancer Lett. 2001;167:39–48. doi: 10.1016/s0304-3835(01)00462-1. [DOI] [PubMed] [Google Scholar]
- 12.Kauppila A. Progestin therapy of endometrial, breast and ovarian carcinoma. A review of clinical observations. Acta Obstet Gynecol Scand. 1984;63:441–450. doi: 10.3109/00016348409156700. [DOI] [PubMed] [Google Scholar]
- 13.Lowe MP, Bender D, Sood AK, Davis W, Syrop CH, Sorosky JI. Two successful pregnancies after conservative treatment of endometrial cancer and assisted reproduction. Fertil Steril. 2002;77:188–189. doi: 10.1016/s0015-0282(01)02937-5. [DOI] [PubMed] [Google Scholar]
- 14.Montz FJ, Bristow RE, Bovicelli A, Tomacruz R, Kurman RJ. Intrauterine progesterone treatment of early endometrial cancer. Am J Obstet Gynecol. 2002;186:651–7. doi: 10.1067/mob.2002.122130. [DOI] [PubMed] [Google Scholar]
- 15.Randall TC, Kurman RJ. Progestin treatment of atypical hyperplasia and well-differentiated carcinoma of the endometrium in women under age 40. Obstet Gynecol. 1997;90:434–40. doi: 10.1016/s0029-7844(97)00297-4. [DOI] [PubMed] [Google Scholar]
- 16.Ferenczy A, Gelfand M. The biologic significance of cytologic atypia in progestogen-treated endometrial hyperplasia. Am J Obstet Gynecol. 1989;160:126–31. doi: 10.1016/0002-9378(89)90103-8. [DOI] [PubMed] [Google Scholar]
- 17.Horn LC, Schnurrbusch U, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestogen treatment. Int J Gynecol Cancer. 2004;14:348–353. doi: 10.1111/j.1048-891x.2004.014220.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang SJ, Amparo EG, Fu YS. Endometrial hyperplasia: histologic classificiation and behavior. Surg Pathol. 1988;1:229. [Google Scholar]
- 19.Lindahl B, Willen R. Spontaneous endometrial hyperplasia. A 5 year follow-up of 82 patients after high-dose gestagen treatment. Anticancer Res. 1994;14:2831–4. [PubMed] [Google Scholar]
- 20.Bergeron C, Nogales FF, Masseroli M, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23:1102–8. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Kendall BS, Ronnett BM, Isacson C, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol. 1998;22:1012–9. doi: 10.1097/00000478-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Skov BG, Broholm H, Engel U, et al. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol. 1997;16:33–7. doi: 10.1097/00004347-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:812–819. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 24.Zaino RJ, Kauderer J, Trimble CL, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106:804–811. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 25.Marsden DE, Hacker NF. Optimal management of endometrial hyperplasia. Best Pract Res Clin Obstet Gynaecol. 2001;15:393–405. doi: 10.1053/beog.2000.0184. [DOI] [PubMed] [Google Scholar]
- 26.Psaty BM, Heckbert SR, Atkins D, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Arch Intern Med. 1994;154:1333–1339. [PubMed] [Google Scholar]
- 27.Pecorelli S, Benedet JL, Creasman WT, Shepherd JH. FIGO staging of gynecologic cancer. 1994-1997 FIGO Committee on Gynecologic Oncology. International Federation of Gynecology and Obstetrics. Int J Gynaecol Obstet. 1999;65:243–249. doi: 10.1016/s0020-7292(99)00070-3. [DOI] [PubMed] [Google Scholar]
- 28.Allison KH, Reed SD, Voigt LF, Jordan CD, Newton KM, Garcia RL. Diagnosing endoemtrial hyperplasia: Why is it so difficult to agree? Am J Surg Pathol. 2008;32:691–698. doi: 10.1097/PAS.0b013e318159a2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril. 1986;46:1062–1066. doi: 10.1016/s0015-0282(16)49880-8. [DOI] [PubMed] [Google Scholar]
- 30.Kurman RJ, Felix JC, Archer DF, Nanavati N, Arce J, Moyer DL. Norethindrone acetate and estradiol-induced endometrial hyperplasia. Obstet Gynecol. 2000;96:373–379. doi: 10.1016/s0029-7844(00)00944-3. [DOI] [PubMed] [Google Scholar]
- 31.Sporrong T, Hellgren M, Samsioe G, Mattsson LA. Comparison of four continuously administered progestogen plus oestradiol combinations for climacteric complaints. Br J Obstet Gynaecol. 1988;95:1042–1048. doi: 10.1111/j.1471-0528.1988.tb06511.x. [DOI] [PubMed] [Google Scholar]
- 32.Stanczyk FZ. Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception. Rev Endocr Metab Disord. 2002;3:211–224. doi: 10.1023/a:1020072325818. [DOI] [PubMed] [Google Scholar]
- 33.Notelovitz M, Varner RE, Rebar RW, et al. Minimal endometrial proliferation over a two-year period in postmenopausal women taking 0.3mg of unopposed esterified estrogens. Menopause: The Journal of the North American Menopause Society. 1997;4:80–88. [Google Scholar]
- 34. ( http://www.cancer.gov/search/ViewClinicalTrials)
- 35.Huang GS, Gebb JS, Einstein MH, Shahabi S, Novetsky AP, Goldberg GL. Accuracy of preoperative endometrial sampling for the detection of high-grade endometrial tumors. Am J Obstet Gynecol. 2007;196:243–245. doi: 10.1016/j.ajog.2006.09.035. [DOI] [PubMed] [Google Scholar]