Abstract
Sunitinib malate (Sutent, SU11248) is a small-molecule receptor tyrosine kinase inhibitor that inhibits cellular signaling of multiple targets such as the platelet-derived growth factor receptors and the vascular endothelial growth factor receptors and is used in the treatment of renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumors. Because tyrosine kinase inhibitors are known to increase the p.o. bioavailability and brain penetration of chemotherapy drugs in animal models, we sought to examine the effect of sunitinib on the ATP-binding cassette (ABC) drug transporters P-glycoprotein (P-gp, ABCB1), the multidrug resistance-associated protein 1 (ABCC1), and ABCG2, which are known to transport a wide variety of anticancer drugs. In this study, we show that sunitinib inhibits P-gp- and ABCG2-mediated efflux of fluorescent substrates in cells overexpressing these transporters. In 4-day cytotoxicity assays, at a nontoxic concentration (2 μM) sunitinib was able to partially reverse drug resistance mediated by P-gp and completely reverse resistance mediated by ABCG2. We further show a direct interaction of sunitinib with the substrate binding pocket of these transporters as it inhibited binding of the photoaffinity substrate [125I]iodoarylazidoprazosin to P-gp (IC50 = 14.2 μM) and ABCG2 (IC50 = 1.33 μM). Sunitinib stimulated the ATP hydrolysis by both transporters in a concentration-dependent manner. Conformation-sensitive antibody binding assays with the P-gp- and ABCG2-specific antibodies, UIC2 and 5D3, respectively, also confirmed the interaction of sunitinib with these transporters. Taken together, this is the first report showing that sunitinib inhibits transport mediated by ABC drug transporters, which may affect the bioavailability of drugs coadministered with sunitinib.
Sunitinib malate (SU011248, Sutent) is an ATP-competitive multitargeted tyrosine kinase (TK) inhibitor with efficacy against renal cell carcinoma and gastrointestinal stromal tumor (Goodman et al., 2007; Rock et al., 2007) that was approved on January 26, 2006 by the Food and Drug Administration for the treatment of advanced renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor (Goodman et al., 2007; Rock et al., 2007). Sunitinib is first anticancer drug simultaneously approved for two different types of cancers. Moreover, it also has anticancer activity in patients with metastatic breast, colon, and neuroendocrine cancer (Faivre et al., 2006; Chow and Eckhardt, 2007).
Sunitinib inhibits cellular signaling by targeting multiple receptor TKs. These include receptor TKs such as platelet-derived growth factor receptors α and β, the vascular endothelial growth factor receptors types 1 and 2, the stem cell factor receptor c-KIT, FMS-like TK-3 receptor (FLT3), and the glial cell-line derived neurotrophic factor receptor (RET) (Chow and Eckhardt, 2007), which play a role in both tumor angiogenesis and tumor cell proliferation. These receptor TKs are transmembrane proteins at the cell surface that possess extracellular ligand-binding domains and an intracellular catalytic domain and transduce extracellular signals to the cytoplasm (Pawson, 2002). Ligand binding induces dimerization of the receptor TKs, resulting in autophosphorylation of the cytoplasmic domains and activation of TK activity. These receptors are important in signal transduction and growth of a number of solid tumors (Bello et al., 2006; Chow and Eckhardt, 2007). Inhibition of these TKs blocks signal transduction, thereby affecting many of the processes involved in tumor growth, progression, metastasis, and angiogenesis (Hanahan and Weinberg, 2000).
The ATP-binding cassette (ABC) drug transporters such as P-glycoprotein (P-gp; ABCB1), multidrug resistance-associated protein (MRP) 1 (ABCC1), and ABCG2 (breast cancer resistance protein, MXR) were first identified based on their role in conferring multidrug resistance (MDR) in cancer (Sarkadi et al., 2006). They are now recognized for their wider role in the absorption, distribution, metabolism, excretion, and toxicity of xenobiotics (Glavinas et al., 2004). It has been recently shown that the MDR-linked ABC transporters, P-gp and ABCG2, interact with different TK inhibitors (TKIs) such as gefitinib, EKI-485, erlotinib, imatinib, nilotinib, CI1033, and INNO-406, and ABCG2 has an especially high affinity for some of these kinase inhibitors (Ozvegy-Laczka et al., 2004, 2005; Burger et al., 2005; Yang et al., 2005; Leggas et al., 2006; Brendel et al., 2007; Shi et al., 2007; Lemos et al., 2008). In animal models, gefitinib has been shown to affect the p.o. absorption of chemotherapy agents (Stewart et al., 2004; Leggas et al., 2006), and imatinib has been shown to enhance efficacy of photodynamic therapy by inhibiting ABCG2 (Liu et al., 2007). Moreover, it has also been shown by several groups that some TKIs are substrates of the two major drug transporters, P-gp and ABCG2, suggesting that the interaction with ABC transporters may also significantly modify the pharmacokinetics and toxicity of TKIs in patients (Illmer et al., 2004; Widmer et al., 2007; Polli et al., 2008; Shukla et al., 2008b).
Although sunitinib has seen early clinical success as a p.o. agent, its interaction with the MDR-linked ABC drug transporters has not been characterized. The objective of this work was to investigate the interaction of sunitinib with two major ABC drug transporters involved in drug disposition, P-gp and ABCG2. We show here that sunitinib inhibits the efflux mediated by P-gp, MRP1, and ABCG2 and that sunitinib completely reverses ABCG2-mediated drug resistance. The biochemical data presented here show that there is a direct interaction of sunitinib with both P-gp and ABCG2 most likely at the drug-binding sites as it prevents the photolabeling of these transporters with [125I]iodoarylazidoprazosin (IAAP), and it also induces a conformational change in the transporters similar to that observed with the known substrates/inhibitors of these transporters.
Materials and Methods
Chemicals. Sunitinib (see Chow and Eckhardt, 2007 and Rock et al., 2007 for chemical structure and formula) was obtained from Pfizer Global Research and Development (La Jolla, CA). Rhodamine 123 and doxorubicin were obtained from Sigma-Aldrich (St. Louis, MO). Pheophorbide A was from Frontier Scientific (Logan, UT). Calcein AM was purchased from Invitrogen (Carlsbad, CA). Fumitremorgin C (FTC) was synthesized by Thomas McCloud, Developmental Therapeutics Program, Natural Products Extraction Laboratory, National Cancer Institute, National Institutes of Health (Bethesda, MD). MK571 was purchased from EMD Biosciences (Gibbstown, NJ). PSC833 (valspodar) was obtained from Novartis (East Hanover, NJ). Flavopiridol and depsipeptide were supplied by the National Cancer Institute anticancer drug screen (Bethesda, MD). Topotecan and SN-38 were obtained from LKT Labs (St. Paul, MN). IAAP (2200 Ci/mmol) was purchased from PerkinElmer Life Sciences (Waltham, MA).
Cell Lines. Human embryonic kidney (HEK)-293 cells stably transfected with pcDNA3.1 vector containing wild-type ABCB1 (P-gp-HEK), ABCC1 (MRP-HEK), or ABCG2 (Arg482-HEK) were maintained as described previously (Müller et al., 2002; Robey et al., 2003). The stable transfectants of HEK-293 cells were maintained in Eagle's minimal essential medium supplemented with 10% fetal bovine serum, penicillin, streptomycin, and 2 mg/ml G418. MCF7 FLV1000 cells (Robey et al., 2001) were grown in the RPMI media supplemented with 10% fetal bovine serum, penicillin, streptomycin, and 1 μM flavopiridol.
Flow Cytometry. Inhibition of P-gp-, MRP1-, or ABCG2-mediated transport was determined by flow cytometry using the fluorescent compounds rhodamine 123, calcein AM, or pheophorbide A, respectively, as described previously (Robey et al., 2004). In brief, cells were trypsinized and incubated with 0.5 μg/ml rhodamine 123, 200 nM calcein AM, or 1 μM pheophorbide A in the presence or absence of the desired inhibitor (indicated concentration of sunitinib or 2.5 μM valspodar for P-gp, 50 μM MK571 for MRP1, or 10 μM FTC for ABCG2) for 30 min. Subsequently, cells were washed and allowed to incubate for 60 min in substrate-free medium continuing with or without inhibitor. Cells were then washed with cold phosphate-buffered saline before analysis (all the incubations and washings were carried out in the subdued light).
Interaction with P-gp and ABCG2 was further examined with the UIC2 shift (for P-gp-expressing cells) or 5D3 shift (for ABCG2-expressing cells) assay, as described earlier (Mechetner et al., 1997; Ozvegy-Laczka et al., 2005), with minor modifications. Three hundred thousand cells were incubated in the presence or absence of varying concentrations of sunitinib, 10 μM cyclosporine A (for P-gp), or 20 μM FTC (for ABCG2) for 5 min at 37°C followed by the addition of UIC2 antibody (3 μg for P-gp; BD Biosciences, San Jose, CA) or 5D3 antibody (1:2500 for ABCG2; eBioscience, San Diego, CA) for 45 min (for P-gp-expressing cells) or 2 h (for ABCG2-expressing cells). Cells were subsequently washed and incubated in fluorescein isothiocyanate (FITC)-labeled anti-mouse secondary antibody (4 μg for P-gp; BD Biosciences) or allophycocyanin (APC)-labeled goat anti-mouse secondary antibody (1:35 for ABCG2; Leinco Technologies, Inc., St. Louis, MO) for 45 min in the dark at 37°C. The cells were washed with cold phosphate-buffered saline and analyzed.
Rhodamine, calcein, and FITC fluorescence were measured on a fluorescence-activated cell-sorting flow cytometer equipped with a 488-nm argon laser and 530-nm band pass filter; pheophorbide A and APC fluorescence were measured with a 635-nm red diode laser and a 561-nm long pass filter. At least 10,000 events were collected for all the samples, and debris was eliminated by gating on forward versus side scatter based on propidium iodide staining.
Cytotoxicity Assays. Cytotoxicity assays were performed based on the sulforhodamine B assay reported by Skehan et al. (1990). Cells were plated at a density of 10,000 cells/well in 96-well plates and allowed to attach overnight at 37°C in 5% CO2. The drugs were subsequently added at various concentrations, and plates were allowed to incubate for 96 h at 37°C in 5% CO2. Cells were then fixed with 50% trichloroacetic acid, washed, and allowed to dry. Plates were then stained with sulforhodamine B solution (0.4% sulforhodamine B w/v in 1% acetic acid) for 30 min and washed three times in 1% acetic acid solution. Sulforhodamine was then solubilized with 10 mM Tris base, and optical densities were read on a plate reader at an absorbance of 570 nm. Each concentration was tested in quadruplicate, and controls were performed in replicates of eight.
Photoaffinity Labeling of P-gp and ABCG2 with IAAP. Crude membranes (1 mg protein/ml) from either P-gp-expressing High-five cells or ABCG2-expressing MCF-7 FLV1000 cells were incubated with 0 to 100 μM sunitinib for 10 min at 21 to 23°C in 50 mM Tris-HCl, pH 7.5. Then 3 to 6 nM [125I]IAAP (2200 Ci/mmol) (PerkinElmer Life Sciences) was added and incubated for an additional 5 min. Both the incubations with sunitinib or another inhibitor and IAAP were carried out under subdued light. The samples were illuminated with a UV lamp (365 nm) for 10 min at room temperature. The labeled ABCG2 was immunoprecipitated as described previously (Shukla et al., 2006). Samples were separated on a 7% Tris-acetate gel at constant voltage, and gels were dried and exposed to X-ray film for 12 to 24 h at –80°C. The incorporation of [125I]IAAP into the ABCG2 or P-gp band was quantified using the STORM 860 PhosphorImager system (GE Healthcare, Little Chalfont, Buckinghamshire, UK) and the software ImageQuaNT (GE Healthcare), as described previously (Sauna et al., 2004).
ATPase Assay. Crude membrane protein (100 μg protein/ml) from High-five insect cells expressing either P-gp or ABCG2 was incubated at 37°C with varying concentrations of sunitinib in the presence and absence of sodium ortho vanadate (0.3 mM for P-gp) or BeFx (0.2 mM beryllium sulfate and 2.5 mM sodium fluoride for ABCG2) in ATPase assay buffer (50 mM KCl, 5 mM NaN3, 2 mM EGTA, 10 mM MgCl2, 1 mM DTT, pH 6.8) for 10 min. The reaction was started by the addition of 5 mM ATP and incubated for 20 min at 37°C. SDS solution (0.1 ml of 5% SDS) was added to terminate the reaction, and the amount of inorganic phosphate released was quantified with a colorimetric reaction, as described previously (Ambudkar, 1998). The specific activity was recorded as either vanadate-sensitive (for P-gp) or BeFx-sensitive (for ABCG2) ATPase activity.
Results
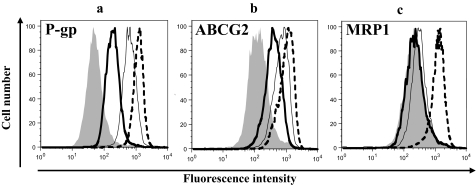
Sunitinib Inhibits P-gp-, MRP1-, and ABCG2-Mediated Efflux of Substrates. Inhibition of ABC transporters seems to be a class effect for the TKIs (Ozvegy-Laczka et al., 2005); for this reason, we evaluated sunitinib for its ability to inhibit transport mediated by P-gp, MRP1, and ABCG2. Accumulation assays with different fluorescent substrates (rhodamine 123 for P-gp, calcein AM for MRP1, and pheophorbide A for ABCG2) in P-gp-, MRP1-, and ABCG2-expressing HEK cells were performed in the presence or absence of 1 or 10 μM sunitinib or specific inhibitors as described under Materials and Methods. As shown in Fig. 1, the presence of sunitinib (1 and 10 μM) inhibited the efflux of rhodamine 123 and pheophorbide A by P-gp and ABCG2, respectively, but 10 μM sunitinib only had a slight effect on calcein AM efflux by MRP1. The inhibition of P-gp and ABCG2 activity with 10 μM sunitinib was comparable with the inhibition observed with valspodar and FTC, respectively, which are known inhibitors of these transporters. The control pcDNA3.1-HEK cells did not show any significant differences in the accumulation of rhodamine 123, calcein AM, and pheophorbide A at the tested concentrations of sunitinib, and the cells treated with sunitinib alone also did not show any detectable intrinsic fluorescence in FL1, FL2, FL3, or FL4 setting in the flow cytometer (data not shown).
Fig. 1.
Effect of sunitinib on the accumulation of fluorescent drug substrates in P-gp- and ABCG2-expressing cells: P-gp-HEK (a), Arg482-HEK (b), or MRP1-HEK (c) cells (300,000/tube) were incubated with 0.5 μg/ml rhodamine 123 (a), 1 μM pheophorbide A (b), or 200 nM calcein AM (c) for 30 min at 37°C in the dark, in the absence (gray-filled) or presence of 1 μM (bold line) or 10 μM (thin line) sunitinib and specific inhibitors [dashed line, 2.5 μM valspodar (PSC833) for P-gp, 10 μM FTC for ABCG2 or 50 μM MK571 for MRP1]. The cells were then washed, allowed to incubate for 1 h in substrate-free medium continuing without or with inhibitors, and were subsequently analyzed by flow cytometer as described under Materials and Methods. The histograms represent the intracellular fluorescence intensity of the substrates. Representative histograms from one of three independent histograms are shown.
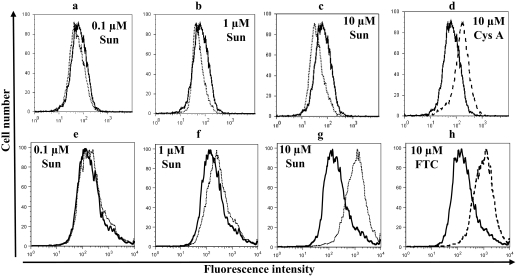
Sunitinib Changes the Binding of Conformation-Sensitive Antibodies to P-gp and ABCG2. We next sought to determine whether the effects of sunitinib were caused by direct interaction with the transporters. UIC2 and 5D3 are P-gp- and ABCG2-specific antibodies, respectively, that recognize extracellular epitope(s) and have been shown to recognize conformation changes in these transporters that result in increased binding in the presence of substrates or inhibitors in the case of UIC2, or inhibitors alone in the case of 5D3 (Mechetner et al., 1997; Ozvegy-Laczka et al., 2005; Robey et al., 2007). Binding of these antibodies to P-gp and ABCG2 in the presence and absence of varying concentrations of sunitinib was examined. As shown in Fig. 2, whereas 10 μM cyclosporine A increased the binding of UIC2 to P-gp (Fig. 2d), 0.1 to 10 μM sunitinib decreased the binding of this antibody to this transporter (Fig. 2, a–c) similar to the other known inhibitors such as vanadate, which blocks the transport cycle of P-gp (Druley et al., 2001). On the other hand, 0.1 to 10 μM sunitinib increased the binding of 5D3 antibody to ABCG2 (Fig. 2h), which was similar to the increase in the antibody binding caused by 10 μM FTC (Fig. 2, e–g). Taken together, the above data suggest that sunitinib interacts with both P-gp and ABCG2 and induces conformation changes similar to other substrates or inhibitors of these transporters.
Fig. 2.
Effect of sunitinib on the binding of UIC2 antibody to P-gp and 5D3 antibody to ABCG2: KB-V1 (a–d) or Arg482-HEK (e–h) cells were incubated in the absence (bold line) or presence of 0.1 μM sunitinib (a, e: dashed line), 1 μM sunitinib (b, f: dashed line), 10 μM sunitinib (c, g: dashed line) sunitinib, 10 μM Cys A (d: broken line) or 10 μM FTC (h: broken line) for 5 min at 37°C. Three micrograms of P-gp-specific UIC2 antibody (a–d) or 1:3500 dilution of 5D3 (e–h) antibody was added, and the cells were incubated at 37°C for 20 min. The cells were washed and subsequently incubated with 3 μg of FITC-conjugated (a–d) or 1:100 dilution of APC-labeled secondary antibody (e–h). The cells were then washed and analyzed by flow cytometer as described under Materials and Methods. Representative histograms from one of at least three independent experiments are shown. Sun, sunitinib; Cys A, cyclosporine A.
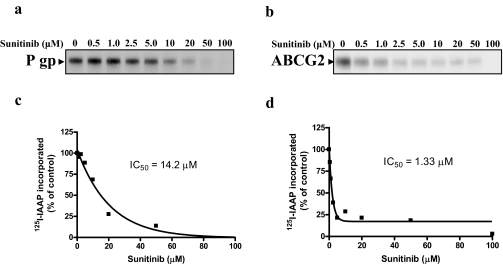
Sunitinib Inhibits Photo Cross-Linking of [125I]IAAP to P-gp and ABCG2. To determine whether sunitinib interacts at the substrate ([125I]IAAP) binding sites of these two transporters, its effect on photolabeling of P-gp and ABCG2 with 1[125I]IAAP was also studied. [125I]IAAP is known to photolabel both P-gp and ABCG2, and this labeling is inhibited by some substrates and inhibitors of these transporters (Sauna and Ambudkar, 2000; Shukla et al., 2006). The crude membranes of P-gp-expressing High-five insect cells (Fig. 3a) or ABCG2-expressing MCF-7 FLV1000 cells (Fig. 3b) were incubated with varying concentrations (0–100 μM) of sunitinib for 5 min at 21 to 23°C. [125I]IAAP (3–6 nM) was added, and the membranes were incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp (365 nm) for 10 min and processed as described under Materials and Methods. It was observed that sunitinib inhibited the photolabeling of both P-gp (Fig. 3a) and ABCG2 (Fig. 3b) by [125I]IAAP, suggesting that it competes for the IAAP binding site on P-gp and ABCG2. This inhibition of [125I]IAAP binding to P-gp and ABCG2 by sunitinib was concentration-dependent, with IC50 values of 14.2 μM (Fig. 3c) and 1.33 μM (Fig. 3d), respectively. The IC50 values obtained for inhibition of 1[125I]IAAP binding to P-gp and ABCG2 are comparable with those reported for imatinib and other known inhibitors of these transporters [imatinib 3.18 μM (Shukla et al., 2008a), disulfiram 3.5–6.3 μM (Sauna et al., 2004) for P-gp; imatinib 0.47 μM (Shukla et al., 2008a), FTC 5.7 μM (Wu et al., 2007) for ABCG2] and indicate direct interaction with both proteins.
Fig. 3.
Effect of sunitinib on photoaffinity labeling of P-gp and ABCG2 with [125I]IAAP. Crude membranes (500 μg/ml) from High-five cells expressing P-gp (a) or MCF-7 FLV1000 (b) cells were incubated with 0 to 100 μM sunitinib for 5 min at 21 to 23°C in 50 mM Tris-HCl, pH 7.5. Three to 6 nM [125I]IAAP (2200 Ci/mmol) was added and incubated for an additional 5 min under subdued light. The samples were then illuminated with a UV lamp (365 nm) for 10 min and were processedas described under Materials and Methods. Representative autoradiograms from one experiment are shown, and similar results were obtained in two additional experiments. The arrows show the position of the P-gp and ABCG2 band. c and d, the incorporation of [125I]IAAP (from autoradiogram, y-axis) into the P-gp (c) and ABCG2 (d) band was quantified by estimating the radioactivity of this band using the STORM 860 PhosphorImager system and the ImageQuaNT software and plotted as a function of concentration of sunitinib (x-axis).
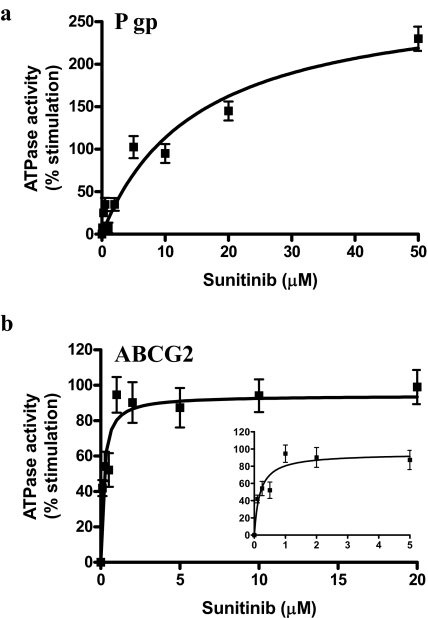
Effect of Sunitinib on ATP Hydrolysis by P-gp and ABCG2. The effect of sunitinib was monitored on the vanadate-sensitive (for P-gp) or BeFx-sensitive (for ABCG2) ATPase activity in crude membranes isolated from High-five cells transfected with ABCB1 (P-gp overexpressing) or ABCG2. The crude membranes expressing P-gp and ABCG2 were incubated with varying concentrations of sunitinib (0–50 μM) for 10 min at room temperature, and the ATPase activity at 37°C was determined as described under Materials and Methods. Sunitinib stimulated the ATPase activity of P-gp to 3-fold (Fig. 4a) and ABCG2 to 2-fold (Fig. 4b). Thus, results with IAAP provide yet another confirmation that sunitinib directly interacts with the transporter proteins P-glycoprotein and ABCG2 (Apparent Kd: P-gp 15.1 ± 3.1 μM, ABCG2 0.18 ± 0.05 μM).
Fig. 4.
Effect of sunitinib on ATPase activity of P-gp and ABCG2. Crude membrane protein (100 μg protein/ml) from High-five cells expressing P-gp (a) or ABCG2 (b) was incubated at 37°C with varying concentrations of sunitinib in the presence or absence of 0.3 mM sodium orthovanadate (a) or BeFx (0.2 mM beryllium sulfate and 2.5 mM sodium fluoride) (b) in ATPase assay buffer for 10 min. The reaction was started by the addition of 5 mM ATP at 37°C and was stopped by the addition of 0.1 ml of 5% SDS solution. The amount of inorganic phosphate released, and the vanadate or BeFx-sensitive ATPase activity was determined as described under Materials and Methods. The basal activity (10–20 nmol Pi/min/mg for P-gp and 25–35 nmol Pi/min/mg for ABCG2) was subtracted to calculate percent stimulation in the presence of indicated concentrations of sunitinib. The average from three experiments is shown here, and the error bars represent S.E. Inset in b shows the percentage stimulation of ABCG2 ATPase activity plotted as a function of concentration in the range of 0 to 5 μM sunitinib.
Sunitinib Reverses Resistance in P-gp- and ABCG2-Expressing Cells. As shown in Fig. 1, sunitinib inhibited efflux of substrates in P-gp-, MRP1-, and ABCG2-expressing HEK cells. We next sought to determine whether sunitinib could reverse P-gp-, MRP1-, or ABCG2-mediated resistance to anticancer agents in 4-day cytotoxicity assays. The toxicity of sunitinib in empty vector-transfected pcDNA3.1-HEK and ABCB1-, ABCC1-, and ABCG2-transfected HEK cells was determined in cytotoxicity assays as described under Materials and Methods. Sunitinib had similar IC50 values (9–10 μM) in all the sublines (data not shown) with no evidence of cross-resistance.
Based on these data, a nontoxic concentration of sunitinib (2 μM; >90% cells were viable for all the transfectants) was used in further cytotoxicity experiments with empty vector-, ABCB1-, ABCC1-, and ABCG2-transfected HEK-293 cells. We combined 2 μM sunitinib with varying concentrations of the substrate drugs depsipeptide and doxorubicin for cells expressing P-gp, etoposide for cells expressing MRP1, and topotecan and SN-38 for cells expressing ABCG2. The P-gp-expressing cells were 800- and 80-fold resistant to depsipeptide and doxorubicin, respectively, and ABCG2-expressing cells were 19- and 28-fold resistant to topotecan and SN-38, respectively. Whereas the presence of sunitinib slightly reversed P-gp-mediated resistance to depsipeptide and had little effect on resistance to doxorubicin (Table 1), it completely inhibited resistance to topotecan and SN-38 in ABCG2-expressing cells (Table 2). Although the discrepancy between reversal of resistance mediated by P-gp or ABCG2 could be because of the difference in cross-resistance to substrate drugs, the findings are in agreement with our data above showing a lower IC50 value for inhibition of IAAP binding to ABCG2 compared with P-gp. These data suggest that sunitinib will probably be more effective in inhibiting ABCG2 than P-gp function. This may further explain the above observation where sunitinib was not able to reverse the P-gp-mediated drug resistance but was effective in inhibiting ABCG2-mediated drug resistance. It is possible that depsipeptide and daunorubicin bind to different regions in the drug-binding pocket, and sunitinib had higher affinity for the depsipeptide binding region compared with daunorubicin binding site. Sunitinib had no effect on MRP1-mediated etoposide resistance at the same concentration, which is in agreement with the flow cytometry data in Fig. 1, where sunitinib at 10 μM was able to only slightly increase calcein fluorescence in MRP1-transfected cells. The decrease in IC50 values by sunitinib was 2- to 3-fold for P-gp-expressing cells and >10-fold for ABCG2-expressing cells. This suggests that sunitinib can inhibit the function of drug transporters, thereby making the cells sensitive to these and other drugs that are substrates for these transporters.
TABLE 1.
Reversal of cytotoxicity in P-gp-expressing cells by sunitinib
|
Cell Line
|
Depsipeptide,
IC50a
(Relative
Resistance)b
|
Doxorubicin,
IC50a
(Relative
Resistance)b
|
||
|---|---|---|---|---|
| Control (DMSO) | +2 μM Sunitinib | Control (DMSO) | +2 μM Sunitinib | |
| μM | ||||
| pcDNA3.1-HEK | 0.0012 ± 0.0006 | 0.0012 ± 0.0006 | 0.055 ± 0.033 | 0.14 ± 0.010 |
| P-gp-HEK | 0.96 ± 0.23 | 0.38 ± 0.17 | 4.4 ± 2.4 | 4.8 ± 3.3 |
| (800) | (317) | (80) | (87) | |
DMSO, dimethyl sulfoxide.
The values represent the mean ± S.D. of three independent experiments performed in triplicate.
Relative resistance values (in parentheses) were obtained by dividing the IC50 value of the P-gp-HEK cells by the IC50 value of the empty vector pcDNA3.1-transfected HEK cell line.
TABLE 2.
Reversal of cytotoxicity in ABCG2-expressing cells by sunitinib
|
Cell Line
|
Topotecan,
IC50a
(Relative
Resistance)b
|
SN-38,
IC50a
(Relative
Resistance)b
|
||
|---|---|---|---|---|
| Control (DMSO) | +2 μM Sunitinib | Control (DMSO) | +2 μM Sunitinib | |
| μM | ||||
| pcDNA3.1-HEK | 0.011 ± 0.0041 | 0.014 ± 0.0078 | 0.0017 ± 0.0011 | 0.0026 ± 0.0017 |
| Arg482-HEK (ABCG2) | 0.21 ± 0.0066 | 0.019 ± 0.0036 | 0.048 ± 0.0038 | 0.0022 ± 0.00047 |
| (19) | (1.7) | (28) | (1.3) | |
DMSO, dimethyl sulfoxide.
The values represent the mean ± S.D. of three independent experiments performed in triplicate.
Relative resistance values (in parentheses) were obtained by dividing the IC50 value of the Arg482-HEK293 cells by the IC50 value of the empty vector pcDNA3.1-transfected HEK cell line.
Discussion
Sunitinib is a rationally designed and developed receptor TKI that, like others in its class, inhibits multiple TKs through competitive inhibition of ATP binding, a key step in the phosphorylation activity of the kinases. One surprising finding that emerged during the development of TKIs has been that imatinib was first shown to be a transport substrate of P-gp (Mahon et al., 2000), and imatinib along with EKI-785 was shown to interact with P-gp or MRP1, respectively (Hegedus et al., 2002). Likewise, CI1033 (Erlichman et al., 2001) and gefitinib (Elkind et al., 2005) were shown to be substrates and inhibitors of ABCG2, and TKIs such as erlotinib have been shown to inhibit P-gp- and ABCG2-mediated transport (Shi et al., 2007). Thus, interaction with ABC transporters seems to be a class effect of these compounds, thus leading us to examine the interaction between sunitinib and P-gp, MRP1 and ABCG2.
In this study, we show that sunitinib at low micromolar concentrations inhibited the efflux of fluorescent substrates mediated by P-gp or ABCG2 in a manner comparable with established inhibitors of these transporters. Conformation-sensitive antibody binding assays with UIC2 and 5D3 antibodies also suggested that sunitinib interacts directly with P-gp and ABCG2, respectively, and induces conformational changes similar to those observed with substrates/inhibitors of these transporters. Sunitinib also inhibited the photolabeling of P-gp and ABCG2 by [125I]IAAP, which is also a substrate of these transporters (Shukla et al., 2006). This observation clearly indicated that sunitinib also interacts at the substrate-binding pocket of both transporters. The IC50 of sunitinib for inhibition of IAAP binding was 14.2 and 1.33 μM for P-gp and ABCG2, respectively. Sunitinib interaction at the substrate-binding pocket was further monitored by ATP hydrolysis assays. The concentration required for 50% stimulation of ATP hydrolysis by ABCG2 was lower compared with P-gp (IC50 = ABCG2, 0.18 ± 0.05 μM; P-gp, 15.1 ± 3.10 μM), which indicates that sunitinib exhibits higher affinity for ABCG2 compared with P-gp. This is in agreement with previous studies, as it is known that other TKIs have higher affinity for ABCG2 compared with P-gp (Ozvegy-Laczka et al., 2004, 2005).
In combination cytotoxicity assays, we also found that a nontoxic concentration of sunitinib (2 μM) in combination with a P-gp- or an ABCG2-specific substrate was able to partially inhibit drug resistance mediated by P-gp and completely inhibit resistance mediated by ABCG2 in transfected HEK-293 cells. These results were similar to those obtained in short-term substrate accumulation assays using flow cytometry, which showed higher accumulation of tested drug in the presence of sunitinib. The results also mirrored those obtained in the IAAP competition and ATP hydrolysis assays, where sunitinib was found to have a higher affinity for ABCG2 than P-gp. Taken together, these results are convincing evidence that sunitinib inhibits ABCG2 and inhibits P-gp as well, albeit more weakly. The question is whether this could be clinically relevant. One immediate implication is that p.o. administered sunitinib in combination with an ABCG2 or P-gp substrate could affect their absorption. Indeed, in vivo animal studies have shown that combining substrate drugs with TKIs can result in increased p.o. bioavailability (Stewart et al., 2004; Breedveld et al., 2005), and it can be perceived that combining sunitinib with substrate drugs may also lead to enhanced systemic exposure of other anticancer agents by inhibiting gastrointestinal efflux or biliary/renal excretion of these agents. Whether sunitinib could be used with substrate drugs to improve clinical outcome is a hypothesis worthy of study.
Another question is whether sunitinib could, in some settings, be a substrate for ABC transporters and resistance mediated by them. One postulated mechanism of resistance to p.o. administered kinase inhibitors is the active extrusion of these agents by ABC drug transporters (Lemos et al., 2008). This not only could limit the concentration of the agents inside cells but also the clinically significant gastrointestinal expression of ABC transporters could affect the bioavailability of kinase inhibitors (Lemos et al., 2008). The role of kinase inhibitors as substrate of ABC drug transporters is controversial and is under current investigation. For instance, although several groups have shown that the resistance to one such TKI, imatinib mesylate, is because of its low intracellular level accumulation (Illmer et al., 2004; Brendel et al., 2007; Widmer et al., 2007), other reports suggested that the resistance is not dependent on the drug efflux by ABC drug transporters (Ferrao et al., 2003; Zong et al., 2005). Our results showed that sunitinib stimulates ATPase activity of both P-gp and ABCG2 and inhibits the photolabeling with IAAP, suggesting that sunitinib most likely is a transport substrate of both transporters. To confirm these findings, the efflux of radiolabeled sunitinib from the P-gp- and ABCG2-expressing cells or ATP-dependent accumulation of labeled sunitinib in membrane vesicles needs to be carried out. In this case, the p.o. bioavailability of sunitinib might be affected by single nucleotide polymorphisms of the ABCB1 or ABCG2 genes that have been shown to influence transport efficacy (Cascorbi, 2006). Furthermore, if sunitinib was a transport substrate, this would suggest that inhibition of P-gp or ABCG2 might lead to increased bioavailability.
The detailed characterization of the interaction of sunitinib with transporters will not only provide information about the involvement of transporters in drug-drug interactions between sunitinib and other anticancer agents but also would add information about the impact of transporters on the efficacy, toxicity, and pharmacokinetics of sunitinib. The results from these studies would also provide a better understanding of the potential drug interactions between sunitinib and other anticancer agents, which not only would result in altered pharmacokinetics of sunitinib but also would affect the pharmacokinetics, efficacy, or toxicity of the known anticancer agents or substrates of these transporters.
In conclusion, this study provides the first in vitro evidence that sunitinib interacts with the two major ABC drug transporters, P-gp and ABCG2. This interaction of sunitinib with the drug transporters may affect treatment outcome for sunitinib and could also influence the disposition and toxicity of this drug in cancer therapy.
Acknowledgments
We thank Dr. Krishnamachary Nandigama for providing P-gp- and ABCG2-expressing High-five insect cell crude membranes, George Leiman for editorial assistance, and Dr. James Christensen (Pfizer Global Research and Development, La Jolla Laboratories, La Jolla, CA) for comments on the manuscript.
This research was supported by the Intramural Research Program of the National Institutes of Health National Cancer Institute, Center for Cancer Research.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024612.
ABBREVIATIONS: Sunitinib, N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide; TK, tyrosine kinase; ABC, ATP-binding cassette; P-gp, P-glycoprotein; MRP, multidrug resistance-associated protein; MDR, multidrug resistance; TKI, tyrosine kinase inhibitor; IAAP, iodoarylazidoprazosin; FTC, fumitremorgin C; HEK, human embryonic kidney; FITC, fluorescein isothiocyanate; MK571, 3-[[3-[2-(7-chloroquinolin-2-yl)vinyl]phenyl]-(2-dimethylcarbamoylethylsulfanyl)methylsulfanyl] propionic acid; SN-38, 7-ethyl-10-hydroxycamptothecin; EKI-485, N-[4-[(3-bromophenyl)amino]-6-quinazolinyl]-2-butynamide; EKI-785, N-[4-[(3-bromophenyl)-amino]-6-quinazolinyl]-2-butynamide; CI1033, N-[4-[(3-chloro-4-fluorophenyl)amino]-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide dihydrochloride; INNO-406, 4-[[(3S)-3-dimethylaminopyrrolidin-1-yl]methyl]-N-[4-methyl-3-[(4-pyrimidin-5-ylpyrimidin-2-yl)amino]phenyl]-3-(trifluoromethyl)-benzamide; APC, allophycocyanin.
References
- Ambudkar SV (1998) Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells. Methods Enzymol 292 504–514. [DOI] [PubMed] [Google Scholar]
- Bello CL, Sherman L, Zhou J, Verkh L, Smeraglia J, Mount J, and Klamerus KJ (2006) Effect of food on the pharmacokinetics of sunitinib malate (SU11248), a multi-targeted receptor tyrosine kinase inhibitor: results from a phase I study in healthy subjects. Anticancer Drugs 17 353–358. [DOI] [PubMed] [Google Scholar]
- Breedveld P, Pluim D, Cipriani G, Wielinga P, van Tellingen O, Schinkel AH, and Schellens JH (2005) The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res 65 2577–2582. [DOI] [PubMed] [Google Scholar]
- Brendel C, Scharenberg C, Dohse M, Robey RW, Bates SE, Shukla S, Ambudkar SV, Wang Y, Wennemuth G, Burchert A, et al. (2007) Imatinib mesylate and nilotinib (AMN107) exhibit high-affinity interaction with ABCG2 on primitive hematopoietic stem cells. Leukemia 21 1267–1275. [DOI] [PubMed] [Google Scholar]
- Burger H, van Tol H, Brok M, Wiemer EA, de Bruijn EA, Guetens G, de Boeck G, Sparreboom A, Verweij J, and Nooter K (2005) Chronic imatinib mesylate exposure leads to reduced intracellular drug accumulation by induction of the ABCG2 (BCRP) and ABCB1 (MDR1) drug transport pumps. Cancer Biol Ther 4 747–752. [DOI] [PubMed] [Google Scholar]
- Cascorbi I (2006) Role of pharmacogenetics of ATP-binding cassette transporters in the pharmacokinetics of drugs. Pharmacol Ther 112 457–473. [DOI] [PubMed] [Google Scholar]
- Chow LQM and Eckhardt SG (2007) Sunitinib: from rational design to clinical efficacy. J Clin Oncol 25 884–896. [DOI] [PubMed] [Google Scholar]
- Druley TE, Stein WD, and Roninson IB (2001) Analysis of MDR1 P-glycoprotein conformational changes in permeabilized cells using differential immunoreactivity. Biochemistry 40 4312–4322. [DOI] [PubMed] [Google Scholar]
- Elkind NB, Szentpetery Z, Apati A, Ozvegy-Laczka C, Varady G, Ujhelly O, Szabo K, Homolya L, Varadi A, Buday L, et al. (2005) Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res 65 1770–1777. [DOI] [PubMed] [Google Scholar]
- Erlichman C, Boerner SA, Hallgren CG, Spieker R, Wang X-Y, James CD, Scheffer GL, Maliepaard M, Ross DD, Bible KC, et al. (2001) The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydroxycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux. Cancer Res 61 739–748. [PubMed] [Google Scholar]
- Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, et al. (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24 25–35. [DOI] [PubMed] [Google Scholar]
- Ferrao PT, Frost MJ, Siah SP, and Ashman LK (2003) Overexpression of P-glycoprotein in K562 cells does not confer resistance to the growth inhibitory effects of imatinib (STI571) in vitro. Blood 102 4499–4503. [DOI] [PubMed] [Google Scholar]
- Glavinas H, Krajcsi P, Cserepes J, and Sarkadi B (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1 27–42. [DOI] [PubMed] [Google Scholar]
- Goodman VL, Rock EP, Dagher R, Ramchandani RP, Abraham S, Gobburu JVS, Booth BP, Verbois SL, Morse DE, Liang CY, et al. (2007) Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 13 1367–1373. [DOI] [PubMed] [Google Scholar]
- Hanahan D and Weinberg RA (2000) The hallmarks of cancer. Cell 100 57–70. [DOI] [PubMed] [Google Scholar]
- Hegedus T, Orfi L, Seprodi A, Váradi A, Sarkadi B, and Kéri G (2002) Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1. Biochim Biophys Acta 1587 318–325. [DOI] [PubMed] [Google Scholar]
- Illmer T, Schaich M, Platzbecker U, Freiberg-Richter J, Oelschlägel U, von Bonin M, Pursche S, Bergemann T, Ehninger G, and Schleyer E (2004) P-glycoprotein-mediated drug efflux is a resistance mechanism of chronic myelogenous leukemia cells to treatment with imatinib mesylate. Leukemia 18 401–408. [DOI] [PubMed] [Google Scholar]
- Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, and Stewart CF (2006) Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res 66 4802–4807. [DOI] [PubMed] [Google Scholar]
- Lemos C, Jansen G, and Peters GJ (2008) Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. Br J Cancer 98 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Baer MR, Bowman MJ, Pera P, Zheng X, Morgan J, Pandey RA, and Oseroff AR (2007) The tyrosine kinase inhibitor imatinib mesylate enhances the efficacy of photodynamic therapy by inhibiting ABCG2. Clin Cancer Res 13 2463–2470. [DOI] [PubMed] [Google Scholar]
- Mahon FX, Deininger MW, Schultheis B, Chabrol J, Reiffers J, Goldman JM, and Melo JV (2000) Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood 96 1070–1079. [PubMed] [Google Scholar]
- Mechetner EB, Schott B, Morse BS, Stein WD, Druley T, Davis KA, Tsuruo T, and Roninson IB (1997) P-glycoprotein function involves conformational transitions detectable by differential immunoreactivity. Proc Natl Acad Sci U S A 94 12908–12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Yong M, Peng XH, Petre B, Arora S, and Ambudkar SV (2002) Evidence for the role of glycosylation in accessibility of the extracellular domains of human MRP1 (ABCC1). Biochemistry 41 10123–10132. [DOI] [PubMed] [Google Scholar]
- Ozvegy-Laczka C, Cserepes J, Elkind NB, and Sarkadi B (2005) Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat 8 15–26. [DOI] [PubMed] [Google Scholar]
- Ozvegy-Laczka C, Hegedus T, Várady G, Ujhelly O, Schuetz JD, Váradi A, Kéri G, Orfi L, Német K, and Sarkadi B (2004) High-affinity interaction of tyrosine kinase inhibitors with the ABCG2 multidrug transporter. Mol Pharmacol 65 1485–1495. [DOI] [PubMed] [Google Scholar]
- Pawson T (2002) Regulation and targets of receptor tyrosine kinases. Eur J Cancer 38 (Suppl 5): S3–S10. [DOI] [PubMed] [Google Scholar]
- Polli JW, Humphreys JE, Harmon KA, Castellino S, O'Mara MJ, Olson KL, John-Williams LS, Koch KM, and Serabjit-Singh CJ (2008) The role of efflux and uptake transporters in N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]-amino}-methyl)-2-furyl]-4-quinazolinamine (GW572016, Lapatinib) disposition and drug interactions. Drug Metab Dispos 36 695–701. [DOI] [PubMed] [Google Scholar]
- Robey RW, Honjo Y, Morisaki K, Nadjem TA, Runge S, Risbood M, Poruchynsky MS, and Bates SE (2003) Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer 89 1971–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey RW, Medina-Pérez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, and Bates SE (2001) Overexpression of the ATP-binding cassette half-transporter, ABCG2 (Mxr/BCrp/ABCP1), in flavopiridol-resistant human breast cancer cells. Clin Cancer Res 7 145–152. [PubMed] [Google Scholar]
- Robey RW, Shukla S, Steadman K, Obrzut T, Finley EM, Ambudkar SV, and Bates SE (2007) Inhibition of ABCG2-mediated transport by protein kinase inhibitors with a bisindolylmaleimide or indolocarbazole structure. Mol Cancer Ther 6 1877–1885. [DOI] [PubMed] [Google Scholar]
- Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, and Bates SE (2004) Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64 1242–1246. [DOI] [PubMed] [Google Scholar]
- Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, Dagher R, Justice R, and Pazdur R (2007) Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist 12 107–113. [DOI] [PubMed] [Google Scholar]
- Sarkadi B, Homolya L, Szakács G, and Váradi A (2006) Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev 86 1179–1236. [DOI] [PubMed] [Google Scholar]
- Sauna ZE and Ambudkar SV (2000) Evidence for a requirement for ATP hydrolysis at two distinct steps during a single turnover of the catalytic cycle of human P-glycoprotein. Proc Natl Acad Sci U S A 97 2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauna ZE, Peng XH, Nandigama K, Tekle S, and Ambudkar SV (2004) The molecular basis of the action of disulfiram as a modulator of the multidrug resistance-linked ATP binding cassette transporters MDR1 (ABCB1) and MRP1 (ABCC1). Mol Pharmacol 65 675–684. [DOI] [PubMed] [Google Scholar]
- Shi Z, Peng X-X, Kim I-W, Shukla S, Si Q-S, Robey RW, Bates SE, Shen T, Ashby CR Jr, Fu L-W, et al. (2007) Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance. Cancer Res 67 11012–11020. [DOI] [PubMed] [Google Scholar]
- Shukla S, Robey RW, Bates SE, and Ambudkar SV (2006) The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry 45 8940–8951. [DOI] [PubMed] [Google Scholar]
- Shukla S, Sauna ZE, and Ambudkar SV (2008a) Evidence for the interaction of imatinib at the transport-substrate site (s) of the multidrug-resistance-linked ABC drug transporters ABCB1 (P-glycoprotein) and ABCG2. Leukemia 22 445–447. [DOI] [PubMed] [Google Scholar]
- Shukla S, Wu CP, and Ambudkar SV (2008b) Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol 4 205–223. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, and Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82 1107–1112. [DOI] [PubMed] [Google Scholar]
- Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, Daw N, Jenkins JJ 3rd, Gilbertson R, Germain GS, et al. (2004) Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice. Cancer Res 64 7491–7499. [DOI] [PubMed] [Google Scholar]
- Widmer N, Rumpold H, Untergasser G, Fayet A, Buclin T, and Decosterd LA (2007) Resistance reversal by RNAi silencing of MDR1 in CML cells associated with increase in imatinib intracellular levels. Leukemia 21 1561–1562. [DOI] [PubMed] [Google Scholar]
- Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, and Ambudkar SV (2007) Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter. Mol Cancer Ther 6 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CJ, Yang CS, Chu YC, Cheng AL, Whang-Peng J, and Yang PC (2005) Gefitinib reverses chemotherapy resistance in gefitinib-insensitive multidrug resistant cancer cells expressing ATP-binding cassette family protein. Cancer Res 65 6943–6949. [DOI] [PubMed] [Google Scholar]
- Zong Y, Zhou S, and Sorrentino BP (2005) Loss of P-glycoprotein expression in hematopoietic stem cells does not improve responses to imatinib in a murine model of chronic myelogenous leukemia. Leukemia 19 1590–1596. [DOI] [PubMed] [Google Scholar]