Abstract
2,2-Bis(bromomethyl)-1,3-propanediol (BMP) is a brominated flame retardant, previously shown to be a multisite carcinogen in experimental animals. Studies were performed to characterize the dispositional and metabolic fate of BMP after oral or intravenous administration to male Fischer-344 rats. After a single oral administration of [14C]BMP (10 or 100 mg/kg) >80% of the low dose and 48% of the high dose were excreted by 12 h in the urine predominantly as a glucuronide metabolite. After repeated daily oral doses for 5 or 10 days, route and rate of elimination were similar to those obtained after single administrations of BMP. In all studies, the radioactivity recovered in feces was low (<15%). The total amount of radioactivity remaining in tissues at 72 h after a single oral administration of BMP (100 mg/kg) was less than 1% of the dose, and repeated daily dosing did not lead to retention in tissues. After intravenous administration, the radiolabel found in blood decreased rapidly. Excretion profiles were similar to those after oral administration. Parent BMP and BMP glucuronide were present in blood plasma after oral or intravenous dosing. After an intravenous dose of BMP (15 mg/kg) the hepatic BMP glucuronide was primarily exported into the bile (>50% within 6 h), but it underwent enterohepatic recycling with subsequent elimination in the urine. These data indicate that the extensive extraction and rapid glucuronidation by the liver limits exposure of internal tissues to BMP by greatly reducing its systemic bioavailability after oral exposure.
2,2-Bis(bromomethyl)-1,3-propanediol (BMP) is a brominated flame retardant (BFR) found in unsaturated polyester resins, molded products, and rigid polyurethane foam. It is also used as an additive during manufacture of plastic polymers and as a chemical intermediate for other flame retardants (Larsen, 1969). Between 1986 and 2002 the estimated annual aggregate production of BMP in the United States was 1 to 10 million pounds, and it is classified as a high-volume chemical (National Toxicology Program, 1996; U.S. Environmental Protection Agency, Non-Confidential IUR Production Volume Information, 2002, http://www.epa.gov/opptintr/iur/tools/data/2002-vol.htm).
The compound has a unique aliphatic neopentyl structure containing no hydrogen atoms adjacent to the carbon bonded to the bromine (Fig. 1) and is slightly soluble in water (2 g/l at 25°C). BMP may enter the environment through wastewater or dust particles and is expected to be environmentally persistent (National Toxicology Program, 1996; U.S. Environmental Protection Agency, 2002). According to a study conducted in the Ramat Hovav aquitard, its half-life is estimated to be more than 100 years (Ezra et al., 2006).
Fig. 1.
Chemical structure and molecular weight of BMP.
The acute oral toxicity of BMP in rats is reported to be low (oral LD50: 3.5 g/kg) (Keyes et al., 1979). Studies in experimental animals conducted by the National Toxicology Program in the 1980s and 1990s indicate that BMP is a multisite carcinogen in rats and mice. In a 13-week subchronic study kidney and urinary bladder lesions were reported in both rats and mice receiving BMP either in feed (0–3000 mg/kg) or by oral gavage (0–800 mg/kg). Lesions were observed mostly at the two highest doses of BMP for each the type of administration and test animal (Elwell et al., 1989). Furthermore, in a 2-year dietary toxicity study in Fischer-344 (F-344) rats, neoplasms of the skin, subcutaneous tissue, mammary gland, Zymbal's gland, oral cavity, esophagus, forestomach, small and large intestine, mesothelium, kidney, urinary bladder, lung, thyroid gland, seminal vesicle, hematopoietic system, and pancreas were observed in animals dosed with BMP (National Toxicology Program, 1996; Dunnick et al., 1997). Based on evidence of carcinogenicity from these studies, BMP is reasonably anticipated to be a human carcinogen (Report on Carcinogens, 2004).
Little information is available on the disposition and metabolism of BMP and their relevance to BMP toxicity. The studies presented here report on the absorption, distribution, metabolism, and elimination of BMP and describe the pharmacokinetics of BMP after oral or intravenous administration to male F-344 rats. Additional studies were designed to determine whether repeated oral administration of BMP alters its disposition profile.
Materials and Methods
Chemicals. U-14C-labeled BMP (lot 10426-17-34), in absolute ethanol (1 mCi/ml) was obtained from Midwest Research Institute (Kansas City, MO). The radiochemical purity of BMP was determined by reversed-phase HPLC-UV/visible-radiometric analysis to be 97.3%. The specific activity was reported to be 65.1 mCi/mmol (247 μCi/mg). Nonradiolabeled BMP (lot 04119MD) was obtained from Sigma-Aldrich (St. Louis, MO). Chemical purity of unlabeled BMP was 98%. Soluene-350 and Solvable tissue solubilization solvents and Pico-Fluor 40 scintillation cocktail solution were received from Perkin-Elmer (Torrance, CA). Hydrogen peroxide (30%) was obtained from VWR (West Chester, PA). Absolute ethanol was from Decon Laboratories, Inc. (King of Prussia, PA). Cremophore EL, β-glucuronidase (EC 3.2.1.31, type B-1 from bovine liver), sulfatase (EC 3.1.6.1, type VI from Aerobacter aerogenes), d-saccharic acid-1,4-lactone, glycine/HCl, acetonitrile, and all other chemicals and reagents were obtained from Sigma-Aldrich. All chemicals and reagents were HPLC or analytical grade.
Animal Studies. Animals. Male F-344 rats 8 to 9 weeks of age (182–236 g) without surgical alteration (conventional) or with an indwelling jugular vein cannula (JVC) were obtained from Harlan (Indianapolis, IN). All animals, with the exception of rats with surgically implanted bile duct cannulas (BDCs) (study described below), were housed in The University of Arizona Animal Care facility, which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The animals were maintained in a temperature-controlled (25°C) room under a 12-h light/dark cycle. Conventional animals were acclimated for 5 to 7 days after receipt. Animals with surgically implanted JVCs were acclimated only for 1 day after arrival to ensure the patency of the cannula. Animals were allowed food (National Toxicology Program; Zeigler Brothers, Inc., Gardners, PA) and water ad libitum except for a 12-h fasting period before a single administration of BMP. Food was returned 2 h after dosing. Animals used in the repeated oral administration studies were not fasted. Animals were placed in Nalgene metabolism cages 24 h before administration of BMP. Food was provided as a powder to reduce contamination of fecal matter.
Dose selection. The doses used in the in vivo studies reported herein were selected on the basis of the results of toxicity studies published by Elwell et al. (1989). Subtoxic doses of 10, 100, 150, 300, and 600 mg/kg were chosen to assess the effect of dose on the rate and route of excretion after oral gavage. Doses of 10 and 15 mg/kg were selected for the intravenous route of administration. For repeated dose studies, 100 mg/kg was administered daily by oral gavage for 1, 5, or 10 days. Doses provided 25 to 200 μCi/kg [14C]BMP. In each study, [14C]BMP was administered to four animals (n = 4) in a solution of Cremophore EL-absolute ethanol-water (3:1:1, v/v/v), with the exception of the BDC study (n = 3).
Sample collection and preparation. After dosing of BMP, the animals were maintained in Nalgene metabolism cages for collection of urine and feces. In single dose studies, urine was collected at 6, 12, 24, 36, 48, and 72 h; feces were collected at 12, 24, 36, 48, and 72 h. In the repeated dose studies, urine was collected at 6, 12, and 24 h after each dose, whereas feces were collected at 12 and 24 h after each administration. The metabolism cages were rinsed with methanol after the collection of urine. Radioactivity recovered in cage rinses was added to that determined for urine.
After collections, triplicate aliquots of urine (10–100 μl) and cage rinse (1 ml) were directly analyzed for 14C equivalent content by liquid scintillation counting (LSC). In addition, an aliquot of urine (20–100 μl) was mixed with an equal volume of deionized water and centrifuged (1000g, 5 min), and the supernatant was subjected to HPLC-radiometric analysis. Samples of urine for LC-MS/MS analysis were from animals that were administered nonradiolabeled BMP. These urine samples were prepared using the same methods as described for HPLC-radiometric analysis. Feces were mixed with water to form a homogeneous mixture. To determine total 14C radioactivity content, triplicate aliquots of fecal samples (100–200 mg) were solubilized using Soluene-350 according to Thompson and Burns (1996).
At the end of each study, animals were euthanized by CO2 inhalation. Blood was collected from the posterior vena cava into a heparinized syringe, and the animals were subjected to necropsy. When bladder urine was available at necropsy, samples were prepared as described for urine and analyzed by HPLC with radiometric detection. All tissues collected (adipose, brain, cecum, cecum contents, heart, intestine, intestinal contents, kidneys, liver, lung, muscle, spleen, stomach, stomach contents, skin, and testes) were stored at –20°C until analysis. Blood was processed immediately. Triplicate aliquots from each collected tissue (100–200 mg) and blood (50–100 μl) were solubilized with Solvable (Thompson and Burns, 1996). Body composition estimates of 11% adipose tissue, 8% blood, 50% muscle, and 16% skin were used to estimate total masses of these tissues (Birnbaum et al., 1980). After solubilization and addition of 15 ml of Pico-Fluor 40, all samples were stored in the dark for 48 h to control for chemiluminescence and were corrected for background. Total 14C radioactivity in all samples was determined by LSC.
Biliary excretion study. This study was conducted at the National Institute of Environmental Health Sciences (Research Triangle Park, NC), in accordance with institutional guidelines. The animal facilities at the National Institute of Environmental Health Sciences are Association for Assessment and Accreditation of Laboratory Animal Care-accredited. F-344 rats, obtained from Charles River Laboratories (Raleigh, NC) were 3 months old with a weight range of 310 to 336 g. Each rat was anesthetized with 50 mg/kg i.p. pentobarbital at a dose volume of 1 ml/kg. After anesthetization, a midline incision in the abdomen was made, and the bile duct was isolated, distally ligated, and punctured with a 25-gauge needle. A beveled PE10 cannula was inserted through the puncture site into the bile duct for a distance of 1 cm and secured with a suture. Bile flow was confirmed, and the incision was closed with wound clips. [14C]BMP (15 mg/kg; 25 μCi/kg; 1 ml/kg) was administered by tail vein injection, and bile was collected on ice at time points from 0.025 to 6 h. The bile samples (10-μl aliquots) were analyzed by LSC for 14C content and stored at –80°C for later analysis by HPLC. Rats were placed on heated pads (40°C), which were changed hourly to maintain a constant body temperature throughout the procedure. The body temperature of the rats, monitored hourly, ranged from 35 to 38°C. Each rat remained anesthetized throughout the procedure as a result of hourly intraperitoneal injections of 0.1 ml of pentobarbital. Concurrent intraperitoneal injections of 300 to 500 μl of 0.85% saline containing 2.5 mM taurocholic acid maintained hydration of the animals. Euthanasia was by CO2 inhalation. For HPLC-radiometric analysis, the bile was centrifuged, and an aliquot (10 μl) of the supernatant was directly injected into the HPLC system.
Blood kinetics after intravenous and oral administration of BMP. For determination of intravenous blood kinetics [14C]BMP (10 or 15 mg/kg; 50 μCi/kg; 2 ml/kg) was administered intravenously through the JVC [in a solution of Cremophore EL-absolute ethanol-water (3:1:1, v/v/v)]. Blood (100 μl) was then drawn into the cannula and returned to the circulation, and the cannula was flushed with normal saline (1 ml/kg). For oral blood kinetic studies, [14C]BMP (10 mg/kg; 200 μCi/kg; 4 ml/kg) was administered by oral gavage to F-344 rats with JVCs. Blood samples (300 μl) were collected via the JVC at 0.083, 0.125, 0.25, 0.5, 1, 1.5, 3, 6, 9, 12, 24, 36, and 48 h into heparinized syringes. The aliquots of blood removed were replaced with an equal volume of saline. The volume of 300 μl of blood per time point was chosen on the basis of recommendations made by Diehl et al. (2001), who was demonstrated that in short-term pharmacokinetic studies, the removal of 20% of the blood volume over 24 h produces minimal disturbance of normal physiological function. Aliquots (2 × 50 μl) from these blood samples were solubilized and 14C radioactivity was quantified by LSC.
After intravenous administration the blood samples obtained were centrifuged (750g; 15 min) to separate plasma from red blood cells. An aliquot (50 μl) of each plasma sample was mixed with 50 μl of acetonitrile, vortexed, and centrifuged to precipitate proteins. The precipitate contained 3% of the radioactivity. To the resulting supernatant 50 μl of acetonitrile was added, and the mixture was recentrifuged. The final supernatant was concentrated under a stream of nitrogen to a volume ≤50 μl and subjected to HPLC analysis with radiometric detection.
Aliquots (150 μl) of each blood sample obtained after oral administration were mixed with 450 μl of ethyl acetate, vortexed for 5 min, and centrifuged (1000g, 5 min). Extractions were performed three times. Supernatants were pooled, and an aliquot (20 μl) was analyzed by LSC. The remaining supernatant was evaporated to dryness (MiVac; Genevac, Valley Cottage, NY) under reduced pressure, reconstituted in 150 μl of methanol, and analyzed by HPLC with radiometric detection.
Pharmacokinetic analysis. The blood concentration-time data after intravenous (parent BMP) and oral administration (total [14C]BMP and parent BMP) were analyzed using a computer modeling program (WinNonlin Professional, version 5.1; Pharsight, Mountain View, CA). The program was used to fit the data to a suitable multicompartment model using nonlinear regression analysis, assuming first-order kinetics for all processes. The model was used to calculate values for the half-life of distribution (t1/2α), terminal half-life for elimination (t1/2β), area under the blood concentration-time curve from zero to infinity, and maximum oral bioavailability (F). Only samples containing quantities of compound above the limit of quantification (LOQ) were used in pharmacokinetic analyses.
Identification of phase II metabolites. To determine the presence of glucuronide conjugates of BMP, an aliquot of urine was mixed with an equal volume of 0.15 M acetate buffer (pH 5.0) and incubated with 5000 Fishman units of β-glucuronidase (type B-1) before HPLC-radiometric analysis. For the analysis of sulfate conjugates of BMP, an aliquot of urine was mixed with an equal volume of 0.1 M phosphate buffer (pH 7.1) containing 0.1 U of sulfatase (type VI) before HPLC-radiometric analysis. Control incubations contained an aliquot of identical urine but no enzyme.
Studies for the identification of 14C radioactivity in blood and liver. To characterize the identity of 14C equivalents in blood by HPLC-radiometric analysis, total blood from rats after intravenous or oral administration of BMP was used for extraction. Four male F-344 rats were administered [14C]BMP (10 mg/kg; 200 μCi/kg; 2 ml/kg) by oral gavage. Two of these animals were sacrificed at 40 min [calculated maximum concentration in blood (Cmax)] postdose and two at 6 h after dosing. In addition, two rats were administered [14C]BMP intravenously via the lateral tail vein (10 mg/kg; 200 μCi/kg; 1 ml/kg). These animals were euthanized at 30 min after dosing. At the end of each experiment, the liver and whole blood were collected. The collected blood (5–6 ml/animal) was separated by centrifugation (750g, 15 min) into blood plasma and blood cells. The cell pellet was washed with normal saline (two 2.5-ml washes), and the washes were added to the plasma. The plasma-saline mixture (6–7 ml) was acidified with an equal volume of 0.7 M glycine/HCl buffer (pH 1.2), and 10 ml of ethyl acetate were added. The mixture was vortexed for 30 min and centrifuged (1000g, 5 min) for phase separation. The extraction was repeated at least 5 times. The combined organic extracts were evaporated to dryness under reduced pressure and reconstituted in 300 μl of methanol, and an aliquot (80 μl) was used for HPLC-radiometric analysis. In addition, the extracted aqueous phases were analyzed by HPLC-radiometric analysis after partial evaporation and pH adjustment. An aliquot of the liver was homogenized and extracted under acidic conditions as described above for blood plasma, and the reconstituted extracts and aqueous phases were analyzed by HPLC with radiometric detection.
Analytical Methods. HPLC analysis. The HPLC system consisted of an Agilent 1100 quaternary pump, thermostated column compartment, thermostated autosampler, and diode array detector (Agilent Technologies, Palo Alto, CA) coupled with a flow-through β-RAM detector for 14C radioactivity (IN/US Systems, Tampa, FL) and to an in-line fraction collector (Gilson Inc., Middleton, CA). Data were acquired and analyzed using HP ChemStation software for LC 3D [revision B.01.01; Agilent Technologies] and WinFlow software [version 1.5; LabLogic, Sheffield, UK]. In addition, fractions of the HPLC effluent were collected at 1-min intervals and analyzed with a Beckman LS 3801 liquid scintillation counter (Beckman Coulter, Fullerton, CA).
Separation was performed on a 250 × 4.6 mm i.d., 5 μm, reversed-phase Luna C18 column coupled with a 4.0 × 3.0 mm i.d. SecurityGuard C18 guard cartridge (Phenomenex, Torrance, CA). The mobile phases consisted of Nanopure water and acetonitrile containing 0.1% formic acid each. The gradient was run from 10% acetonitrile and 90% Nanopure water for the 1st min, then up to 40% acetonitrile over 12 min, and then up to 90% acetonitrile in 1 min and held for 5 min. The column was reequilibrated to initial conditions for 10 min between injections. The flow rate was 0.9 ml/min at 25°C. The autosampler temperature was maintained at 10°C. The sample injection volume was 20 to 100 μl. The limit of detection using radiometric detection was 0.72 μg/ml and the LOQ was 2.15 μg/ml. The limit of detection and LOQ for 14C equivalents using LSC were 2.9 and 11.3 ng, respectively, as determined by the equation described by Zhu et al. (2005).
LC-MS/MS analysis. The HPLC system was the same as that described above for HPLC analysis but coupled with an Agilent MSD-Trap SL ion trap mass spectrometer (Agilent Technologies) and LC/MSD Trap software (version 5.3) for data collection and analysis (Bruker Daltonics, Billerica, MA). The mass spectrometer operated in the positive electrospray ionization mode over a scan range of m/z 50 to 500. The flow rate of the nitrogen drying gas was 11 l/min, the nebulizer pressure was set to 60 psi, the drying temperature was maintained at 350°C, the HV capillary voltage was 3500 V, and the capillary current was 42.7 nA. MS and MS/MS experiments were performed in multiple reaction monitoring mode. Two masses were selected for analysis: m/z 262.9 (BMP) and m/z 438.9 (BMP monoglucuronide). The first scan was a full MS scan, and then one or two precursor ions were selected, isolated, and selectively fragmented in the ion trap.
Results
Tissue Distribution of BMP. The distribution of 14C radioactivity to tissues after single and repeated oral administrations is shown in Table 1. After a single oral administration of 100 mg/kg [14C]BMP to food-deprived rats, the total percentage of dose retained in tissues at 72 h was less than 1% (0.13–0.28 nmol/g of tissue). When the same dose of BMP was administered orally to nonfasted animals, 4.2% of total radioactivity was recovered from tissues at 24 h postdose. Adipose tissues, liver, kidneys, muscle, and skin contained 0.2, 0.7, 0.1, 0.3, and 0.3% of dose, respectively. The majority of the unexcreted 14C equivalents (11.9% of dose) were found in the contents of the intestine and cecum. Concentration in nanomoles per gram of tissues and contents ranged from 2.2 to 10.4. After 5 or 10 days of repeated oral administration (100 mg/kg/day, not fasted) total tissue levels of 14C equivalents at 24 h after the last dose were in the same range. In addition, the tissue distribution was similar, with the majority of the dose remaining in the gastrointestinal (GI) tract. Tissue disposition of BMP was not determined at the lower dose of 10 mg/kg.
TABLE 1.
Percentage of dose recovered from tissues and excreta after oral administration of [14C]BMP (100 mg/kg) for 1, 5, or 10 daily administrations to male F-344 rats
Data are mean ± S.D.
|
Fasted (72
ha): 1
Administration (n = 4)
|
Unfasted (24
ha)
|
|||
|---|---|---|---|---|
| 1 Administration (n = 3) | 5 Administrations (n = 4) | 10 Administrations (n = 4) | ||
| Adipose tissues | 0.13 ± 0.05 | 0.18 ± 0.06 | 0.06 ± 0.02 | 0.04 ± 0.01 |
| Bladder | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.00 ± 0.00 |
| Bladder urine | 0.02 ± 0.02 | 0.60 ± 0.53 | 0.05 ± 0.03 | 0.03 ± 0.03 |
| Blood | 0.20 ± 0.02 | 0.34 ± 0.11 | 0.22 ± 0.04 | 0.17 ± 0.01 |
| Brain | N.D. | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Cecum | 0.01 ± 0.00 | 0.26 ± 0.07 | 0.11 ± 0.04 | 0.03 ± 0.00 |
| Cecum contents | 0.10 ± 0.05 | 4.08 ± 2.18 | 0.99 ± 0.33 | 0.35 ± 0.13 |
| Cecum rinse | 0.01 ± 0.01 | 0.55 ± 0.47 | 0.04 ± 0.03 | 0.01 ± 0.00 |
| Heart | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Intestine | 0.05 ± 0.03 | 1.78 ± 0.95 | 0.49 ± 0.16 | 0.14 ± 0.05 |
| Intestine contents | 0.18 ± 0.06 | 7.23 ± 1.94 | 2.30 ± 0.24 | 0.99 ± 0.41 |
| Kidneys | 0.01 ± 0.00 | 0.05 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| Liver | 0.05 ± 0.01 | 0.65 ± 0.40 | 0.15 ± 0.04 | 0.09 ± 0.03 |
| Lung | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Muscle | 0.20 ± 0.03 | 0.34 ± 0.29 | 0.27 ± 0.09 | 0.18 ± 0.02 |
| Skin | 0.11 ± 0.02 | 0.25 ± 0.06 | 0.12 ± 0.02 | 0.08 ± 0.01 |
| Spleen | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Stomach | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.00 ± 0.00 |
| Stomach contents | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Testes | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| GI tract tissues | 0.07 ± 0.03 | 2.05 ± 0.96 | 0.60 ± 0.21 | 0.18 ± 0.05 |
| GI tract contents | 0.29 ± 0.12 | 11.86 ± 3.98 | 3.33 ± 0.42 | 1.35 ± 0.43 |
| Tissues total | 0.89 ± 0.18 | 16.05 ± 5.72 | 4.63 ± 0.70 | 1.97 ± 0.49 |
| Urine | 58.05 ± 4.28 | 53.40 ± 9.43 | 30.39 ± 5.75 | 19.13 ± 6.21 |
| Cage rinse | 22.73 ± 5.20 | 17.32 ± 0.30 | 44.56 ± 3.41 | 58.23 ± 3.18 |
| Feces | 9.68 ± 1.93 | 7.86 ± 1.05 | 14.22 ± 3.48 | 14.51 ± 6.31 |
| Excreta total | 90.46 ± 1.49 | 78.62 ± 8.42 | 89.17 ± 3.37 | 91.88 ± 2.79 |
| Total recovery | 91.56 ± 1.67 | 95.01 ± 4.10 | 94.05 ± 3.26 | 94.02 ± 2.42 |
N.D., not determined.
Time after administration.
Blood levels of 14C equivalents at 72 h after oral or intravenous administration of BMP (10 mg/kg) were 0.1% of dose (data not shown). After a single oral administration of 100 mg/kg BMP, 0.3 and 0.2% of dose were recovered in the blood by 24 and 72 h, respectively. After repeated oral dosing of BMP (100 mg/kg/day) for 5 or 10 days 14C blood levels were 0.2% of dose at 24 h after the last dose (Table 1).
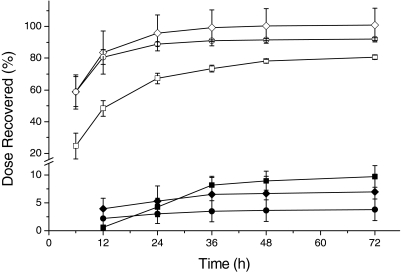
Cumulative Elimination of BMP. The results for the cumulative excretion of BMP-derived 14C equivalents after intravenous and oral administration are summarized in Fig. 2. When [14C]BMP was administered intravenously (10 mg/kg), the predominant route of its elimination was urinary. The peak excretion in the urine occurred between 6 and 12 h, and the majority of the dose (>80%) was eliminated by 12 h. By 24 h greater than 89% of the administered radioactivity had been recovered in the urine. Only trace amounts were excreted in the feces by 72 h (<4% of dose). After a single oral bolus dose (10 mg/kg) the excretion rate of BMP-derived 14C equivalents in urine was similar to that after intravenous administration. The fecal elimination accounted for less than 10% of the dose at 72 h. Administration of a 10-fold higher dose of BMP (100 mg/kg) resulted in a similar excretion profile, but urinary excretion was slower; within the first 12 h only 48% of the administered 14C equivalents were excreted. The urinary excretion rate at higher oral doses of BMP (150–600 mg/kg) paralleled those of 100 mg/kg (data not shown).
Fig. 2.
Cumulative excretion of radioactivity after intravenous [10 mg/kg (○, •)] and oral [10 mg/kg (⋄, ♦) or 100 mg/kg (□, ▪)] administration of [14C]BMP to male F-344 rats (intravenous: n = 4; oral: n = 4; mean ± S.D.; urine: open symbols; feces: closed symbols).
After the rate and route of elimination of BMP after a single oral administration were defined, the effects of repeated daily oral dosing of [14C]BMP on excretion of BMP-derived 14C equivalents were determined (Table 1). After 5 or 10 days of repeated daily dosing of 100 mg/kg [14C]BMP, the elimination profile of BMP was not notably altered from that of a single dose. The average cumulative amounts of 14C equivalents found in urine including cage rinses were 71, 75, and 77% of dose, respectively. The cumulative fecal excretion of BMP from animals receiving 5 or 10 consecutive administrations was slightly increased (14% of dose). In addition, it was noted that the radioactivity recovered in feces at 24 h after administration was increased to 7 to 8% of dose in nonfasted rats compared with 4% in fasted animals (Fig. 2).
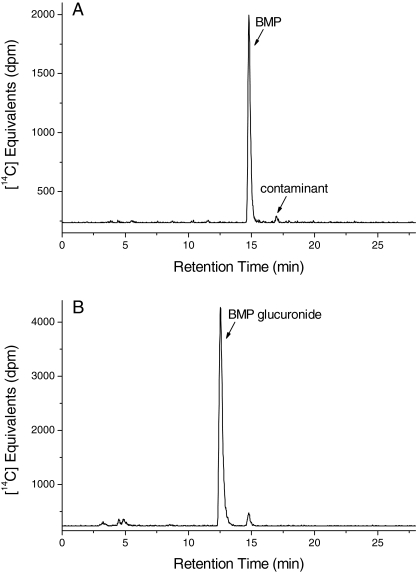
Identification of 14C Radioactivity in Urine. Urine collected from rats treated orally with [14C]BMP was analyzed by HPLC with radiometric detection. A representative radiochromatogram of 14C radioactivity present in a pooled urine sample is shown in Fig. 3. The major peak that eluted earlier than BMP from the reversed-phase column with a retention time (RT) of 12.4 min was observed in all samples and at all time points (Fig. 3B). Figure 3A shows the elution of the [14C]BMP standard. In a number of the urine samples a small radioactive peak (1–5% of total 14C equivalents) that coeluted with authentic BMP at 14.4 min was detected. The HPLC profiles of urine samples obtained from animals after intravenous administration were similar to those after oral gavage; however, BMP was not detected. Bladder urine also contained the polar metabolite exclusively, and therefore the presence of parent BMP in urine from orally treated rats was suggested to be due to cross-contamination from feces. In addition, three minor (≤5% of total 14C equivalents) and more polar products eluted between 3 and 6 min. The chemical identity of these products was not determined. The product at 5.5 min seems to be a degradation product of the 14C contaminant originally present in the radiolabeled BMP standard, because the appearance of this peak was accompanied by the loss of the contaminant peak in aging [14C]BMP standards. Incubation of urine with β-glucuronidase before HPLC-radiometric analysis resulted in the loss of the polar metabolite with a concurrent increase of parent BMP. Thus, the metabolite was tentatively identified as a glucuronide of BMP.
Fig. 3.
Representative HPLC radiochromatograms of (A) [14C]BMP standard and (B) a pooled urine sample collected 6 h after oral administration of [14C]BMP (100 mg/kg) to male F-344 rats.
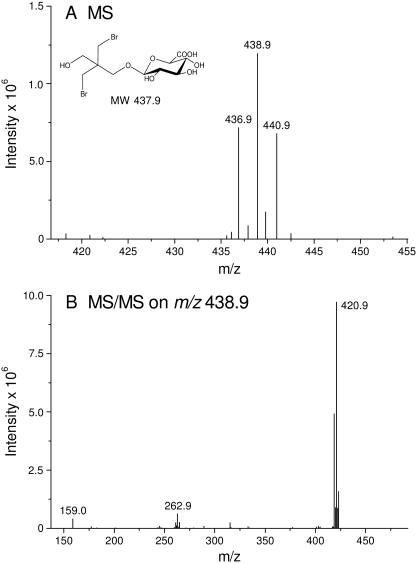
On the basis of data obtained from β-glucuronidase assays of urine, animals were administered unlabeled BMP by oral gavage. LC-MS analysis of urine from these studies confirmed the conclusion that the metabolite present in urine was BMP monoglucuronide (Fig. 4). MS/MS analysis of the metabolite revealed a molecular ion of m/z438.9 ([M + H]+) consistent with the mass of a glucuronic acid conjugate of BMP (Fig. 4A). In addition, the isotope pattern obtained for this ion was indicative of molecules containing two bromine atoms. The daughter ion spectra obtained by MS/MS analysis from the molecular ion of m/z 438.9 was dominated by the loss of one water molecule indicated by m/z 420.9 (Fig. 4B).
Fig. 4.
Representative (A) electrospray ionization-LC/MS and (B) MS/MS spectra of BMP glucuronide (HPLC: RT = 12.4 min).
Identification of 14C Radioactivity in Liver and Bile. When liver homogenates from rats treated intravenously or orally with [14C]BMP (10 mg/kg) were extracted into ethyl acetate in the presence of glycine/HCl buffer at 30 min (intravenous) and 40 min (oral) postdose and analyzed by HPLC with radiometric detection, the 14C radioactivity in these liver extracts consisted of parent BMP and the glucuronide conjugate identified in urine. After intravenous administration of [14C]BMP (15 mg/kg) to BDC rats, more than 50% of the dose was excreted in the bile within 6 h (data not shown). HPLC-radiometric analysis showed that greater than 99% of the BMP-derived 14C equivalents excreted in bile over time consisted of a single peak, identified by cochromatography as the glucuronide conjugate of BMP detected in urine (data not shown).
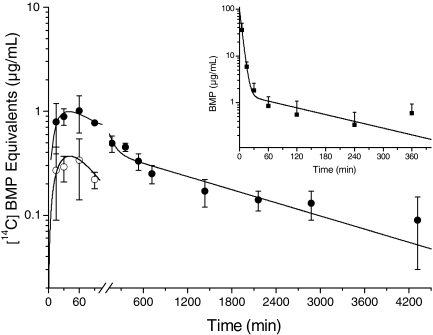
Pharmacokinetics of BMP and Identification of 14C Radioactivity in Blood. Blood kinetics after a single intravenous administration of [14C]BMP (15 mg/kg) indicated that parent BMP rapidly disappeared from the systemic circulation. The concentration-time profile of [14C]BMP in blood plasma after intravenous administration was best described by a biexponential equation that is consistent with a two-compartment model with first-order elimination (Fig. 5, inset). After a rapid initial distribution of [14C]BMP, indicated by a short theoretical half-life of distribution (t1/2α: 3.4 min), a significantly slower elimination (t1/2β: 2 h) occurred. Blood plasma concentrations of BMP at times later than 30 min were very low (<1 μg/ml).
Fig. 5.
Time course of total BMP 14C equivalents (•) and extracted parent BMP (○) in blood after oral administration of [14C]BMP (10 mg/kg) to male F-344 rats (n = 4, mean ± S.D.). Inset, time course of parent [14C]BMP (▪) in blood after intravenous administration of [14C]BMP (15 mg/kg) to male F-344 rats (n = 4, mean ± S.D.).
When [14C]BMP (10 mg/kg) was administered orally and extracts obtained from small aliquots of whole blood (150 μl per time point) were analyzed by LSC, the quantities of 14C equivalents were close to or below the LOQ. Extracted BMP was quantifiable only at the first four time points (Fig. 5). The area under the blood concentration-time curve for these four points, compared with that after intravenous administration and adjusted for dose, suggests an oral bioavailability into the systemic circulation of less than 10%. The blood concentration-time profile after oral administration is presented for parent BMP at the quantifiable time points and for total [14C]BMP equivalents (Fig. 5). After oral administration absorption was rapid with Cmax reached at 40 min. 14C equivalents were detectable in the blood, although at very low levels, throughout to termination at 72 h.
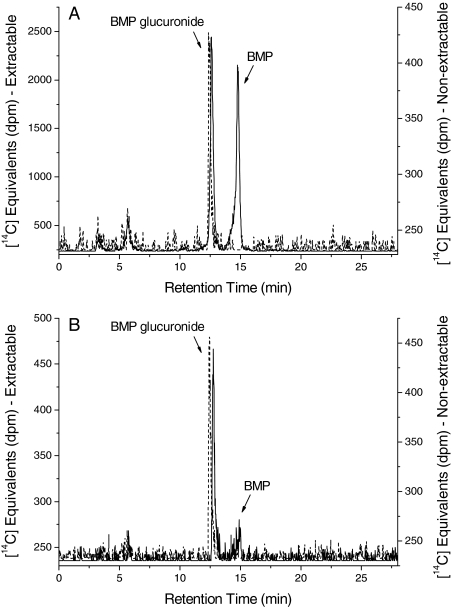
To elucidate the identity of the 14C equivalents in blood after oral or intravenous administration of [14C]BMP (10 mg/kg), blood plasma (2–3 ml) from rats was extracted with ethyl acetate in the presence of glycine/HCl buffer and analyzed by HPLC with radiometric detection (Fig. 6). After a single intravenous administration of [14C]BMP (10 mg/kg), both parent BMP and BMP glucuronide were detected in blood plasma (Fig. 6A). At 5 min after the intravenous dose the majority (>90%) of the 14C equivalents were present as the parent compound, whereas the amounts of BMP glucuronide (26–57%) were notably increased at all later time points. After oral administration the 14C radioactivity detected in plasma extracts showed both BMP-glucuronide and parent BMP. Because the extraction of the 14C radioactivity in plasma into ethyl acetate was incomplete (33–56% extraction efficiency), the nonextractable 14C equivalents remaining in the aqueous phase were analyzed by HPLC with radiometric detection and were identified as BMP glucuronide. By Cmax (40 min (Fig. 6B) the majority of the 14C radioactivity in blood plasma was BMP glucuronide.
Fig. 6.
Representative HPLC-radiochromatograms of blood plasma after (A) intravenous (30 min postdose) and (B) oral (40 min postdose) administration of [14C]BMP (10 mg/kg) to male F-344 rats (solid line, extractable 14C radioactivity; dashed line, nonextractable 14C radioactivity). Minor shifts in retention time of the nonextractable moiety were due to matrix effects.
Discussion
These studies represent the first comprehensive description of the ADME and pharmacokinetic profile of the brominated flame retardant BMP. After single intravenous administration to rats, parent BMP disappeared rapidly from the blood. It seems that BMP is efficiently extracted by the liver where it undergoes extensive glucuronidation. The resulting BMP glucuronide is excreted primarily in the bile, but because it is ultimately eliminated predominantly in the urine, some percentage of this glucuronide must also be exported from liver to blood. Thus, glucuronidation seems to be the key physiological process that governs the clearance of BMP and subsequent exposure to BMP in rats.
Likewise, after oral administration of single or multiple (5 or 10) doses of BMP to rats, the major route of elimination was urinary as a glucuronide. In fact, pharmacokinetic analysis after oral administration showed that Cmax in blood was reached quickly, but parent BMP was only detectable at the very early time points after administration. The majority of the 14C equivalents recovered from blood plasma represents BMP metabolite. Thus, these data demonstrate the critical role of glucuronidation (hepatic and possibly intestinal) in determining the systemic bioavailability of BMP.
Our data show that the hepatic BMP glucuronide was efficiently excreted into the bile but only a low percentage of the dose was eliminated in the feces. These findings suggest that the effectiveness of biliary excretion of BMP glucuronide is greatly limited by enterohepatic recirculation of BMP. After the glucuronide is excreted via the bile into the small intestine it seems to undergo hydrolysis by microbial β-glucuronidases. The released parent molecule is reabsorbed, subjected to glucuronidation, and ultimately excreted in the urine. This enterohepatic recycling explains the persistent low blood levels of [14C]BMP equivalents and suggests that the long terminal elimination phases observed in the pharmacokinetic studies most likely result from reabsorption of the aglycone from the intestine rather than retention and slow release from tissues. The effective elimination of BMP glucuronide in bile and enterohepatic recycling results in a low systemic bioavailability of BMP in rats.
The major route of elimination was urinary, regardless of BMP dose level, route of administration, or number of doses. However, a slight increase in the rate of fecal excretion was observed after repeated daily dosing (Table 1). At oral doses of 100 mg/kg BMP and higher (150–600 mg/kg; data not shown), the urinary excretion rate, when expressed as a percentage of dose, was slower than that of a dose of 10 mg/kg. The radioactivity in urine was excreted predominantly as a glucuronide. Formation of glucuronide conjugates is generally regarded as a high-capacity pathway for xenobiotic metabolism that is not readily saturable (Hjelle and Klaassen, 1984). However, studies on the glucuronidation of acetaminophen have demonstrated that certain UGT enzymes with high affinity and low capacity involved in acetaminophen glucuronidation demonstrate concentration-dependent saturation (Court et al., 2001). The decreased elimination rate of BMP glucuronide observed at higher doses may also result from a dose-dependent saturation of relevant UGTs. Alternatively, the UGT enzyme(s) responsible for the glucuronidation of BMP may be expressed at lower levels. In addition, glucuronides are transported from the hepatocyte into the plasma and the bile, which have been shown to be saturable as well (Sakamoto et al., 2008).
Tissues were collected at 72 h after oral administration of the high dose (100 mg/kg) and at 24 h after the last dose in repeated dose studies. There was no selective retention of 14C radioactivity in tissues reported to develop neoplasms (National Toxicology Program, 1996; Dunnick et al., 1997) compared with nontarget tissues. Internal tissues such as liver, kidney, and adipose depots contained minor levels of [14C]BMP equivalents. Radioactivity that had not been excreted within the first 24 h was mainly associated with tissues and contents of the intestine and cecum. Unpublished data (J. M. Sanders; data not shown) of a previous study of BMP indicated no preferential distribution to tissues within 24 h after intravenous administration (15 mg/kg) to F-344 rats. The results of these studies demonstrate that BMP does not accumulate in tissues of rats after oral exposure to low and high doses of BMP.
The aliphatic BMP, with two free hydroxyl groups, behaves somewhat similarly to tetrabromobisphenol A (TBBPA), an aromatic BFR that possesses two phenolic hydroxyl groups. TBBPA is readily absorbed from the GI tract and extensively conjugated in the liver, and the glucuronide metabolite is then eliminated in the bile (Schauer et al., 2006; Kuester et al., 2007). The molecular weights of the glucuronides of both BMP (mol. wt. 438) and TBBPA (mol. wt. 718) exceed 325, the threshold for biliary elimination in rats (Riviere, 1999). This explains the extensive excretion of these conjugates into bile. However, in contrast to the bulkier TBBPA glucuronide, which is predominantly eliminated via the fecal route (Kuester et al., 2007), the ultimate route of elimination of the BMP glucuronide is urinary. It seems that these two glucuronides are dramatically different with respect to serving as substrates for microbial β-glucuronidases. It is interesting to note that the molecular weight of the BMP glucuronide is below the threshold for biliary elimination in humans (mol. wt. >450), and therefore BMP glucuronide, if formed in human liver, would most likely be released into blood for excretion in the urine without undergoing enterohepatic recirculation.
The disposition of two other higher molecular weight BFRs, TBBPA-2,3-dibromopropyl ether and 1,2-bis(2,4,6-tribromophenoxy)ethane, differs significantly from the ADME data reported here for BMP and for TBBPA. These BFRs do not contain free hydroxyl groups, which enhances their lipophilicity and greatly limits their absorption from the GI tract. Both compounds are minimally absorbed, not metabolized, and therefore are excreted in the feces mainly as parent compound (Nomeir et al., 1993; Knudsen et al., 2007).
As stated previously, BMP has been reported to cause malignant tumor formation at multiple tissue sites in rats and mice (National Toxicology Program, 1996). Another known multisite carcinogen in rodents is the aliphatic BFR 2,3-dibromo-1-propanol (Eustis et al., 1995). It is a direct-acting mutagen and also is bioactivated to reactive intermediates that bind to nucleophilic sites of biological macromolecules. In contrast to 2,3-dibromo-1-propanol, there was no indication that BMP formed reactive metabolite(s) in vivo; only an aliphatic glucuronide conjugate was generated. Preliminary results of in vitro studies with isolated rat hepatocytes demonstrate essentially stoichiometric conversion of BMP to BMP glucuronide. Likewise, in other in vitro studies, no evidence for cytochrome P450-mediated monooxygenation or/and glutathione conjugation of BMP was obtained (R. K. Kuester, unpublished data). Most likely the glucuronide of BMP, an ether glucuronide, can be considered as an inactive metabolite because it is lacking the electrophilic character of acyl glucuronides (Zia-Amirhosseini et al., 1994). In addition, there was no evidence for bioaccumulation in tissues, even after repeated daily administration of BMP. In conclusion, the ADME data described here do not provide clues as to the mechanism(s) by which BMP-induced carcinogenesis is initiated. In addition, it has to be noted that the BMP preparation used in the 2-year feed study conducted by the National Toxicology Program contained 21.2% impurities (National Toxicology Program, 1996), whereas in the studies reported here the minimum purity of BMP was 97.3%. Whether or not these additional components may contribute to the carcinogenic effects assigned to BMP is not known.
In summary, the data presented here indicate that BMP is rapidly absorbed from the GI tract into the portal circulation and efficiently metabolized in the liver to a glucuronide conjugate that is primarily excreted in the urine of rats. Because of the extensive metabolism the likelihood of systemic exposure of BMP after ingestion of BMP is low. The studies clearly describe the fate of BMP in rats but do not establish mechanism(s) for the carcinogenic potential of BMP. Thus, how BMP acts as a multisite carcinogen remains to be determined in further studies.
Acknowledgments
We thank Dr. Michael Cunningham (National Toxicology Program and National Center for Toxicogenomics) and Dr. Robert Kuester for advice and support as well as Leigh Jacobs and Golriz Rad for assistance in various aspects of this project.
This work was supported by the National Toxicology Program/National Institute of Environmental Health Science (NIEHS) (Contract N01-ES-45529) and in part by the Intramural Research Program of the National Institutes of Health and NIEHS. We also acknowledge support provided by the Analytical Core of the NIEHS-funded Southwest Environmental Health Science Center [Grant P30-ES 06694].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.023937.
ABBREVIATIONS: BMP, 2,2-bis(bromomethyl)-1,3-propanediol; BFR, brominated flame retardant; F-344, Fischer 344; HPLC, high-performance liquid chromatography; JVC, jugular vein cannula; BDC, bile duct cannula; LSC, liquid scintillation counting; LC, liquid chromatography; MS/MS, tandem mass spectrometry; LOQ, limit of quantification; MS, mass spectrometry; GI, gastrointestinal; ADME, absorption, distribution, metabolism, and excretion; UGT, UDP glucuronosyltransferase; TBBPA, tetrabromobisphenol A.
References
- Birnbaum LS, Decad GM, and Matthews HB (1980) Disposition and excretion of 2,3,7,8-tetrachlorodibenzofuran in the rat. Toxicol Appl Pharmacol 55 342–352. [DOI] [PubMed] [Google Scholar]
- Court MH, Duan SX, von Moltke LL, Greenblatt DJ, Patten CJ, Miners JO, and Mackenzie PI (2001) Interindividual variability in acetaminophen glucuronidation by human liver microsomes: identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J Pharmacol Exp Ther 299 998–1006. [PubMed] [Google Scholar]
- Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, and van de Vorstenbosch C (2001) A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21 15–23. [DOI] [PubMed] [Google Scholar]
- Dunnick JK, Heath JE, Farnell DR, Prejean JD, Haseman JK, and Elwell MR (1997) Carcinogenic activity of the flame retardant, 2,2-bis(bromomethyl)-1,3-propanediol in rodents, and comparison with the carcinogenicity of other NTP brominated chemicals. Toxicol Pathol 25 541–548. [DOI] [PubMed] [Google Scholar]
- Elwell MR, Dunnick JK, Brown HR, and Montgomery CA (1989) Kidney and urinary bladder lesions in F344/N rats and B6C3F1 mice after 13 weeks of 2,2-bis(bromomethyl)-1,3-propanediol administration. Fundam Appl Toxicol 12 480–490. [DOI] [PubMed] [Google Scholar]
- Eustis SL, Haseman JK, Mackenzie WF, and Abdo KM (1995) Toxicity and carcinogenicity of 2,3-dibromo-1-propanol in F344/N rats and B6C3F1 mice. Fundam Appl Toxicol 26 41–50. [DOI] [PubMed] [Google Scholar]
- Ezra S, Feinstein S, Yakirevich A, Adar E, and Bilkis I (2006) Retardation of organo-bromides in a fractured chalk aquitard. J Contam Hydrol 86 195–214. [DOI] [PubMed] [Google Scholar]
- Hjelle JJ and Klaassen CD (1984) Glucuronidation and biliary excretion of acetaminophen in rats. J Pharmacol Exp Ther 228 407–413. [PubMed] [Google Scholar]
- Keyes DG, Kociba RJ, Schwetz RW, Wade CE, Dittenber DA, Quinn T, Gorzinski SJ, Hermann EA, Momany JJ, and Schwetz BA (1979) Results of a two-year toxicity and oncogenic study of rats ingesting diets containing dibromoneopentyl glycol (FR-1138). J Combust Toxicol 7 77–98. [Google Scholar]
- Knudsen GA, Jacobs LM, Kuester RK, and Sipes IG (2007) Absorption, distribution, metabolism and excretion of intravenously and orally administered tetrabromobisphenol A [2,3-dibromopropyl ether] in male Fischer-344 rats. Toxicology 237 158–167. [DOI] [PubMed] [Google Scholar]
- Kuester RK, Sólyom AM, Rodriguez VP, and Sipes IG (2007) The effects of dose, route, and repeated dosing on the disposition and kinetics of tetrabromobisphenol A in male F-344 rats. Toxicol Sci 96 237–245. [DOI] [PubMed] [Google Scholar]
- Larsen ER (1969) 2,2-Bis(bromomethyl)propanediol-1,3: a light stable fire retardant monomer for condensation polymers. Org Coatings Plast Chem 29 375. [Google Scholar]
- National Toxicology Program (1996) Toxicology and carcinogenesis studies of 2,2-bis(bromomethyl)-1,3-propanediol (FR-1138®) (CAS No. 3296-90-0) in F344 rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 452 1–465. [PubMed] [Google Scholar]
- Nomeir AA, Markham PM, Ghanayem BI, and Chadwick M (1993) Disposition of the flame retardant 1,2-bis(2,4,6-tribromophenoxy)ethane in rats following administration in the diet. Drug Metab Dispos 21 209–214. [PubMed] [Google Scholar]
- Riviere JE (1999) Comparative Pharmacokinetics: Principles, Techniques, and Applications, Iowa State Press, Ames.
- Report on Carcinogens, 11th ed (2004) U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program (2004).
- Sakamoto S, Kusuhara H, Horie K, Takahashi K, Baba T, Ishizaki J, and Sugiyama Y (2008) Identification of the transporters involved in the hepatobiliary transport and intestinal efflux of methyl 1-(3,4-dimethoxyphenyl)-3-(3-ethylvaleryl)-4-hydroxy-6,7,8-trimethoxy-2-naphthoate (S-8921) glucuronide, a pharmacologically active metabolite of S-8921. Drug Metab Dispos 36 1553–1561. [DOI] [PubMed] [Google Scholar]
- Schauer UM, Völkel W, and Dekant W (2006) Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol Sci 91 49–58. [DOI] [PubMed] [Google Scholar]
- Thompson J and Burns DA (1996) CS-003: LSC Sample Preparation by Solubilization. Packard Instruments, Meriden, CT.
- U.S. Environmental Protection Agency (2002b). Test plan for 2,2-bis(bromomethyl)-1,3-propanediol. CAS No. 3296-90-0, High Production Volume (HPV) Chemical Challenge Program, Washington, DC.
- Zia-Amirhosseini P, Spahn-Langguth H, and Benet LZ (1994) Bioactivation by glucuronide-conjugate formation. Adv Pharmacol 27 385–397. [DOI] [PubMed] [Google Scholar]
- Zhu M, Zhao W, Vazquez N, and Mitroka JG (2005) Analysis of low level radioactive metabolites in biological fluids using high-performance liquid chromatography with micro-plate scintillation counting: method validation and application. J Pharm Biomed Anal 39 233–245. [DOI] [PubMed] [Google Scholar]