Abstract
Carbonyl reductase 1 (CBR1) reduces the anticancer drug doxorubicin into the cardiotoxic metabolite doxorubicinol. We documented the hepatic expression of CBR1 in samples from white and black donors. Concordance between ethnicity and geographical ancestry was examined with ancestry informative markers. Livers from blacks and whites showed similar CBR1 mRNA levels (CBR1 mRNAblacks = 4.8 ± 4.3 relative -fold versus CBR1 mRNAwhites = 3.6 ± 3.6 relative -fold; p = 0.217). CBR1 protein levels did not differ between both groups (CBR1blacks = 8.0 ± 3.4 nmol/g cytosolic protein versus CBR1whites = 9.0 ± 4.6 nmol/g cytosolic protein; p = 0.347). The CBR1 3′-untranslated region polymorphism 1096G>A was detected in DNA samples from whites (p = 0.875; q = 0.125), and livers with homozygous G/G genotypes showed a trend toward higher CBR1 mRNA levels compared with samples with heterozygous G/A genotypes [CBR1 1096G>A(G/G) = 4.1 ± 4.1 relative -fold versus CBR1 1096G>A(G/A) = 3.0 ± 2.5 relative-fold; p = 0.266]. CBR1 1096G>A genotype status was associated with CBR1 protein levels (p = 0.030) and CBR activity expressed as the rate of synthesis of doxorubicinol (p = 0.028). Our findings warrant further studies to evaluate the impact of CBR1 1096G>A genotype status on the variable pharmacodynamics of anthracycline drugs.
Carbonyl reductase 1 (CBR1) is a cytosolic short-chain dehydrogenase that catalyzes the two-electron reduction of relevant pharmacological substrates such as the antipsychotic haloperidol and the anticancer anthracyclines doxorubicin and daunorubicin (Forrest and Gonzalez, 2000; Rosemond and Walsh, 2004; Matsunaga et al., 2006). CBR1 reduces doxorubicin into its main circulating C-13 alcohol metabolite doxorubicinol (doxol) by using the cofactor NADPH (Wermuth, 1981; Forrest et al., 1990). On average, 16 to 45% of the total doxorubicin is eliminated as doxorubicinol, and the remainder of the drug is eliminated unchanged. Other minor metabolites such as 7-deoxy aglycones are synthesized by cytochrome P450 reductase and circulate in plasma at low concentrations (1–2% of the parent drug) (Speth et al., 1988; Joerger et al., 2005). The pharmacodynamics of doxorubicin in different cancer settings is variable, and the development of anthracycline-related cardiotoxicity in some patients hampers the clinical use of the drug. It is interesting to note that the metabolite doxorubicinol synthesized by CBR1 activity plays a key role during the pathogenesis of anthracycline-related cardiotoxicity. Doxorubicinol exerts cardiotoxicity by a combination of mechanisms, including inhibition of Ca2+/Mg2+-ATPase in the sarcoplasmic reticulum and inactivation of the cytoplasmic aconitase/iron regulatory protein-1 complex (Olson et al., 1988; Minotti et al., 2004). Olson et al. (1988) reported that an overall 40 to 50% reduction of Cbr1 protein levels in Cbr1(+/–) mice was sufficient to confer protection against anthracycline-related cardiotoxicity. Cbr1(+/–) animals treated with a single injection of doxorubicin (20 mg/kg i.p.) showed lower plasma levels of doxorubicinol than wild-type animals [Cbr1(+/+)]. Furthermore, histopathological analyzes together with echocardiographical assessments demonstrated anthracycline-related cardiotoxicity in Cbr1(+/+) but not in Cbr1(+/–) mice (Olson et al., 2003).
The liver is the major organ for the metabolism of doxorubicin, and various reports including a very recent report by Kassner et al. (2008), have shown that CBR1 is the main source of hepatic doxorubicin reductase activity. The aldo-keto reductases AKR1A1 and AKR1B1 are expressed in liver and also catalyze the reduction of doxorubicin. However, AKR1A1 and AKR1B1 have 7- to 18-fold lower catalytic efficiencies for the reduction of anthracycline substrates compared with CBR1 (Wermuth et al., 1986; Ohara et al., 1995; O'connor et al., 1999; Rosemond and Walsh, 2004; Kassner et al., 2008). Thus, variable hepatic CBR1 expression may affect the unpredictable pharmacodynamics of doxorubicin. Therefore, the first aim of this study was to analyze the expression of CBR1 in a collection of liver tissue samples from white (n = 64) and black donors (n = 32). Toward this end, we documented CBR1 mRNA and CBR1 protein levels by quantitative real-time RT-PCR and immunoblotting with a polyclonal anti-CBR1 antibody, respectively.
Functional single-nucleotide polymorphisms (SNPs) on CBR1 may contribute to variable CBR1 activity. We have characterized the functional impact of a nonsynonymous SNP on CBR1 (CBR1 V88I, rs1143663) that seems to be confined to individuals with African ancestry (q = 0.014). CBR1 V88I results in CBR1 protein variants (CBR1 V88 and CBR1 I88) with distinctive catalytic and thermodynamic properties (Gonzalez-Covarrubias et al., 2007). Further studies demonstrated that the anthracycline reductase activities of CBR1 V88 and CBR1 I88 are differentially inhibited by the cardioprotectant flavonoid monoHER (Gonzalez-Covarrubias et al., 2008). A second nonsynonymous SNP on CBR1 (CBR1 S131P, rs41557318) has been recently reported by the dbSNP database (build 129). In addition, Avramopoulos et al. (1992) identified a relatively common SNP on the CBR1 3′-untranslated region (CBR1 1096G>A, rs9024). Thus, we investigated the presence of CBR1 V88I, CBR1 S131P, and CBR1 1096G>A in paired liver DNA samples; and we determined whether CBR1 1096G>A genotype status dictates variable hepatic CBR1 expression.
Materials and Methods
Human Liver Samples. The Institutional Review Board of the State University of New York at Buffalo, NY, approved this research. Demographic information (e.g., age, gender, and ethnicity) was obtained from medical records (Supplemental Table S1). Human liver tissues from black (n = 32) and white (n = 64) donors were processed at St. Jude Children's Research Hospital (Memphis, TN) and were provided by the Liver Tissue Procurement and Distribution System (National Institutes of Health Contract N01-DK-9-2310) and by the Cooperative Human Tissue Network (http://chtn.nci.nih.gov/), respectively. Liver tissue samples were processed following standardized procedures to obtain cytosolic fractions, RNA, and DNA. DNA and RNA isolations were performed with phenol-chloroform extraction (n = 1; 1% of the total), TRI Reagent (n = 7; 7% of the total; Molecular Research Center, Cincinnati, OH), and QIAGEN DNA/RNA kits (n = 88; 92% of the total; QIAGEN, Valencia, CA).
Ancestry Informative Markers. One hundred and seventy-six autosomal genetic markers showing large differences in allele frequencies between populations with distinctive geographical ancestries were used as ancestry-informative markers (AIM). A subset of DNA samples from white and black donors were selected by blinded operators for AIM genotyping (whites, n = 49, 77% of the total; and blacks, n = 27, 84% of the total). AIM were genotyped by DNAprint Genomics (Sarasota, FL). The results are reported as the estimated percentage of sub-Saharan African, European, Native American, and East Asian ancestry (Kishi et al., 2007).
Hepatic CBR1 mRNA Expression. The expression of CBR1 mRNA was analyzed in 23 total liver RNA samples from blacks (72% of the total) and 42 total liver RNA samples from whites (66% of the total; Supplemental Fig. S1). RNA concentrations were measured with a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Total RNA (100 ng) was reverse-transcribed and amplified by using one-step QuantiTect SYBR Green RT-PCR kits (QIAGEN). RT-PCR reaction mixtures were incubated in a MX3050P thermal cycler equipped with proprietary software for data analyses (MxPro version 3.00; Stratagene, La Jolla, CA). CBR1 primers were 5′-CTGATCCCACACCCTTTCAT-3′ (forward) and 5′-TTAAGGGCTCTGACGCTCAT-3′ (reverse). β-Actin primers were 5′-ACGGCTCCGGCATGTGCAAG-3′ (forward) and 5′-TGACGATGCCGTGCTCGATG-3′ (reverse). CBR1 and β-actin (normalizer) mRNAs were amplified in parallel with the following cycling parameters: 50°C for 30 min (reverse transcription), 95°C for 10 min (Taq DNA polymerase activation), 40 cycles of 95°C for 15 s (denaturation), 51°C for 30 s (annealing), 72°C for 30 s (extension), and 78°C for 30 s (fluorescence collection). Standard curves for CBR1 and β-actin mRNAs (20-fold dynamic range) were run in parallel to ensure accurate mRNA quantifications. In all cases, the regression coefficients of the standard curves were r2 ≥ 0.94. Amplification efficiencies for CBR1 and β-actin mRNAs were comparable and ranged between 125 and 175%. Experimental samples and standards for calibration curves were analyzed in quadruplicate. The relative amount of CBR1 mRNA in each liver sample was automatically calculated with the comparative quantitation algorithm by using individual β-actin mRNA levels as normalizers. CBR1 mRNA values were expressed relative to the normalized CBR1 mRNA content of liver sample 237. Liver sample 237 was randomly selected for data normalization (Blanquicett et al., 2002; Bustin, 2002; Zamber et al., 2003).
Hepatic CBR1 Protein Expression. Randomly selected liver cytosols from white (n = 28) and black (n = 28) donors were analyzed by quantitative immunoblotting (Supplemental Fig. S1). Liver cytosols (150 μg) and recombinant CBR1 standards (0.05, 0.08, 0.10, 0.15, 0.20, and 0.30 μg) were heated in a boiling water bath for 5 min. Recombinant CBR1 was obtained as described previously (Gonzalez-Covarrubias et al., 2007). Nanoliquid chromatography coupled to triple quadrupole mass spectroscopy showed that the purity of CBR1 was ≥96%. Samples were supplemented with Laemmli buffer and 5% β-mercaptoethanol (Bio-Rad, Hercules, CA). Samples were loaded into 4 to 10% Precise-protein gels (Pierce Chemical, Rockford, IL) and separated by electrophoresis at 90 V for 90 min in a Bio-Rad mini-cell apparatus. Proteins were transferred into Hybond-polyvinylidene difluoride membranes (GE Healthcare, Little Chalfont, Buckinghamshire, UK). Membranes were blocked at room temperature for 1 h with StartingBlock T20 (Pierce Chemical). After blocking, membranes were incubated for 1 h with a specific polyclonal anti-human CBR1 antibody (1:2000 dilution; Abcam Inc., Cambridge, MA) or with an anti-β-actin monoclonal antibody (1:5000 dilution; Sigma-Aldrich, St. Louis, MO). Next, membranes were incubated for 1 h with a secondary anti-IgG antibody conjugated with horseradish peroxidase (1:10,000 dilution; Sigma-Aldrich). Immunoreactive bands were visualized with the ECL Plus Western blotting detection system (GE Healthcare). CBR1 band intensity values (pixels per square millimeter) were quantified with a ChemiDoc XRS gel documentation system equipped with Quantity One software (Bio-Rad). Hepatic CBR1 levels were estimated by direct extrapolation from the calibration curves with recombinant CBR1. The limit of quantification was 0.02 μg, and the limit of detection was 0.01 μg. Detection of CBR1 was linear (range, 0.05–0.30 μg; r2 > 0.85; coefficient of variation = 9.5%). No immunoreactive bands were detected in immunoblots of recombinant human CBR3 (purity ≥90%) probed with the polyclonal anti-human CBR1 antibody. CBR3 was obtained as described previously (Lakhman et al., 2005).
Hepatic CBR Activity. Maximal CBR activities were measured in liver cytosols from whites (n = 64) and blacks (n = 32) with the substrate doxorubicin at 37°C. Validation experiments with liver cytosols from black and white donors showed that 400 μM doxorubicin [S] ensured conditions of Vmax and/or maximal CBR activity (zero-order kinetics). Thus, maximal CBR activities (reaction rates) were directly proportional to the amount of cytosolic CBR1 enzyme. CBR activity with the substrate doxorubicin was linear within the following total protein concentration range: 0.2 to 5.0 mg/ml (r2 = 0.99). Incubation mixtures (final volume, 1 ml) contained potassium phosphate buffer (0.1 M; pH 7.4), NADPH (200 μM; Sigma-Aldrich), and doxorubicin (400 μM; Sigma-Aldrich). Kinetic reactions were started by the addition of liver cytosols (100 μl, total protein concentration: 1.2 ± 0.6 mg/ml; range, 0.1–3.7 mg/ml). The oxidation rates of the NADPH cofactor were recorded at 340 nm (NADPH molar absorption coefficient, 6220 M–1 cm–1) for 3.0 min at an acquisition speed of 5 readings/s (900 readings) in a Cary Varian Bio 300 UV-Vis (Varian, Inc., Palo Alto, CA) spectrophotometer equipped with thermal control and proprietary software for enzyme kinetics analysis (Wermuth, 1981; Covarrubias et al., 2006). Enzymatic velocities (V0) were automatically calculated by linear regression of the ΔAbs/Δtime points (r2 ≥ 0.95). After the kinetic measurements, the reaction mixtures were immediately frozen at –70°C for doxorubicinol quantification. Direct quantification of doxorubicinol with a high-performance liquid chromatography and tandem mass spectrometry assay adapted for human liver cytosols was performed in a subset of randomly selected reactions mixtures from whites (n = 40; 63% of the total) and blacks (n = 20; 63% of the total) (DiFrancesco et al., 2007). Doxorubicinol was extracted from the cytosolic reaction mixtures by solid phase extraction using reversed-phase sorbent cartridges (Oasis HLB; Waters, Milford, MA). The high-performance liquid chromatography system consisted of an autosampler, a degasser, and an LC pump (1100 series; Agilent Technologies, Santa Clara, CA) coupled to an Applied Biosystems PE/Sciex API 3000 mass spectrometer (Applied Biosystems, Foster City, CA). The mass spectrometer was operated in the mixed reaction-monitoring positive ion mode using a turbo ionspray interface. The desolvation temperature of the interface was 350°C, and the ion current was 4000 V. Chromatographic separation was performed in a C18 2.1 × 30-mm column (Waters Symmetry, Milford, MA) protected by a C18 2.1 × 10-mm guard column (Waters Symmetry). Separation of doxorubicinol and doxorubicin was achieved at a flow rate of 250 μl/min using a mobile phase gradient of 75% mobile phase A (5 mM acetate buffer, pH 3.5; 5% methanol) and 25% mobile phase B (5 mM acetate buffer, pH 3.5; 95% methanol), with a transition to 25% mobile phase A and 75% mobile phase B in 8 min. The anthracycline daunorubicin was used as an internal standard (240 ng/ml). The range of linearity for the quantification of doxorubicinol was 50 to 1000 ng/ml (r2 > 0.990). The inter- and intraday coefficients of variations were <15%, and the accuracy range for doxorubicinol was 107 to 112%. Quality control points were routinely prepared by spiking doxorubicinol (Toronto Research Chemicals, Toronto, ON, Canada) into pooled liver cytosols at the following final concentrations: 50, 100, 200, and 400 ng/ml. The matrix effects of plasma and liver cytosols were comparable (percentage of recovery plasma, 99% and percentage of recovery cytosols, 103%). Cytosolic protein concentrations were determined with an assay based on the Bradford (1976) technique using bovine serum albumin as standard (Bio-Rad). Maximal hepatic CBR activities were expressed as doxorubicinol synthesis rates (nanomoles of doxol per minute per milligram).
CBR1 Genotyping. The CBR1 V88I polymorphism (rs1143663, 391G>A) was analyzed with a validated assay for allelic discrimination with specific fluorescent probes as described previously (Gonzalez-Covarrubias et al., 2007). The CBR1 3′-UTR 1096G>A (rs9024, 1096G>A) and CBR1 S131P (rs41557318, 520C>T) polymorphisms were investigated by allelic discrimination with fluorescent probes followed by real-time PCR (Assays-by-Designs; Applied Biosystems). Supplemental Table 2 lists the nucleotide sequences of genotyping primers and probes. Genotyping reactions were performed according to the manufacturer's protocol in a Bio-Rad iQ5 thermal cycler (Bio-Rad). All genotyping runs included appropriate negative (no DNA template) and positive controls (DNA samples from the Coriell Institute for Medical Research (Camden, NJ) with known CBR1 V88I, CBR1 1096G>A, and CBR1 S131P genotypes). For quality control purposes, CBR1 3′-UTR 1096G>A was further investigated by direct sequencing on 33 CBR1 full-length cDNA samples from black (n = 13) and white (n = 20) donors as described previously (Gonzalez-Covarrubias et al., 2007).
Human Lymphoblastoid Cell Lines. Nine human lymphoblastoid cell lines (GM10853, GM10845, GM10857, GM10858, GM10860, GM17240, GM16654, GM16688, and GM16689) derived from individual donors with Chinese (n = 3) or European (n = 6) ancestries were purchased as live cultures from the Coriell Institute for Medical Research. Cultures were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 2 mM l-glutamine and 15% fetal bovine serum (Invitrogen). Total RNA was extracted from lymphoblastoid cell cultures (1 × 106 cells/ml) with Illustra RNA Mini Spin kits according to the manufacturer's instructions (GE Healthcare). The relative amount of CBR1 mRNA in each lymphoblastoid cell line was determined as described above. CBR1 mRNA levels in lymphoblastoid cells were expressed relative to the normalized CBR1 mRNA content of cell lines with CBR1 1096G>A homozygous G/G genotypes (cell lines: GM10857 and GM16688). Genomic DNA was extracted with QIAGEN kits. Cell cultures (1 × 106 cells/ml) were centrifuged at 3000 rpm for 15 min for the preparation of cytosolic fractions. The resulting pellets were treated with 2.0 ml of lysis buffer [320 mM sucrose, 10 mM potassium phosphate, 1 mM EDTA, and 1 mM tris(2-carboxyethyl)phosphine, pH 7.4] supplemented with protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). Pellets were homogenized with a handheld tissue homogenizer (BioSpec Products, Inc., Bartlesville, OK). Cell homogenates were sequentially centrifuged at 10,000 rpm (15 min), 15,000 rpm (30 min), and 45,000 (1 h). Cytosols were obtained by collecting the supernatants from the last centrifugation step. Synthesis of doxorubicinol by lymphoblastoid cell lines was determined by incubating cytosolic fractions with the substrate doxorubicin (400 μM) and NADPH (200 μM) for 5 h. Reactions were started by the addition of cytosols (volume, 600 μl; total protein concentration, 3 mg/ml). Doxorubicinol concentrations were determined as described above.
Statistical Analysis. Descriptive statistics (e.g., group means, variances, standard deviations, group ranges, and group percentiles) were computed with Excel 2000 version 9.0 (Microsoft Office; Microsoft, Redmond, WA) and GraphPad Prism version 4.03 (GraphPad Software Inc., San Diego, CA). The D'Agostino and Pearson omnibus normality test was used to examine data normality with α levels <0.05. Unpaired Student's t tests were used to compare population means of data sets normally distributed. The Mann-Whitney U test was used to compare population means of data sets with non-normal distributions. In all cases, differences were considered to be statistically significant at p < 0.050. Pearson's coefficient of correlation (rp) was used to analyze data sets with normal distributions, and Spearman's coefficient of correlation (rs) was used for data sets with non-normal distributions. Genotype and phenotype data sets will be available at the PharmGKB database (http://www.pharmgkb.org).
Results
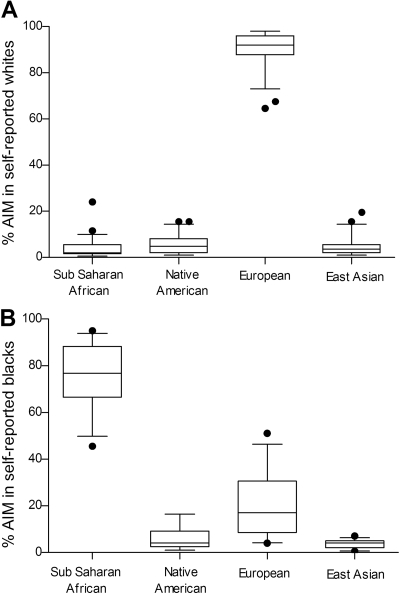
AIM in Samples from Black and White Liver Donors. First, we sought to examine the extent of concordance between self-reported ethnicity and geographical ancestry by genotyping 176 AIM in a subset of randomly selected liver DNA samples from black (n = 27) and white (n = 49) donors (Supplemental Fig. S1). Samples from black donors showed an average AIM score of 76 ± 13% for the sub-Saharan African panel, whereas samples from white donors showed an average AIM score of 91 ± 8% for the European panel (Fig. 1). Further analysis showed that the hepatic expression of CBR1 (CBR1 mRNA, CBR1 protein, and CBR activity) was similar whether stratifying by ethnicity or AIM-determined geographical ancestry (Table 1). Thus, the hepatic expression of CBR1 was analyzed after stratifying samples by self-reported ethnicity.
Fig. 1.
AIM scores in samples from white (n = 49; A) and black (n = 27; B) liver donors. Boxes indicate the 5th and 95th percentiles, horizontal bars indicate medians, and whiskers indicate the range after excluding outliers (circles).
TABLE 1.
Hepatic CBR1 expression in samples stratified by ethnicity and AIM-determined geographical ancestry
Number of samples is indicated in parentheses.
| Ethnicity Black | White | AIM-Determined Geographical Ancestry Sub-Saharan African | European | p (Black vs. Sub-Saharan African) | p (White vs. European) | |
|---|---|---|---|---|---|---|
| CBR1 mRNA (relative -fold) | 4.8 ± 4.3 (23) | 3.6 ± 3.6 (30) | 4.8 ± 4.3 (23) | 3.9 ± 3.8 (33) | 1.000 | 0.728 |
| CBR1 protein (nmol/g cytosolic protein) | 8.0 ± 3.4 (28) | 9.0 ± 4.6 (28) | 8.1 ± 3.5 (25) | 9.9 ± 4.8 (20) | 0.897 | 0.522 |
| Maximal CBR activity (nmol doxol/min · mg) | 4.2 ± 2.3 (20) | 3.9 ± 2.1 (40) | 4.2 ± 2.3 (20) | 3.9 ± 2.2 (29) | 1.000 | 0.991 |
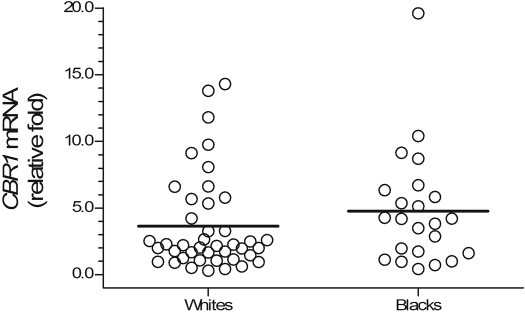
CBR1 mRNA and CBR1 Protein Expression in Liver Tissues from Black and White Donors. Hepatic CBR1 mRNA expression was analyzed in 23 total liver RNA samples from blacks and 42 total liver RNA samples from whites, respectively. The number of RNA samples represented 72% (blacks) and 66% (whites) of the total number of liver samples available for each ethnic category (Supplemental Fig. S1). Hepatic CBR1 mRNA levels varied widely in blacks (49-fold; CBR1 mRNAblacks range, 0.4–19.6 relative -fold) and whites (48-fold; CBR1 mRNAwhites range, 0.3–14.3 relative -fold). Statistical comparisons demonstrated that the relative expression of hepatic CBR1 mRNA was similar between samples from black and white donors (CBR1 mRNAblacks = 4.8 ± 4.3 relative -fold versus CBR1 mRNAwhites = 3.6 ± 3.6 relative -fold; p = 0.217) (Fig. 2).
Fig. 2.
Hepatic CBR1 mRNA expression in samples from white (n = 42) and black (n = 23) donors. Relative CBR1 mRNA levels were determined with the comparative quantitation method (see text for details). Individual β-actin mRNA levels were used as normalizers. Samples and standards for calibration curves (r2 > 0.94) were analyzed in quadruplicates. Each circle depicts the average of individual samples. Horizontal lines indicate group means (p = 0.217).
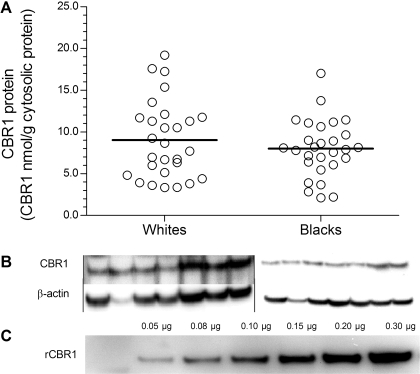
A randomly selected subset of liver cytosols from blacks (n = 28) and whites (n = 28) was examined for CBR1 protein expression by immunoblot analysis (Supplemental Fig. S1). CBR1 was detected as a single band of approximately 30 kDa in all samples. β-Actin expression was also examined to assess the integrity of each cytosolic sample. The expression of CBR1 varied by 8-fold in samples from blacks (range, 2.2–17.0 nmol/g cytosolic protein) and by 6-fold in samples from whites (range, 3.3–19.2 nmol/g cytosolic protein). CBR1 protein levels did not differ between blacks and whites (CBR1blacks = 8.0 ± 3.4 nmol/g cytosolic protein versus CBR1whites = 9.0 ± 4.6 nmol/g cytosolic protein; p = 0.347) (Fig. 3). Correlation analysis showed no association between CBR1 mRNA and CBR1 protein expression in samples from black (rp = 0.244; p = 0.305) and white (rp = 0.296; p = 0.150) liver donors (Supplemental Fig. S2).
Fig. 3.
Hepatic CBR1 protein expression in samples from white (n = 28) and black (n = 28; A) donors. Each circle represents individual liver samples. Horizontal lines indicate group means (p = 0.347). B, immunodetection of hepatic CBR1 and β-actin in human liver cytosols. C, immunodetection of recombinant CBR1 standards (rCBR1) from a typical calibration curve.
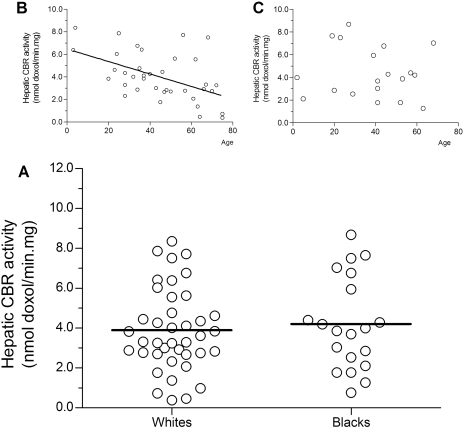
CBR Activities in Liver Cytosols from Blacks and Whites. Hepatic CBR activities for the substrate doxorubicin were assessed by measuring the oxidation rates of the NADPH cofactor in liver cytosols from white (n = 64) and black liver donors (n = 32). Variable levels of CBR activity were observed in samples from whites (range, <0.1–10.6 nmol/min · mg) and blacks (range, 0.9–14.0 nmol/min · mg), respectively. Statistical comparisons demonstrated that maximal CBR activities were similar between both groups (CBRblacks = 4.0 ± 2.5 nmol/min · mg versus CBRwhites = 3.9 ± 2.2 nmol/min · mg; p = 0.820; Supplemental Fig. S3). Direct quantification of the C-13 alcohol metabolite doxorubicinol was performed in a subset of randomly selected cytosolic reaction mixtures from whites (n = 40; 63% of the total) and blacks (n = 20; 63% of the total). There was a significant correlation between the values of maximal CBR activities obtained with the spectrophotometric assay and those measured by direct quantification of doxorubicinol (n = 60; rs = 0.270; p = 0.039; Supplemental Fig. S3). Statistical comparisons showed that maximal CBR activities expressed as the rate of synthesis of doxorubicinol were similar between blacks and whites (CBRblacks = 4.2 ± 2.3 nmol doxol/min · mg versus CBRwhites = 3.9 ± 2.1 nmol doxol/min · mg, p = 0.610) (Fig. 4). Further correlation analyses showed no association between CBR1 protein and CBR activity in samples from white (rp = 0.203; p = 0.377) and black (rp = 0.055; p = 0.826) liver donors (Supplemental Fig. S4). Likewise, there was no association between hepatic CBR1 mRNA expression and CBR activity in samples from white (rp = 0.325; p = 0.070) and black (rp = 0.357; p = 0.175) donors (Supplemental Fig. S5).
Fig. 4.
A, maximal CBR activities with the substrate doxorubicin in liver cytosols from white (n = 40) and black (n = 20) donors. Each sample was analyzed in duplicates. Each circle depicts the average of individual samples. Group means are indicated by horizontal lines (p = 0.610). Linear regression analyzes of age versus maximal CBR activity in samples from white (B) and black liver donors (C).
Regression analyses showed a significant negative correlation between maximal CBR activities expressed as the rate of synthesis of doxorubicinol and the donors' age in samples from whites (rp = –0.439; p = 0.006). Similar analyses in samples from black donors showed no correlation between CBR activity and age (rs = 0.008; p = 0.715) (Fig. 4). We also determined whether a relationship existed between age and cytosolic protein yield. Cytosolic protein yield was not significantly associated with age in samples from blacks (rp = 0.126; p = 0.325) and whites (rp = 0.231; p = 0.340), respectively.
CBR1 Genotype-Phenotype Associations in Liver Samples from Black and White Donors. To pinpoint genetic determinants of hepatic CBR1 expression, paired liver DNA samples were genotyped for the nonsynonymous SNPs CBR1 V88I and CBR1 S131P, and for the 3′-UTR SNP CBR1 1096G>A, respectively (Avramopoulos et al., 1992; Gonzalez-Covarrubias et al., 2007). The variant alleles for CBR1 V88I (A) and CBR1 S131P (T) were absent in DNA samples from white and black liver donors. The CBR1 1096G>A polymorphism was detected in samples from whites but not in samples from blacks (Table 2). CBR1 1096G>A genotype distributions were in Hardy-Weinberg equilibrium (χ2 test; p = 0.565). Genetic surveys in small DNA human diversity panels from the Coriell Institute for Medical Research revealed that the CBR1 1096G>A polymorphism seems to be relatively common (q > 0.12) among individuals with distinctive geographical ancestries, such as Chinese, Japanese, and South East Asians (Table 2).
TABLE 2.
CBR1 1096G>A genotype distributions in liver donors and human DNA diversity panels
| Population | Na | G/G | G/A | A/A | pb | qc |
|---|---|---|---|---|---|---|
| Black liver donors | 32 | 32 | 1.000 | |||
| White liver donors | 64 | 48 | 16 | 0.875 | 0.125 | |
| U.S. African ancestryd | 69 | 67 | 2 | 0.990 | 0.010 | |
| U.S. European ancestryd | 50 | 38 | 11 | 1 | 0.870 | 0.130 |
| Indo-Pakistanid | 9 | 7 | 2 | 0.889 | 0.111 | |
| Middle Easternd | 10 | 2 | 8 | 0.900 | 0.100 | |
| Africans north of the Saharad | 7 | 6 | 1 | 0.929 | 0.071 | |
| Africans south of the Saharad | 9 | 9 | 1.000 | |||
| Chinesed | 10 | 6 | 3 | 1 | 0.750 | 0.250 |
| Japanesed | 10 | 3 | 6 | 1 | 0.600 | 0.400 |
| Mexicand | 10 | 9 | 1 | 0.950 | 0.050 | |
| Asia Pacificd | 7 | 3 | 3 | 1 | 0.642 | 0.357 |
| South American Andesd | 10 | 6 | 3 | 1 | 0.750 | 0.250 |
| South East Asiad | 10 | 4 | 6 | 0.800 | 0.200 |
N, number of DNA samples.
p denotes the G allele.
q denotes the A allele.
DNA human diversity panels from the Coriell Institute for Medical Research.
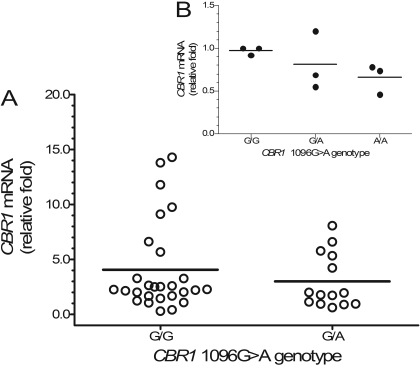
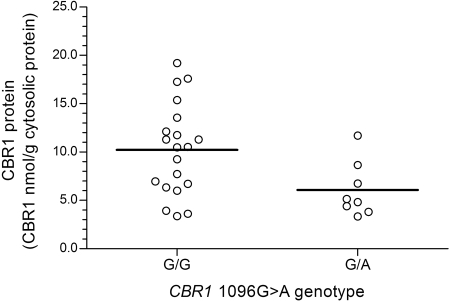
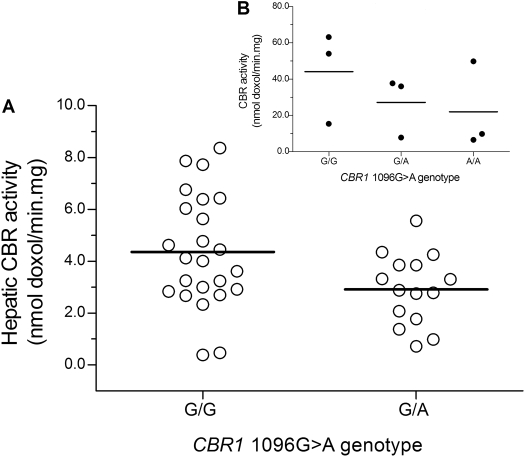
On average, relative CBR1 mRNA levels tended to be higher in samples from white donors with CBR1 1096G>A homozygous G/G genotypes compared with samples from donors with heterozygous G/A genotypes, but the differences between genotype groups did not reach statistical significance [CBR1 mRNA1096G>A(G/G) = 4.1 ± 4.1 relative -fold versus CBR1 mRNA1096G>A(G/A) = 3.0 ± 2.5 relative -fold; p = 0.266] (Fig. 5). Further analysis showed statistically significant differences in cytosolic CBR1 protein levels between both CBR1 1096G>A genotype groups [CBR1 protein1096G>A(G/G) = 10.2 ± 4.7 nmol/g cytosolic protein versus CBR1 protein1096G>A(G/A) = 6.1. ± 2.8 nmol/g cytosolic protein; p = 0.030] (Fig. 6). In line, liver cytosols with CBR1 1096G>A homozygous G/G genotypes showed higher maximal rates of doxorubicinol synthesis (1.5-fold) compared with samples with heterozygous G/A genotypes [CBR activity1096G>A(G/G) = 4.4 ± 2.2 nmol doxol/min · mg versus CBR activity1096G>A(G/A) = 2.9 ± 1.4 nmol doxol/min · mg; p = 0.028] (Fig. 7).
Fig. 5.
A, impact of CBR1 1096G>A genotype on the hepatic expression of CBR1 mRNA. Each circle represents individual liver samples. Horizontal lines indicate group means (p = 0.266). B, impact of CBR1 1096G>A genotype on the expression of CBR1 mRNA in cultures of lymphoblastoid cell lines. Each circle represents the average of quadruplicate measurements of relative CBR1 mRNA levels in individual cell lines (p ≈ 0.04 for G/G versus A/A).
Fig. 6.
Impact of CBR1 1096G>A genotype on hepatic CBR1 protein expression. Each circle represents individual liver samples. Horizontal lines indicate group means (p = 0.030).
Fig. 7.
A, impact of CBR1 1096G>A genotype on maximal cytosolic CBR activities for the substrate doxorubicin (p = 0.028). B, impact of CBR1 1096G>A genotype on maximal cytosolic CBR activities in cultures of lymphoblastoid cell lines. Each circle represents the average of duplicate measurements of maximal CBR activities in individual cell lines (p ≈ 0.02 for G/G versus A/A).
The impact of the CBR1 1096G>A polymorphism was further investigated in cultures of lymphoblastoid cell lines with known CBR1 1096G>A genotype status. CBR1 mRNA levels in lymphoblastoid cells were expressed relative to the normalized CBR1 mRNA content of cell lines: GM10857 and GM16688 (CBR1 1096G>A G/G genotypes). Cell lines with CBR1 1096G>A homozygous G/G genotypes exhibited higher CBR1 mRNA relative expression levels than cell lines with CBR1 1096G>A homozygous A/A genotypes (CBR1 mRNA1096G>A(G/G) = 1.0 ± 0.1 relative -fold versus CBR1 mRNA1096G>A(A/A) = 0.7 ± 0.2 relative -fold; p ≈ 0.04) (Fig. 5). Furthermore, cell lines with CBR1 1096G>A homozygous G/G genotypes synthesized 2-fold more doxorubicinol than cell lines with homozygous A/A genotypes [CBR activity1096G>A(G/G) = 44.2 ± 25.4 nmol doxol/min · mg versus CBR activity1096G>A(A/A) = 22.0 ± 24.0 nmol doxol/min · mg; p ≈ 0.02] (Fig. 7).
Discussion
The reduction of carbonyl groups by hepatic CBR1 activity is an important step during the metabolism of clinically relevant drugs such as haloperidol (antipsychotic), doxorubicin (anticancer), dolasteron (antiemetic), and pentoxyfilline (hemorheologic) (Rosemond and Walsh, 2004). On the other hand, differences in the average expression of drug-metabolizing enzymes between ethnic groups with distinctive geographical ancestries may affect drug response and toxicity (Wilson et al., 2001; Daar and Singer, 2005; Diczfalusy et al., 2008). Therefore, our first aim was to document the hepatic expression of CBR1 in a cohort of liver tissue samples from white and black donors. Recent pharmacogenetic studies suggest that in certain cases, discrete population labels based on self-reported ethnicity and/or skin color may be inappropriate surrogates of geographical ancestry due to population admixture (Suarez-Kurtz et al., 2007a,b). Thus, we sought to examine 176 autosomal genetic markers of geographical ancestry to refine the stratification criterion. Stratification by ethnicity or AIM-determined geographical ancestry did not modify the average expression values of hepatic CBR1 mRNA, CBR1 protein, and CBR activity (Table 1). Therefore, in this group of samples, self-reported ethnicity seems to be an accurate indicator of geographical ancestry.
CBR1 mRNA and CBR1 protein were detected in all liver samples. On average, CBR1 mRNA expression varied by 48-fold (Fig. 2). Further analysis showed that the hepatic expression of CBR1 mRNA varied by 13- and 23-fold in samples from black and white donors after the elimination of outlier values located above the 95th or below the 5th percentiles (blacks, n = 2; whites, n = 3). Hepatic CBR1 protein expression varied by 7-fold, and CBR1 protein was detected as a single immunoreactive band after electrophoretic separation in 4 to 10% gradient polyacrylamide gels (Fig. 3). In a recent study, Kassner et al. (2008) reported that CBR1 expression in liver cytosols varied by >70-fold. The 10-fold discrepancy between both studies may be due in part to different strategies for CBR1 immunodetection. That is, Kassner et al. (2008) performed electrophoretic separations by using 12% polyacrylamide gels, and CBR1 was detected as a triple band after immunoblotting due to the binding of 2-oxocarbonyl acids to lysine 239 (Wermuth et al., 1993; Kassner et al., 2008). We also found that hepatic doxorubicin reductase activity varied by 22-fold in samples from blacks and whites (Fig. 4). In this case, the extent of variable CBR activity for the substrate doxorubicin is in agreement with the 22-fold value reported by Kassner et al. (2008). We observed a modest although significant correlation between CBR1 activities determined by UV-spectrophotometry versus direct quantification of doxorubicinol (Supplemental Fig. S5). The comparatively low specificity of UV-spectrophotometric methods in complex biological matrices (i.e., liver cytosols) may have affected the extent of correlation between both measurements of CBR1 activity (Tietz, 1999).
In a recent study, we reported that CBR1 mRNA, CBR1 protein, and CBR activity are concomitantly up-regulated by strong ligands of the aryl hydrocarbon receptor (AHR) such as β-naphtoflavone and 2,3,7,8-tetrachlorodibenzo-p-dioxin (Lakhman et al., 2007). Here, we found no significant relationships between CBR1 mRNA, CBR1 protein, and CBR activity levels in liver samples from white and black donors. Based on these observations, it is possible to hypothesize that the hepatic expression of CBR1 under “basal conditions” (i.e., absence of AHR ligands) may be regulated by factors that operate at various levels (e.g., transcriptional and/or post-transcriptional), whereas the expression of CBR1 under “nonbasal conditions” (i.e., presence of AHR ligands) would be primarily driven by AHR through coupled transcriptional-translational regulation.
The CBR1 1096G>A polymorphism was common in samples from white donors, and we pinpointed a significant association between CBR1 1096G>A genotype status and the hepatic expression of CBR1 protein. Furthermore, CBR1 1096G>A genotype status in whites was significantly associated with the maximal rates of synthesis of the cardiotoxic C-13 metabolite doxorubicinol (Fig. 7). It should be noted that Kassner et al. (2008) did not detect associations between CBR1 1096G>A genotype status and doxorubicin reductase activity or CBR1 protein expression in 57 liver samples. The reasons for this discrepancy are unclear and highlight the need for confirmatory studies. Nevertheless, it is also interesting to note that the impact of the CBR1 1096G>A polymorphism on the maximal rates of synthesis of doxorubicinol was also apparent in cultures of lymphoblastoid cell lines. That is, cell lines with CBR1 1096G>A homozygous G/G genotypes synthesized 2-fold more doxorubicinol per unit of time compared with cell lines with homozygous A/A genotypes. This observation is limited by the small number of cell lines analyzed (n = 3 per each genotype combination), but it provides the rationale to test whether peripheral blood lymphocytes would serve as surrogates to predict the impact of CBR1 1096G>A genotype status on variable hepatic CBR activity.
We found that on average, the hepatic expression of CBR1 mRNA, CBR1 protein, and CBR1 activity was similar in samples from black and white donors (Figs. 2, 3, 4). However, these findings need to be interpreted with caution by considering 1) sample size limitations, 2) the potential functional impact of CBR1 1096G>A, and 3) the distribution of the variant CBR1 1096 A allele among individuals with distinctive geographical ancestry (Table 2). For example, cytosols from whites with CBR1 1096G>A homozygous G/G genotype and the entire group of cytosols from black donors exhibited similar doxorubicinol synthesis rates [CBR activity 1096G>A(G/G)-whites = 4.4 ± 2.2 nmol doxol/min · mg, n = 24 versus CBR activity 1096G>A(G/G)-blacks = 4.2 ± 2.3 nmol doxol/min · mg, n = 20; p = 0.826]. Thus, the survey of larger sample sizes from white (i.e., more samples with the variant allele) and black donors may pinpoint differences in the hepatic expression of CBR1 between both groups. It is interesting to note that clinical studies in pediatric cancer survivors treated with anthracyclines identified higher incidence of anthracycline-related cardiotoxicity among black patients (Krischer et al., 1997; Grenier and Lipshultz, 1998). Therefore, it is tempting to speculate that the comparatively higher risk of anthracycline-related cardiotoxicity among black cancer patients may be due in part to the scarcity of the variant allele (A) among individuals with African ancestry.
A pharmacokinetic study in patients receiving concomitant administration of doxorubicin and cyclophosphamide showed a significant negative correlation between age and the clearance of doxorubicin (rp = –0.46; p = 0.00037) (Li and Gwilt, 2003). In addition, Joerger et al. (2007) detected a negative correlation between the clearance of doxorubicin and age by using a physiologically based pharmacokinetic model. They concluded that a 10-year increase in patient age led to a 9% decrease in doxorubicin clearance (Li and Gwilt, 2003; Joerger et al., 2007). In this study, we detected a significant negative correlation between age and maximal CBR activity in samples from whites (rp = –0.439; p = 0.006). Regression analysis without stratification by CBR1 1096G>A genotype demonstrated that age would account for approximately 16% of the variation in doxorubicin reductase activity (Fig. 4). Further analysis after stratification by CBR1 1096G>A genotype status showed that maximal CBR activity was significantly associated with age only in the group of samples with CBR1 1096G>A homozygous G/G genotypes (rp = –0.505; p = 0.014) (Supplemental Fig. S6). Thus, age would account for 25% of the variation in doxorubicin reductase activity among subjects with homozygous G/G genotypes. In this group, samples from donors 1 to 25 years old showed 1.9-fold higher maximal rates of doxorubicinol synthesis compared with those from donors older than 55 years (CBR1-25 years old = 6.7 ± 1.5 nmol doxol/min · mg, n = 5 versus CBR1>55 years = 3.0 ± 3.0 nmol doxol/min · mg, n = 5; p = 0.039). Age did not correlate with doxorubicinol synthesis rates in the group of samples with heterozygous G/A genotypes (rp = 0.054; p = 0.410; Supplemental Fig. S6). These findings are intriguing and highlight the potential functional impact of the CBR1 1096G>A polymorphism. Recent studies on genes of pharmacogenetic relevance indicate that certain 3′-UTR polymorphisms affect the binding of specific microRNAs (miRNA) and are associated with differential gene expression (Mishra et al., 2007; Gow et al., 2008). In consequence, we performed bioinformatics searches by using the PolymiRTS and miRBase databases to test whether the polymorphic CBR1 3′-UTR sequence constitutes a potential target for miRNA. The searches revealed that the presence of the A allele at the polymorphic position (5′-ACTAATATACTAC-3′) creates a potential binding site for miR-656 (Glazov et al., 2008). Thus, we hypothesize that CBR1 1096G>A regulates the steady-state concentrations of hepatic CBR1 mRNA levels through the binding of specific miRNA species such as miR-656 (He and Hannon, 2004).
Anthracycline C-13 alcohol metabolites circulate in plasma and are devoid of significant tumor cell killing activity (Forrest and Gonzalez, 2000). Therefore, it will be of clinical relevance to explore whether CBR1 1096G>A genotype status influence 1) the therapeutic efficacy of anthracycline drugs and 2) the risk of anthracycline-related cardiotoxicity. Our studies support the notion that information on specific genetic determinants of variable CBR activity may assist to optimize anticancer therapy with anthracycline drugs.
Supplementary Material
Acknowledgments
The excellent technical assistance of Donna M. Ruszaj, Julie Donnelly, and Erick Vasquez is gratefully acknowledged.
This work was supported by National Institutes of Health National Institute of General Medical Sciences [Grant GM73646].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024547.
ABBREVIATIONS: CBR, carbonyl reductase; doxol, doxorubicinol; RT-PCR, reverse transcription-polymerase chain reaction; SNP, single-nucleotide polymorphism; AIM, ancestry informative marker(s); UTR, untranslated region; AHR, aryl hydrocarbon receptor; miRNA, microRNA.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
References
- Avramopoulos D, Cox T, Forrest GL, Chakravarti A, and Antonarakis SE (1992) Linkage mapping of the carbonyl reductase (CBR) gene on human chromosome 21 using a DNA polymorphism in the 3′ untranslated region. Genomics 13 447–448. [DOI] [PubMed] [Google Scholar]
- Blanquicett C, Johnson MR, Heslin M, and Diasio RB (2002) Housekeeping gene variability in normal and carcinomatous colorectal and liver tissues: applications in pharmacogenomic gene expression studies. Anal Biochem 303 209–214. [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254. [DOI] [PubMed] [Google Scholar]
- Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol 29 23–39. [DOI] [PubMed] [Google Scholar]
- Covarrubias VG, Lakhman SS, Forrest A, Relling MV, and Blanco JG (2006) Higher activity of polymorphic NAD(P)H:quinone oxidoreductase in liver cytosols from blacks compared to whites. Toxicol Lett 164 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar AS and Singer PA (2005) Pharmacogenetics and geographical ancestry: implications for drug development and global health. Nat Rev Genet 6 241–246. [DOI] [PubMed] [Google Scholar]
- Diczfalusy U, Miura J, Roh HK, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allqvist A, Jande M, Kim JW, et al. (2008) 4beta-Hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics 18 201–208. [DOI] [PubMed] [Google Scholar]
- DiFrancesco R, Griggs JJ, Donnelly J, and DiCenzo R (2007) Simultaneous analysis of cyclophosphamide, doxorubicin and doxorubicinol by liquid chromatography coupled to tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 852 545–553. [DOI] [PubMed] [Google Scholar]
- Forrest GL, Akman S, Krutzik S, Paxton RJ, Sparkes RS, Doroshow J, Felsted RL, Glover CJ, Mohandas T, and Bachur NR (1990) Induction of a human carbonyl reductase gene located on chromosome 21. Biochim Biophys Acta 1048 149–155. [DOI] [PubMed] [Google Scholar]
- Forrest GL and Gonzalez B (2000) Carbonyl reductase. Chem Biol Interact 129 21–40. [DOI] [PubMed] [Google Scholar]
- Glazov EA, McWilliam S, Barris WC, and Dalrymple BP (2008) Origin, evolution, and biological role of miRNA cluster in DLK-DIO3 genomic region in placental mammals. Mol Biol Evol 25 939–948. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Covarrubias V, Ghosh D, Lakhman SS, Pendyala L, and Blanco JG (2007) A functional genetic polymorphism on human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic activity and NADPH binding affinity. Drug Metab Dispos 35 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Covarrubias V, Kalabus JL, and Blanco JG (2008) Inhibition of polymorphic human carbonyl reductase 1 (CBR1) by the cardioprotectant flavonoid 7-monohydroxyethyl rutoside (monoHER). Pharm Res 25 1730–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow JM, Chinn LW, and Kroetz DL (2008) The effects of ABCB1 3′-untranslated region variants on mRNA stability. Drug Metab Dispos 36 10–15. [DOI] [PubMed] [Google Scholar]
- Grenier MA and Lipshultz SE (1998) Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol 25 72–85. [PubMed] [Google Scholar]
- He L and Hannon GJ (2004) MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5 522–531. [DOI] [PubMed] [Google Scholar]
- Joerger M, Huitema AD, Meenhorst PL, Schellens JH, and Beijnen JH (2005) Pharmacokinetics of low-dose doxorubicin and metabolites in patients with AIDS-related Kaposi sarcoma. Cancer Chemother Pharmacol 55 488–496. [DOI] [PubMed] [Google Scholar]
- Joerger M, Huitema AD, Richel DJ, Dittrich C, Pavlidis N, Briasoulis E, Vermorken JB, Strocchi E, Martoni A, Sorio R, et al. (2007) Population pharmacokinetics and pharmacodynamics of doxorubicin and cyclophosphamide in breast cancer patients: a study by the EORTC-PAMM-NDDG. Clin Pharmacokinet 46 1051–1068. [DOI] [PubMed] [Google Scholar]
- Kassner N, Huse K, Martin HJ, Gödtel-Armbrust U, Metzger A, Meineke I, Brockmöller J, Klein K, Zanger UM, Maser E, et al. (2008) Carbonyl reductase 1 is a predominant doxorubicin reductase in the human liver. Drug Metab Dispos 36 2113–2120. [DOI] [PubMed] [Google Scholar]
- Kishi S, Cheng C, French D, Pei D, Das S, Cook EH, Hijiya N, Rizzari C, Rosner GL, Frudakis T, et al. (2007) Ancestry and pharmacogenetics of antileukemic drug toxicity. Blood 109 4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, and Lipshultz SE (1997) Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 15 1544–1552. [DOI] [PubMed] [Google Scholar]
- Lakhman SS, Chen X, Gonzalez-Covarrubias V, Schuetz EG, and Blanco JG (2007) Functional characterization of the promoter of human carbonyl reductase 1 (CBR1). Role of XRE elements in mediating the induction of CBR1 by ligands of the aryl hydrocarbon receptor. Mol Pharmacol 72 734–743. [DOI] [PubMed] [Google Scholar]
- Lakhman SS, Ghosh D, and Blanco JG (2005) Functional significance of a natural allelic variant of human carbonyl reductase 3 (CBR3). Drug Metab Dispos 33 254–257. [DOI] [PubMed] [Google Scholar]
- Li J and Gwilt PR (2003) The effect of age on the early disposition of doxorubicin. Cancer Chemother Pharmacol 51 395–402. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Shintani S, and Hara A (2006) Multiplicity of mammalian reductases for xenobiotic carbonyl compounds. Drug Metab Pharmacokinet 21 1–18. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, and Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56 185–229. [DOI] [PubMed] [Google Scholar]
- Mishra PJ, Humeniuk R, Mishra PJ, Longo-Sorbello GS, Banerjee D, and Bertino JR (2007) A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc Natl Acad Sci U S A 104 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'connor T, Ireland LS, Harrison DJ, and Hayes JD (1999) Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem J 343 487–504. [PMC free article] [PubMed] [Google Scholar]
- Ohara H, Miyabe Y, Deyashiki Y, Matsuura K, and Hara A (1995) Reduction of drug ketones by dihydrodiol dehydrogenases, carbonyl reductase and aldehyde reductase of human liver. Biochem Pharmacol 50 221–227. [DOI] [PubMed] [Google Scholar]
- Olson LE, Bedja D, Alvey SJ, Cardounel AJ, Gabrielson KL, and Reeves RH (2003) Protection from doxorubicin-induced cardiac toxicity in mice with a null allele of carbonyl reductase 1. Cancer Res 63 6602–6606. [PubMed] [Google Scholar]
- Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, and Boucek RJ Jr (1988) Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A 85 3585–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosemond MJ and Walsh JS (2004) Human carbonyl reduction pathways and a strategy for their study in vitro. Drug Metab Rev 36 335–361. [DOI] [PubMed] [Google Scholar]
- Speth PA, van Hoesel QG, and Haanen C (1988) Clinical pharmacokinetics of doxorubicin. Clin Pharmacokinet 15 15–31. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G, Perini JA, Bastos-Rodrigues L, Pena SD, and Struchiner C (2007a) Impact of population admixture on the distribution of the CYP3A5*3 polymorphism. Pharmacogenomics 8 1299–1306. [DOI] [PubMed] [Google Scholar]
- Suarez-Kurtz G, Vargens DD, Struchiner CJ, Bastos-Rodrigues L, and Pena SD (2007b) Self-reported skin color, genomic ancestry and the distribution of GST polymorphisms. Pharmacogenet Genomics 17 765–771. [DOI] [PubMed] [Google Scholar]
- Tietz N (1999) Tietz Texbook of Clinical Chemistry, 3rd ed. (Burtis CA and Ashwood ER, series eds), W. B. Saunders Company, Philadelphia, PA.
- Wermuth B (1981) Purification and properties of an NADPH-dependent carbonyl reductase from human brain. Relationship to prostaglandin 9-ketoreductase and xenobiotic ketone reductase. J Biol Chem 256 1206–1213. [PubMed] [Google Scholar]
- Wermuth B, Bohren KM, and Ernst E (1993) Autocatalytic modification of human carbonyl reductase by 2-oxocarboxylic acids. FEBS Lett 335 151–154. [DOI] [PubMed] [Google Scholar]
- Wermuth B, Platts KL, Seidel A, and Oesch F (1986) Carbonyl reductase provides the enzymatic basis of quinone detoxication in man. Biochem Pharmacol 35 1277–1282. [DOI] [PubMed] [Google Scholar]
- Wilson JF, Weale ME, Smith AC, Gratrix F, Fletcher B, Thomas MG, Bradman N, and Goldstein DB (2001) Population genetic structure of variable drug response. Nat Genet 29 265–269. [DOI] [PubMed] [Google Scholar]
- Zamber CP, Lamba JK, Yasuda K, Farnum J, Thummel K, Schuetz JD, and Schuetz EG (2003) Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics 13 19–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.