Abstract
Studies were conducted to characterize the effect of dose and route of administration on the disposition of N-butylpyridinium chloride (NBuPy-Cl), an ionic liquid with solvent properties. Urine was the major route of NBuPy-Cl excretion after intravenous (5 mg/kg), single oral (0.5, 5, or 50 mg/kg), or repeated oral (50 mg/kg/day, 5 days) administration to male F-344 rats and single oral (50 mg/kg) administration to female B6C3F1 mice. Depending on the vehicle, absorption after dermal application (5 mg/kg, 125 μg/cm2) was 10 to 35% at 96 h. After the single intravenous dose, the blood concentration of NBuPy-Cl decreased in a biphasic manner with an elimination half-life of 2.2 h and a clearance of 7 ml/min. After single oral administration of NBuPy-Cl (50 mg/kg), maximum blood concentration was reached at 1.3 h, and the bioavailability was determined to be 47% at 6 h based on the blood toxicokinetics and 67% at 72 h based on urinary excretion. In all the urine and blood samples, only the parent compound was detected. Coadministration of NBuPy-Cl and inulin (by intravenous injection) revealed that the clearance of NBuPy-Cl exceeded the rat glomerular filtration rate. After incubation with Chinese hamster ovary cells expressing human organic cation transporter 2 (hOCT2), NBuPy-Cl was transported effectively (Kt = 18 μM), and also a potent inhibitor of hOCT2 mediated tetraethylammonium transport (IC50 = 2.3 μM). In summary, NBuPy-Cl is partially absorbed from the gastrointestinal tract and eliminated rapidly in the urine as parent compound most likely by renal glomerular filtration and OCT2-mediated secretion.
N-Butylpyridinium chloride (NBuPy-Cl) belongs to a vast list of chemicals known as ionic liquids (ILs). They represent a class of salts that are liquids at room temperature and are typically composed of various nitrogen-containing organic cations and variable inorganic or organic anions. Because of the diversity of the cation and anion constituents, Baker et al. (2005) have estimated that up to 10 trillion combinations are theoretically possible. However, the commonly used cations for ILs are based on an imidazolium, pyridinium, or pyrrolidinium moiety with alkyl chain substituents, whereas the anions include hexafluorophosphate, tetrafluoroborate, nitrate, or chloride (Zhao et al., 2007). Research on ILs has expanded rapidly because of their potential industrial application to replace or reduce the reliance on the commonly used volatile organic solvents (Rogers and Seddon, 2003; Baker et al., 2005). ILs have excellent properties that include nonvolatility, low melting point, thermal stability, high ionic conductivity, high viscosity, and large electrochemical windows (Endres and Zein El Abedin, 2006). In addition, depending on the chemical nature of the cation/anion, ILs with specific characteristics can be formulated.
ILs are considered to be “green chemicals” and environmentally benign because of their much lower vapor pressure than conventional organic solvents (Welton, 1999; Earle and Seddon, 2000). However, increased use of these compounds in large-scale industrial applications could lead to environmental contamination through accidental spills, effluents, or geologic adsorption. Many ILs could be transported unimpeded through the underground water because of their miscibility with water (Couling et al., 2005). Ecotoxicological studies have been conducted in a variety of microorganisms, including algae, diatoms, water fleas (Daphnia magna), freshwater snails (Physa acuta), and marine bacteria (Vibrio fischeri), to assess the potential impact of ILs on the aquatic environment (Ranke et al., 2004; Bernot et al., 2005a,b; Docherty and Kulpa, 2005; Latala et al., 2005). In general, these studies show that toxicity is determined mainly by the cation, not by varying the anion, and that toxicity increases with increasing length of the alkyl chains. In addition, several studies have shown the antimicrobial activity of ILs against various strains of bacteria and fungi (Pernak et al., 2003; Docherty and Kulpa, 2005). In the few studies that compared the toxicity of imidazolium and pyridinium compounds, neither cation type was consistently more toxic than the other (Bernot et al., 2005a,b; Docherty and Kulpa, 2005). However, an enzyme inhibition study showed that purified acetylcholinesterase could be inhibited by imidazolium and pyridinium ionic liquids with EC50 values as low as 13 μM; greater inhibition of acetylcholinesterase activity was observed with pyridinium cations than with imidazolium cations (Stock et al., 2004).
Likewise, little disposition, metabolism, and/or toxicity information is available on ILs in mammalian systems. Ranke et al. (2004) investigated the toxicity of imidazolium ionic liquids on leukemia and glioma rat cell lines and found these ILs were more toxic than conventional solvents, such as acetone and acetonitrile. One IL, 1-butyl-3-methylimidazolium chloride (Bmim-Cl), has been shown to cause dermal irritation when applied topically to rats but produced only minimal contact sensitization when evaluated in the mouse local lymph node assay (Landry et al., 2005). Systemic toxicity was also observed in these animals when Bmim-Cl was applied in a hydrophobic vehicle. In a study by Landry et al. (2005), the acute single oral dose LD50 of Bmim-Cl was reported to be 550 mg/kg.
Three ILs, Bmim-Cl, NBuPy-Cl, and 1-butyl-1-methylpyrrolidinium chloride, were nominated to the National Toxicology Program for toxicological testing because they are representative of the most common cation classes of ILs and are the starting materials for many other ILs. Disposition and toxicokinetic studies were conducted on these compounds to determine the extent of systemic bioavailability, metabolism, distribution, and elimination in rats and mice. We recently reported that systemically available Bmim-Cl was cleared readily from the blood by renal processes and eliminated in the urine as parent compound in rats and mice (Sipes et al., 2008). Because of the rapid renal clearance and the cationic nature of Bmim-Cl, it was speculated that it may be a substrate for organic cation transporters (OCTs). The studies reported here were designed to determine whether NBuPy-Cl had a toxicokinetic profile similar to Bmim-Cl, and to examine directly whether it was a substrate for and/or an inhibitor of OCT2.
Materials and Methods
Chemicals. [14C]NBuPy-Cl in water (27.5 mCi/mmol, 98% purity; Fig. 1) was received from RTI International (Research Triangle Park, NC). [3H]Inulin in water (250 mCi/mmol, >99% purity after dialysis) was purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA), and [3H]tetraethylammonium trifluoroacetate (TEA, 54 Ci/mmol) in aqueous solution containing 10% ethanol was synthesized by GE Healthcare (Little Chalfont, Buckinghamshire, UK). NBuPy-Cl (98% purity) was obtained from Merck KGaA (Darmstadt, Germany). TEA (98% purity) was purchased from Sigma-Aldrich (St. Louis, MO). Dimethylformamide (DMF) was purchased from J. T. Baker (Phillipsburg, NJ). Soluene-350, Solvable, and Pico-Flour scintillation mixture solution were obtained from PerkinElmer Life and Analytical Sciences. All the reagents used for animal studies were analytical grade. Ham's F12 medium, fetal bovine serum, hygromycin B, and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). All the other chemicals used for in vitro studies were purchased from Sigma-Aldrich.
Fig. 1.
Chemical structure of NBuPy-Cl (CAS 1124-64-7). Asterisk indicates the location of the radiolabeled 14C.
Animal Studies. Animals. Nonsurgical (conventional) or in-dwelling jugular vein–cannulated (JVC) male Fischer-344 rats (8–9 weeks of age, 161–220 g) and conventional female B6C3F1 mice (8–10 weeks old, 17–20 g) were purchased from Harlan (Indianapolis, IN). Animals were housed in the Association for Assessment and Accreditation for Laboratory Animal Care-accredited University of Arizona Animal Care Facility with controlled temperature (25°C), humidity (40–60%), and light/dark cycle (12 h). Conventional rats and mice were acclimated for 5 to 7 days and JVC rats for 1 day before experiments with food (NTP 2000; Harlan Teklad, Madison, WI) and water ad libitum. For the single oral and intravenous dose studies, animals were fasted 12 h before administration. Food was returned 2 h after dosing. Animals were not fasted for the dermal and repeated oral dose studies.
Animal dosing. To assess the effect of dose and route on excretion, NBuPy-Cl was administered intravenously (5 mg/kg, 50 μCi/kg) or orally (0.5, 5, or 50 mg/kg, 50 μCi/kg) to male F-344 rats and orally (50 mg/kg, 50 μCi/kg) to female B6C3F1 mice. In the repeat dose study, NBuPy-Cl was administered orally to male F-344 rats 50 mg/kg/day, 50 μCi/kg/day for 1 day or 5 days. For the dermal application studies, rats were shaved at the dorsal back, and a metal trap was adhered to the skin as described by Winter and Sipes (1993). The dose (5 mg/kg, 100 μCi/kg, 125 μg/cm2) was applied to the skin within this circumscribed area in vehicles of DMF/water (55:45, v/v), ethanol/water (55:45, v/v), or water. For the NBuPy-Cl toxicokinetic studies, JVC rats were dosed once with NBuPy-Cl intravenously (5 mg/kg, 100 μCi/kg) or orally (50 mg/kg, 100 μCi/kg). In another study, NBuPy-Cl (5 mg/kg, 50 μCi/kg) and inulin (0.67 mg/kg, 250 μCi/kg) were coadministered intravenously to JVC rats. Saline was used as the dosing vehicle in all the cases except dermal application.
Sample collection and analysis. Samples were collected and analyzed as described by Sipes et al. (2008). In brief, animals were placed into Nalgene metabolism cages (Nalge Nunc International, Rochester, NY) after dosing. Urine and feces were collected up to 48 h (intravenous), 72 h (single oral), 96 h (dermal), or 120 h (repeated oral). After each collection, the cage was rinsed with around 15 ml of water. This cage rinse solution was counted independently but ultimately added to 14C recovered in the urine. For NBuPy-Cl toxicokinetic studies, 300 μl of blood was collected via the JVC at 7.5, 15, 30, 60, 90, 180, 270, 360, 720, and 1440 min. For the coadministration of NBuPy-Cl and inulin, 300 μl of blood was collected at 7.5, 15, 30, 60, 90, 120, 180, 240, 300, 360, and 720 min. Aliquots of blood removed via the JVC were replaced with an equal volume of saline. At the terminus of each study, animals were euthanized by CO2 inhalation, and various tissues were collected.
Aliquots of urine (100–1000 μl) and cage rinse (1000 μl) were counted directly in triplicate using a Beckman liquid scintillation counter (LSC). In addition, the urine samples were diluted with water (1:1) and filtered through a 0.45-μm Whatman (Maidstone, UK) nylon syringe filter. The filtrates were then subjected to high-pressure liquid chromatography (HPLC) analysis to separate parent compound from any metabolites. The HPLC procedure is identical to that reported for Bmim-Cl (Sipes et al., 2008). In brief, the filtrates were applied to a C18 guard column (4.0 × 3.0 mm) connected to a C18 reverse-phase column (250 × 4.6 mm). The mobile phase consisted of acidified (0.1 M trifluoroacetic acid) acetonitrile and water at the flow rate of 1 ml/min for 35 min. Eluting peaks were detected by an INUS (Tampa, FL) radiometric detector. In addition, urine samples obtained from rats administered nonradiolabeled NBuPy-Cl were subjected to liquid chromatography/mass spectrometry to identify the NBuPy-Cl-derived peak appearing in the urine. Analytes were ionized using an electrospray ionization source and dried with nitrogen (10 ml/min) at 350°C, nebulizer pressure of 50 psi, and capillary and endplate currents of 17 and 650 nA, respectively. Tandem mass spectrometry fragmentation was performed at m/z = 4.0 isolation width at 1-V fragmentation amplitude (Sipes et al., 2008).
Fecal samples were homogenized with an equal amount of water, and triplicate aliquots (100–200 mg) were solubilized with Soluene-350 (2 ml). Aliquots of blood (100 μl) and tissue (100–200 mg) samples were solubilized with Solvable (2 ml). After solubilization, beaching with 0.2 ml of H2O2 (30%), and addition of Pico-Fluor (15 ml), samples (feces, blood, and tissue) were stored in dark for 48 h before LSC. In the dermal application study, radioactivity on the skin surface at the dosing site was removed by washing and tape stripping as described in detail by Sipes et al. (2008). This recovered 14C was considered separately from the radiolabel absorbed into the skin at this site. Blood samples (150 μl) collected from the NBuPy-Cl toxicokinetic studies were extracted three times with acetonitrile (750 μl), and the extracts were combined. After evaporation of solvents, the samples were reconstituted to 150 μl in water/acetonitrile (93:7, v/v). Then 75 μl was analyzed by radio-HPLC as described for urine sample analysis. Fractions of eluent collected at 1-min interval were quantified by LSC. The peak that coeluted with [14C]NBuPy-Cl standard was analyzed. For the NBuPy-Cl and inulin coadministration study, blood plasma samples (100 μl) were collected and quantified by LSC directly.
NBuPy-Cl Blood Binding Assay. Heparinized blood from male F-344 rats was incubated with [14C]NBuPy-Cl (0.015, 0.15, and 1.5 mg/ml blood) for 30 min at 37°C. After incubation, blood cells were separated from the plasma by centrifugation (GPR centrifuge; Beckman Coulter, Fullerton, CA) at 750g for 15 min. Blood cells were washed with saline three times. After each wash, the samples were centrifuged at 750g for 15 min, and the supernatant (saline solution) was collected and counted separately. This 14C was considered part of the plasma fraction. Plasma proteins were removed by centrifugal filtration (14,000g for 30 min) through a filter device (YM-10; Millipore Corporation, Billerica, MA) (molecular weight cutoff, 5000). Unbound [14C]NBuPy-Cl was recovered in the filtrate. Radioactivity in saline washes, plasma, and plasma proteins was counted directly by LSC. The washed blood cells were solubilized and counted as described in the sample analysis section.
In Vitro Transport Studies. To determine the transport of TEA or NBuPy-Cl by human organic cation transporter 2 (hOCT2), Chinese hamster ovary (CHO) cells and CHO cells with stably expressed hOCT2 (CHO_hOCT2) were incubated with [3H]TEA (15 nM) or [14C]NBuPy-Cl (18 μM) and increasing concentrations of unlabeled TEA (0–1000 μM) or NBuPy-Cl (0–750 μM), respectively. For characterization of the inhibitory effect of NBuPy-Cl on hOCT2, transport of [3H]TEA (15 nM) in the CHO_hOCT2 cells was measured in the presence of various concentrations of unlabeled NBuPy-Cl (0–100 μM).
CHO cells and CHO_hOCT2 cells were maintained in Ham's F12 medium with fetal bovine serum (10%, v/v), penicillin (100 unit/ml), streptomycin (100 μg/ml), and hygromycin B (100 μg/ml, only for CHO_hOCT2 cells) (Pelis et al., 2006). For the transport assay, cells in 12-well cell culture plates (550,000 cells/ml/well) were maintained in the incubator at 37°C for 24 h. The medium was then aspirated carefully before the experiment, and the cells were immediately rinsed twice with 1 ml of Waymouth's buffer (WB, in mM: 135 NaCl, 13 HEPES-NaOH, 28 d-glucose, 5 KCl, 1.2 MgCl2, 2.5 CaCl2, and 0.8 MgSO4, pH 7.4) at room temperature. To initiate the reaction, TEA and/or NBuPy-Cl were added to the cells in 0.4 ml of WB. After 30 s, the incubation solution was removed, and the cells were rinsed three times with 1 ml of ice-cold WB. The cells were then solubilized for 30 min by adding 0.4 ml of NaOH (0.5 M) with 1% SDS. After neutralization by addition of HCl (0.2 ml, 1 M), 0.5 ml of the resulting cell lysate solution was counted by the LSC.
Data Analyses. Data are presented as percentage of dose recovered or as nanomole per gram, nanomole per milliliter, and nanogram per milliliter. The latter were determined by the specific activity of appropriate dosing solution (dpm/nmol or dpm/ng). Only samples above the limit of quantification for LSC (dpm 132) were used for data analysis (Sipes et al., 2008). WinNonlin software (version 5.1; Pharsight, Mountain View, CA) was used to define the concentration time curve using a two-compartment (intravenous) or one-compartment (oral) toxicokinetic model. For the transport studies, apparent Michaelis-Menten constant (Kt) values were determined by the modified Michaelis-Menten eq. 1 (Malo and Berteloot, 1991; Pelis et al., 2006). Estimates of the IC50 for inhibition of [3H]TEA transport used eq. 2, a modification of eq. 1. It presumes that the interaction of NBuPy-Cl and TEA with hOCT2 involves competitive inhibition, i.e., IC50 approximates the Ki for inhibition, an assumption consistent with the observation that NBuPy-Cl proved to be a transported substrate of hOCT2. The numerical data were analyzed using Student's t test or one-way analysis of variance with the Tukey's post-test by GraphPad Software Inc. (San Diego, CA) Prism 4 Statistics Program. A level of p < 0.05 was considered significant. Individual data points are presented as mean ± S.E.M.
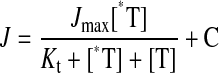 |
(1) |
where J is the measured rate of radiolabeled compound transported from a concentration of labeled substrate equal to [*T]; Jmax is the maximum rate of transport; Kt is the concentration that results in half-maximal transport; [T] is the concentration of unlabeled substrate; and C is a constant representing the component of total uptake that is not saturable over the concentration range tested. This nonsaturable component probably reflects the combined influence of diffusive flux, nonspecific binding, and/or incomplete rinsing of the cell layer.
 |
(2) |
where Jmapp is the Jmax for TEA transport times the ratio of Ki/Kt, and IC50 is equal to Ki(1 + [T*/Kt]) (Groves et al., 1994).
Results
Disposition Studies. Intravenous administration. After a single intravenous dose (5 mg/kg), NBuPy-Cl was rapidly excreted in the urine of male F-344 rats (Fig. 2A). Greater than 80% of the administered radioactivity was recovered from the urine by 12 h. By 48 h, 97% of the dose was recovered in urine (93%) and feces (4%). Radioactivity detected in the blood samples at 48 h was negligible.
Fig. 2.
Cumulative excretion of radioactivity after a single administration of [14C]NBuPy-Cl (♦ total, ▾ urine, • feces). Data are expressed as mean ± S.E.M. A, intravenous administration (5 mg/kg) to male F-344 rats (n = 7); B, oral administration (50 mg/kg) to male F-344 rats (n = 8); C, oral administration (50 mg/kg) to female B6C3F1 mice (n = 6).
Oral administration. To determine the effect of dose on the disposition of NBuPy-Cl, a single bolus dose providing 0.5, 5, or 50 mg/kg NBuPy-Cl was administered to male F-344 rats by oral gavage. NBuPy-Cl (50 mg/kg) was well absorbed and eliminated rapidly from the body (Fig. 2B). Dose did not affect the rate or route of excretion of 14C equivalents (Table 1). For those three doses, 83 to 88% of the administered radioactivity was recovered within 24 h with 55 to 66% in urine and 20 to 25% in feces. By 72 h, 87 to 93% of the dose was excreted from the body, of which 62 to 68% was in the urine. The total 14C remaining in the liver, kidneys, and lung at 72 h was less than 0.01% of dose in the animals administered with 50 mg/kg NBuPy-Cl.
TABLE 1.
Dose recovered (%) in 72 h for male F-344 rats (R) and in 96 h for female B6C3F1 mice (M) after a single oral administration of [14C]NBuPy-Cl
Data are presented as mean ± S.E.M.
|
Dose Recovered
|
||||
|---|---|---|---|---|
| 0.5 mg/kgR, n = 4 | 5 mg/kgR, n = 4 | 50 mg/kgR, n = 8 | 50 mg/kgM, n = 6 | |
| % | % | % | % | |
| Feces | 25.0 ± 4.8 | 21.2 ± 2.7 | 25.9 ± 2.2 | 10.3 ± 1.2* |
| Urine | 62.3 ± 6.0 | 68.0 ± 2.5 | 66.7 ± 1.9 | 71.6 ± 5.0 |
| Blood | <0.05 | <0.05 | <0.05 | <0.05 |
| Total recovery | 87.3 ± 7.4 | 89.3 ± 3.8 | 92.5 ± 2.8 | 81.9 ± 4.3 |
p < 0.05 compared with rats dosed with 50 mg/kg NBuPy-Cl.
NBuPy-Cl (50 mg/kg) was orally administered to female B6C3F1 mice to characterize the disposition of NBuPy-Cl in a different species. At 96 h, 82% of the dosed NBuPy-Cl was found in the urine (72%) and feces (10%) as shown in Table 1. The urinary elimination profile was similar to that observed in rats, but the fecal recovery was lower (Fig. 2C). No urine was collected at 6 h. The recovery at this time point was obtained from 14C present in the cage rinse. The lower recovery of fecal samples observed in mice (compared with that of rats) was also reported for Bmim-Cl and related to some contamination of feed with feces (Sipes et al., 2008). The total amount of radioactivity detected in all the tissues after 96 h in mice was less of 0.2% of dosed [14C]NBuPy-Cl.
After daily oral administration of NBuPy-Cl (50 mg/kg/day) for 5 days, the elimination profile of NBuPy-Cl was not altered in male F-344 rats (Fig. 3). Essentially, 93 to 99% of each dose was eliminated within 24 h after its administration, with 51 to 59% appearing in the urine. At 24 h after the fifth dose, 97% of the total administered dose had been excreted (Table 2). At this time, analyzed tissues contained less than 1% of the total dose. Concentration of [14C]NBuPy-Cl equivalents in tissues ranged from 1.5 to 21 nmol/g (Table 3).
Fig. 3.
Cumulative excretion of radioactivity after serial daily oral administration of [14C]NBuPy-Cl (50 mg/kg/day) to male F-344 rats. Data are expressed as mean ± S.E.M.; n = 4 per dosing group. Open symbols, 1 dose; closed symbols, 1 dose/day for 5 days; ⋄/♦, total; ▿/▾, urine; ○/•, feces; stepwise line, total cumulative dose.
TABLE 2.
Dose recovered (%) at 24 h after final dose following a single or five serial daily oral administrations of [14C]NBuPy-Cl (50 mg/kg/day) to male F-344 rats
Data are presented as mean ± S.E.M.
|
Dose Recovered
|
||
|---|---|---|
| One Administration, n = 4 | Five Administrations, n = 4 | |
| % | % | |
| Feces | 45.5 ± 1.7 | 41.4 ± 1.0 |
| Urine | 43.6 ± 2.0 | 54.0 ± 2.2 |
| Blood | <0.05 | <0.05 |
| Tissues | 2.4 ± 0.1 | 0.9 ± 0.1 |
| Gastrointestinal contents | 1.8 ± 0.3 | 0.3 ± 0.1 |
| Total recovery | 93.3 ± 0.4 | 96.6 ± 0.7 |
TABLE 3.
Concentration in nanomoles per gram of [14C]NBuPy-Cl equivalents in selected tissues (except blood, which was given in nmol/ml) at 24 h after a single oral administration or 24 h after the fifth daily oral administration of [14C]NBuPy-Cl (50 mg/kg/day) to male F-344 rats
Data are presented as mean ± S.E.M.
|
NBuPy-Cl
|
||
|---|---|---|
| One Administration, n = 4 | Five Administrations, n = 4 | |
| nmol/g | nmol/g | |
| Adipose | 3.6 ± 0.7 | 3.5 ± 0.4 |
| Blood (nmol/ml) | 0.8 ± 0.1 | 3.2 ± 0.1* |
| Brain | 0.5 ± 0.1 | 1.5 ± 0.3* |
| Gastrointestinal tract tissues | 10.0 ± 1.0 | 20.9 ± 3.5* |
| Kidney | 3.5 ± 0.3 | 7.7 ± 0.2* |
| Liver | 5.1 ± 0.3 | 10.7 ± 0.2* |
| Muscle | 10.1 ± 0.4 | 17.9 ± 1.1* |
| Skin | 4.7 ± 0.8 | 10.5 ± 0.6* |
p < 0.05 compared with single dose.
Dermal administration. NBuPy-Cl showed variable percutaneous absorption after dermal application in various vehicles as assessed by excretion in the urine and feces (Fig. 4A). Total absorption (presence of 14C in urine, feces, and tissues) was greatest (35% of dose) after 96 h when NBuPy-Cl (5 mg/kg) was applied in a vehicle of DMF/water (55:45, v/v), and less (16 and 10% of dose) when applied in vehicles of ethanol/water (55:45, v/v) or water, respectively (Fig. 4B). The majority of dosed NBuPy-Cl was not absorbed. It was recovered in skin washes, skin strips, and the dosing trap. No visible irritation was observed at this dose (5 mg/kg, 125 μg/cm2) in any of the animals over the course of the experiment.
Fig. 4.
Dermal application of [14C]NBuPy-Cl (5 mg/kg) to male F-344 rats in three different vehicles. DMF, DMF/water vehicle (55:45, v/v); EtOH, ethanol/water vehicle (55:45, v/v); water, water vehicle. Data are expressed as mean ± S.E.M.; n = 3 per dosing group. A, cumulative excretion of radioactivity in urine and feces over 96 h. B, absorbed (14C recovered from urine, feces, and tissues) and unabsorbed (14C recovered from skin wash, skin stripping, and trap) [14C]NBuPy-Cl at 96 h.
Chemical identification. Analysis of urine samples by radio-HPLC revealed the presence of one predominant peak (retention time 14 min) in both rats and mice after NBuPy-Cl (50 mg/kg) oral administration (Fig. 5B). This peak coeluted with the [14C]NBuPy-Cl standard (Fig. 5A). This pattern was also observed in urine samples collected at later time points (24, 36, 48, and 72 h). Several minor peaks noted only in 6-h urine samples of rats were also observed in the dosing solution. The only exception was the new, small peak at a retention time of 12 min. However, it accounted for less than 1% of the total [14C]NBuPy-Cl administered to the animals. This same radiochromatographic profile was observed in urine of rats after intravenous, single oral (5 or 0.5 mg/kg), repeated oral, and dermal administrations of [14C]NBuPy-Cl. Liquid chromatography/mass spectrometry analysis of urine samples from rats dosed with unlabeled NBuPy-Cl (50 mg/kg) confirmed the molecular mass of the major peak to be NBuPy+ (m/z = 136.2). Collision-induced fragmentation of NBuPy+ resulted in a molecular mass of m/z 80.2 (pyridinium) after loss of m/z 56 (butyl group).
Fig. 5.
Representative radiochromatograms of urine and blood samples. A, [14C]NBuPy-Cl standard. B, urine samples from male F-344 rats at 6 h (dash-dot line) and female B6C3F1 mice at 12 h (solid line) after a single oral administration of [14C]NBuPy-Cl (50 mg/kg). C, blood samples at 7.5 min after a single intravenous administration of [14C]NBuPy-Cl (5 mg/kg, dash-dot line) and at 90 min after a single oral administration of [14C]NBuPy-Cl (50 mg/kg, solid line) to male F-344 rats.
Toxicokinetics of NBuPy-Cl. After a single intravenous administration (5 mg/kg), the blood concentration of NBuPy-Cl decreased in a biphasic manner and fit a two-compartment model with a distribution half-life (t1/2α) of 0.22 h (Vss 789 ml) and an elimination half-life (t1/2β) of 2.2 h (Fig. 6). Kinetic parameters are listed in Table 4. After oral administration, NBuPy-Cl (50 mg/kg) was absorbed into the systemic blood and reached its maximum concentration (2.6 μg/ml) at 1.3 h. The oral systemic bioavailability at 6 h was determined to be 47% based on area under the curve (AUC)oral/AUCintravenous and adjusted for dose (Shargel and Yu, 1993). Radio-HPLC analysis of the blood extracts after both intravenous and oral administration showed only one peak, which coeluted with [14C]NBuPy-Cl standard (Fig. 5C).
Fig. 6.
Time course of NBuPy-Cl in blood after intravenous (5 mg/kg, n = 7, closed circle) and oral (50 mg/kg, n = 8, open circle) administration of [14C]NBuPyCl to male F-344 rats. Data are expressed as mean ± S.E.M. Predicted values (dash lines) were calculated based on best-fit analyses using two-compartment model fit for intravenous and one-compartment model fit for oral.
TABLE 4.
Kinetic parameters of NBuPy-Cl and inulin in male F-344 rats after intravenous administration
| AUC | t1/2α | t1/2β | CL | Vss | |
|---|---|---|---|---|---|
| μg · min/ml | h | h | ml/min | ml | |
| NBuPy-ClS | 119 | 0.22 | 2.2 | 7.0 | 789 |
| NBuPy-ClC | 121 | 0.21 | 2.7 | 8.5 | 972 |
| InulinC | 97 | 0.28 | 3.4 | 2.8 | 148 |
S, kinetics in blood following a single dose of [14C]NBuPy-Cl (5 mg/kg, 100 μCi/kg); C, kinetics in plasma following coadministration of [14C]NBuPy-Cl (5 mg/kg, 50 μCi/kg) and [3H]inulin (0.67 mg/kg, 250 μCi/kg); CL, systemic clearance.
Characterization of NBuPy-Cl Blood Binding. After 30-min incubation of [14C]NBuPy-Cl with rat blood, around 25% of the applied radioactivity was found to be associated with the blood cells in all three tested NBuPy-Cl concentrations. The other 75% of radioactivity was recovered in the plasma. Negligible amounts of radioactivity (<1% of total radioactivity) were associated with plasma proteins. Therefore, NBuPy-Cl is not bound to the plasma proteins but seems to be partially associated with blood cells.
Kinetics of Intravenous Coadministration of NBuPy-Cl and Inulin. To determine the relationship of NBuPy-Cl urinary excretion to the glomerular filtration rate, a dosing solution containing both [14C]NBuPy-Cl (5 mg/kg, 50 μCi/kg) and [3H]inulin (0.67 mg/kg, 250 μCi/kg) was administered intravenously to male F-344 JVC rats. The toxicokinetic profile of NBuPy-Cl in the presence of inulin was similar to that obtained when NBuPy-Cl was administered alone (Figs. 6 and 7A). Similar AUC, t1/2α, and t1/2β were observed between NBuPy-Cl and inulin, but NBuPy-Cl is distributed more widely than that of inulin by 6-fold (Table 4). The systemic clearance of NBuPy-Cl exceeded that of inulin by 3-fold.
Fig. 7.
Time course of NBuPy-Cl (5 mg/kg; A) and inulin (0.67 mg/kg; B) in blood plasma after intravenous coadministration of [14C]NBuPy-Cl and [3H]inulin to male F-344 rats. Data are expressed as mean ± S.E.M.; n = 4. Predicted values (dash lines) were calculated based on best-fit analyses using two-compartment model fit.
hOCT2-Mediated TEA and NBuPy-Cl Transport. The transport of TEA, a model substrate for hOCT2, and NBuPy-Cl was investigated in CHO cells and CHO_hOCT2 cells. In CHO cells, increasing concentration of unlabeled TEA (0–1000 μM) did not affect the intracellular level of [3H]TEA (Fig. 8A). However, in CHO_hOCT2 cells, the intracellular accumulation of [3H]TEA was decreased dramatically by increasing concentrations of unlabeled TEA (Fig. 8A). The Kt of hOCT2-mediated TEA transport was 27.1 ± 7.3 μM (mean ± S.E.M., n = 3). Transport profiles of [14C]NBuPy-Cl were similar to those of TEA. Only in the CHO_hOCT2 cells was transport of NBuPy-Cl observed. The Kt was determined to be 17.6 ± 3.0 μM (mean ± S.E.M., n = 3) (Fig. 8B).
Fig. 8.
Kinetics of TEA (A) and NBuPy-Cl (B) transport in the CHO cells (○) and CHO_hOCT2 cells (•) and inhibition of TEA transport by NBuPy-Cl (C) in CHO_hOCT2 cells (•). A, Kt(TEA) = 27.1 ± 7.3 μM. B, Kt(NBuPy-Cl) = 17.6 ± 3.0 μM. C, IC50(NBuPy-Cl) = 2.3 ± 0.6 μM. Data are expressed as mean ± S.E.M.; n = 3.
To determine whether NBuPy-Cl could act as an inhibitor of OCT2, increasing concentrations of NBuPy-Cl (0–100 μM) were coincubated with [3H]TEA (15 nM) in the CHO_hOCT2 cells. As shown in Fig. 8C, the presence of NBuPy-Cl decreased the intracellular uptake of [3H]TEA in a concentration-dependent manner. An IC50 value of 2.3 ± 0.6 μM (mean ± S.E.M., n = 3) was obtained.
Discussion
ILs are being increasingly examined in industrial and laboratory chemical processes such as synthesis, catalysis, and enzymatic biocatalysis, electrolysis, and extraction. ILs are also expected to be applied in consumer products, including batteries, photoelectrochemical devices, fuel cells for automotive consumption, and surgical implants (Freemantle, 2000). Therefore, the potential for exposure will increase with increased use of ILs. Because of the low volatility of these agents, exposure would most likely occur orally and/or dermally. Thus, it is important to understand how ILs are absorbed and cleared from the systemic circulation. In a recent article we reported that in rodents the IL, Bmim-Cl, was partially absorbed after oral or dermal administration but readily eliminated as parent compound in the urine. The studies here examine the absorption and tissue retention of another IL, NBuPy-Cl, and provide evidence for an important role of OCT2 in its urinary elimination.
NBuPy-Cl was absorbed from the gastrointestinal tract after oral administration. The oral systemic bioavailability of NBuPy-Cl (50 mg/kg) in rats was calculated to be 47% at 6 h based on the blood AUCoral/AUCintravenous. This is in good agreement with the amount of radiolabel excreted in the urine of rats in 6 h (47 ± 2% of dose) (Fig. 2B). However, the overall bioavailability is greater than this based on the urinary excretion (67% of dose) over 72 h. Blood at times after 6 h could not be used to determine the AUC because they had 14C levels that were above the limit of detection but below the limit of quantification. This extra 20% most likely represents additional NBuPy-Cl absorption from intestine, as well as dose that had distributed to the tissues. Because only a small amount of radiolabel appeared in the feces after intravenous administration of NBuPy-Cl (<5% of dose), it can be concluded that the 22 to 26% of the dose excreted in the feces after oral administration represents unabsorbed compound. Similar results were observed for urinary elimination of NBuPy-Cl administered orally to female mice. Fecal excretion was lower in mice, but this may be because of poor recovery of fecal materials (Sipes et al., 2008).
Over the dose range studied, the magnitude of a single dose did not affect the rate or route of NBuPy-Cl excretion. Repeated daily dosing also had a minimal, if any, effect on the elimination. It seems that nearly the entire daily dose was eliminated within each 24-h interval as the parent compound. At 24 h after the fifth dose, the concentration of NBuPy-Cl increased 2- to 4-fold in most tissues, which reflects, in part, the higher NBuPy-Cl concentration in the blood. However, this does not have a significant influence on the disposition of NBuPy-Cl because the total radioactivity recovered in all the tissues represents approximately 1% of the administered dose. In the particular group of rats used in the repeated dose study, fecal excretion was approximately 20% higher than the other studies (Tables 1 and 2). This occurred in the animals receiving one dose and in those receiving five doses. Because these animals were not fasted, the presence of food most likely reduced absorption.
The extent of NBuPy-Cl absorption after dermal application was affected by the nature of the vehicle. This is similar to observations with Bmim-Cl (Landry et al., 2005; Sipes et al., 2008). With a more hydrophobic vehicle (DMF), NBuPy-Cl was absorbed more extensively. The fast evaporation of ethanol from the ethanol/water vehicle may explain why no significant difference on dermal absorption was observed between it and water. Although the increased urinary elimination observed after 48 h reflects the continued absorption of dose that had penetrated the skin, why the rate increased is not clear. It may be related to NBuPy-Cl remodeling the barrier function of the skin because irritation (redness and slight edema) was observed at the dosing site when a higher dose of NBuPy-Cl (50 mg/kg, 1250 μg/cm2) was applied in water and ethanol vehicle (data not shown).
As observed with Bmim-Cl, no evidence of metabolism was noted for the systemically available NBuPy-Cl. As stated above, NBuPy-Cl was eliminated primarily in the urine as the parent compound in both rats and mice, independent of dose and route of administration. In addition, only NBuPy-Cl was detected in the blood after intravenous or oral administration. The small peak that appeared in the urine (retention time of 12 min) might be a metabolite of a contaminant present in the dosing solution. It was only observed in the 6-h urine samples and accounted for less than 1% of the total dose.
The blood toxicokinetic parameters obtained for NBuPy-Cl after intravenous administration are very similar to those obtained for Bmim-Cl (clearance from blood was 7.4 ml/min for Bmim-Cl and 7.0 ml/min for NBuPy-Cl). NBuPy-Cl was eliminated from the blood in a biphasic manner with t1/2β > t1/2α. The terminal elimination half-life most likely represents the compound that had distributed to various tissues. There it may have undergone ionic interactions with tissue constituents. In support of this are its large volume of distribution and the retention of 14C in the sedimented cellular fraction of blood incubated in vitro with [14C]NBuPy-Cl. It was also observed that the systemic clearance of NBuPy-Cl exceeded that of inulin, a compound that is only eliminated by glomerular filtration. Because more than 95% of the NBuPy-Cl was excreted in the urine after intravenous administration and its clearance exceeded that of inulin, and thus glomerular filtration rate, NBuPy-Cl appears to be excreted by renal secretion.
Based on physiological (rapid renal clearance) and chemical (organic cation) factors, we speculated that NBuPy-Cl may be a substrate for OCTs, a suggestion consistent with the observation that many cationic pyridinium compounds, including 1-methyl-4-phenylpyridinium and paraquat, are substrates for OCTs (Chen et al., 2007; Koepsell et al., 2007). When NBuPy-Cl was incubated with CHO cells expressing hOCT2, it was transported effectively into the cells. Such uptake was not observed in CHO cells not expressing hOCT2. Kinetic analysis revealed that NBuPy-Cl has kinetic properties similar to those of the model OCT2 substrate, TEA. This suggests that the urinary elimination of NBuPy-Cl behaves similarly to TEA, which is secreted by OCT2 in the kidney (Jonker et al., 2003). NBuPy-Cl was also found to be a potent inhibitor of TEA transport by hOCT2. Its IC50 value (2.3 μM) is similar to that observed for the high affinity OCT2 inhibitors, 1-methyl-4-phenylpyridinium and tetrapentylammonium (2 and 10 μM, respectively) (Suhre et al., 2005).
OCTs belong to the solute carrier superfamily 22A and transport a wide array of organic cations and positively charged weak bases (Wright and Dantzler, 2004). OCT1 and OCT2 are the predominate OCT homologs expressed in the basolateral membrane of rodent kidney, and both play a key role in the transport of monovalent, hydrophilic cations with molecular mass less than 400 (Okuda et al., 1996; Slitt et al., 2002; Jonker et al., 2003; Suhre et al., 2005). The observed renal secretion of NBuPy-Cl in the rat argues a fortiori that this compound enters rat renal proximal tubule cells and therefore is likely to be transported by OCT1, OCT2, or both. In the human, OCT2 seems to be the predominant transporter in the entry step in the renal organic cation secretion (Motohashi et al., 2002). Consequently, the high affinity interaction of NBuPy-Cl with hOCT2 supports the hypothesis that NBuPy-Cl is rapidly cleared by the human kidney.
NBuPy-Cl is a hydrophilic and positively charged compound, so its oral absorption could also be facilitated by OCT2-mediated transport in the small intestine (Koepsell et al., 2007). Meanwhile, it should be noted that other OCTs and transporters expressed in the small intestine and kidney may also contribute to its absorption and renal clearance. As mentioned above, OCT2 is probably involved in the uptake of NBuPy-Cl from the systemic circulation to the proximal tubular cells, but other transporter(s) expressed on the apical membrane, such as the multidrug and toxin extrusion transporters, MATE1 and/or MATE2k, probably export the NBuPy-Cl to the tubular lumen, as has been shown for TEA (Otsuka et al., 2005; Terada and Inui, 2008).
OCTs transport various physiological, pharmacological, and toxic chemicals, like dopamine, metformin, and nicotine, respectively (Wright, 2005). Therefore, impeded function of OCTs could alter the blood levels of these chemicals. It has been reported that TEA clearance was significantly decreased in the OCT1 and OCT2 double knockout mice and in the rats with lower OCT1 and OCT2 expression (Jonker et al., 2003; Matsuzaki et al., 2008). Absence of OCT2 has little effect on the pharmacokinetics of TEA in mice because both OCT1 and OCT2 are richly expressed in the mouse kidney, but OCT1 is weakly expressed in human kidney (Motohashi et al., 2002; Jonker et al., 2003). Therefore, because of its potent inhibition of hOCT2, impaired absorption and renal secretion of some cationic drugs might occur in humans exposed to NBuPy-Cl.
In summary, NBuPy-Cl was readily absorbed from the rodent gastrointestinal tract and then widely distributed in the body. Its dermal absorption depended on the vehicle applied. NBuPy-Cl was excreted primarily in the urine as the parent compound independent of dose and route of administration in both rats and mice. Its rapid renal elimination is mediated, in part, by OCT2.
Acknowledgments
We thank Dr. Michael Cunningham (National Toxicology Program and National Center for Toxicogenomics, National Institute of Environmental Health Sciences) for advice and support, as well as Dr. Robert Kuester, Gabriel Knudsen, and Leigh Jacobs for assistance in this project. We also thank Drs. Mike Sanders and Suramya Waidyanatha (National Toxicology Program, National Institute of Environmental Health Sciences) for critical review of this manuscript, and Dr. William Dantzler for providing [3H]inulin and helpful advice.
This work was supported in part by the National Institutes of Health National Institute of Environmental Health Sciences [Grants N01-ES45529, ES06694]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant ZO1-ES045004-11 BB] (Intramural Research Program, Research Project Number 1); and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK58251].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.022681.
ABBREVIATIONS: NBuPy-Cl, N-butylpyridinium chloride; IL, ionic liquid; Bmim-Cl, 1-butyl-3-methylimidazolium chloride; OCT, organic cation transporter; TEA, tetraethylammonium trifluoroacetate; DMF, dimethylformamide; JVC, jugular vein cannula; LSC, liquid scintillation counter; HPLC, high-pressure liquid chromatography; hOCT2, human organic cation transporter 2; CHO, Chinese hamster ovary; WB, Waymouth's buffer; Kt, concentration of substrate that results in half-maximal transport; t1/2α, distribution half-life; Vss, volume of distribution at steady state; t1/2β, elimination half-life; AUC, area under the curve.
References
- Baker GA, Baker SN, Pandey S, and Bright FV (2005) An analytical view of ionic liquids. Analyst 130 800–808. [DOI] [PubMed] [Google Scholar]
- Bernot RJ, Brueseke MA, Evans-White MA, and Lamberti GA (2005a) Acute and chronic toxicity of imidazolium-based ionic liquids on Daphnia magna. Environ Toxicol Chem 24 87–92. [DOI] [PubMed] [Google Scholar]
- Bernot RJ, Kennedy EE, and Lamberti GA (2005b) Effects of ionic liquids on the survival, movement and feeding behavior of the freshwater snail, Physa acuta. Environ Toxicol Chem 24 1759–1765. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang S, Sorani M, and Giacomini KM (2007) Transport of paraquat by human organic cation transporters and multidrug and toxic compound extrusion family. J Pharmacol Exp Ther 322 695–700. [DOI] [PubMed] [Google Scholar]
- Couling DJ, Bernot RJ, Docherty KM, Dixon JK, and Maginn EJ (2005) Assessing the factors responsible for ionic liquid toxicity to aquatic organism via quantitative structure-property relationship modeling. Green Chem 8 82–90. [Google Scholar]
- Docherty MK and Kulpa CF (2005) Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem 7 185–195. [Google Scholar]
- Earle MJ and Seddon KR (2000) Ionic liquids. Green solvents for the future. Pure Appl Chem 72 1391–1398. [Google Scholar]
- Endres F and Zein El Abedin S (2006) Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys 8 2101–2116. [DOI] [PubMed] [Google Scholar]
- Freemantle M (2000) Eyes on ionic liquids. Chem Eng News 78 37–50. [Google Scholar]
- Groves CE, Evans KK, Dantzler WH, and Wright SH (1994) Peritubular organic cation transport in isolated rabbit proximal tubules. Am J Physiol 266 F450–F458. [DOI] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, and Schinkel AH (2003) Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol 23 7902–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, and Volk C (2007) Polyspecific organic cation transporters: structure, function, physiological roles and biopharmaceutical implications. Pharm Res 24 1227–1251. [DOI] [PubMed] [Google Scholar]
- Landry TD, Brooks K, Poche D, and Woolhiser M (2005) Acute toxicity profile of 1-butyl-3-methylimidazolium chloride. Bull Environ Contam Toxicol 74 559–565. [DOI] [PubMed] [Google Scholar]
- Latała A, Stepnowski P, Nedzi M, and Mrozik W (2005) Marine toxicity assessment of imidazolium ionic liquids: acute effects on the Baltic algae Oocystis submarina and Cyclotella meneghiniana. Aquat Toxicol 73 91–98. [DOI] [PubMed] [Google Scholar]
- Malo C and Berteloot A (1991) Analysis of kinetic data in transport studies: new insights from kinetic studies of Na(+)-D-glucose cotransport in human intestinal brush-border membrane vesicles using a fast sampling, rapid filtration apparatus. J Membr Biol 122 127–141. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Morisaki T, Sugimoto W, Yokoo K, Sato D, Nonoguchi H, Tomita K, Terada T, Inui K, Hamada A, et al. (2008) Altered pharmacokinetics of cationic drugs caused by down-regulation of renal rOCT2 (Slc22a2) and rMATE1 (Slc47a1) in ischemia/reperfusion-induced acute kidney injury. Drug Metab Dispos 36 649–654. [DOI] [PubMed] [Google Scholar]
- Motohashi H, Sakurai Y, Saito H, Masuda S, Urakami Y, Goto M, Fukatsu A, Ogawa O, and Inui K-I (2002) Gene expression levels and immunolocalization of organic anion transporters in the human kidney. J Am Soc Nephrol 13 866–874. [DOI] [PubMed] [Google Scholar]
- Okuda M, Saito H, Urakami Y, Takano M, and Inui K (1996) cDNA cloning and functional expression of a novel rat kidney organic cation transporter, OCT2. Biochem Biophys Res Commun 224 500–507. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, and Moriyama Y (2005) A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A 102 17923–17928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelis RM, Suhre WM, and Wright SH (2006) Functional influence of N-glycosylation in OCT2-mediated tetraehylammonium transport. Am J Physiol Renal Physiol 290 F1118–F1126. [DOI] [PubMed] [Google Scholar]
- Pernak J, Sobaszkiewicz K, and Mirska I (2003) Anti-microbial activities of ionic liquids. Green Chem 5 52–56. [Google Scholar]
- Ranke J, Mölter K, Stock F, Bottin-Weber U, Poczobutt J, Hoffmann J, Ondruschka B, Filser J, and Jastorff B (2004) Biological effects of imidazolium ionic liquids with varying chain lengths in acute Vibrio fischeri and WST-1 cell viability assays. Ecotoxicol Environ Saf 58 396–404. [DOI] [PubMed] [Google Scholar]
- Rogers RD and Seddon KR (2003) Chemistry. Ionic liquids—solvents for the future. Science 302 792–793. [DOI] [PubMed] [Google Scholar]
- Shargel L and Yu A (1993) Applied Biopharmaceutics and Pharmacokinetics, 3rd ed, Appleton & Lange, Norwalk, CT.
- Sipes IG, Knudsen GA, and Kuester RK (2008) The effects of dose and route on the toxicokinetics and disposition of 1-butyl-3-methylimidazolium chloride in male F-344 rats and female B6C3F1 mice. Drug Metab Dispos 36 284–293. [DOI] [PubMed] [Google Scholar]
- Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, and Klaassen CD (2002) Tissue distribution and renal developmental changes in rat organic cation transport mRNA levels. Drug Metab Dispos 30 212–219. [DOI] [PubMed] [Google Scholar]
- Stock F, Hoffmann J, Ranke J, Stormann R, Ondruschka B, and Jastorff B (2004) Effects of ionic liquids on the acetylcholinesterase—a structure-activity relationship consideration. Green Chem 6 286–290. [Google Scholar]
- Suhre WM, Ekins S, Chang C, Swaan PW, and Wright SH (2005) Molecular determinants of substrate/inhibitor binding to the human and rabbit renal organic cation transporters hOCT2 and rbOCT2. Mol Pharmacol 67 1067–1077. [DOI] [PubMed] [Google Scholar]
- Terada T and Inui K (2008) Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem Pharmacol 75 1689–1696. [DOI] [PubMed] [Google Scholar]
- Welton T (1999) Room-temperature ionic liquids. Solvent for synthesis and catalysis. Chem Rev 99 2017–2084. [DOI] [PubMed] [Google Scholar]
- Winter SM and Sipes IG (1993) The disposition of acrylic acid in the male Sprague-Dawley rat following oral or topical administration. Food Chem Toxicol 31 615–621. [DOI] [PubMed] [Google Scholar]
- Wright SH (2005) Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol Appl Pharmacol 204 309–319. [DOI] [PubMed] [Google Scholar]
- Wright SH and Dantzler WH (2004) Molecular and cellular physiology of renal organic cation and anion transport. Physiol Rev 84 987–1049. [DOI] [PubMed] [Google Scholar]
- Zhao D, Liao Y, and Zhang Z (2007) Toxicity of ionic liquids. CLEAN—Soil, Air, Water 35 42–48. [Google Scholar]










