Abstract
UDP-glucuronosyltransferases (UGTs) catalyze the addition of glucuronic acid to endo- and xenobiotics, increasing hydrophilicity and enhancing elimination. Gender-divergent glucuronidation rates are observed in humans and rats, and gender differences in UGT mRNA levels have been observed in rodents. The purpose of this study was to establish the hormonal regulation of gender-dependent Ugt mRNA expression in mouse liver and kidney. Therefore, three mouse models were used to characterize the involvement of sex hormones and gender-specific growth hormone (GH) secretion patterns, including 1) hypophysectomized mice treated with male- or female-pattern GH, testosterone, or 17β-estradiol; 2) GH releasing hormone receptor-deficient little (lit/lit) mice treated with male- or female-pattern GH; and 3) gonadectomized mice treated with testosterone or 17β-estradiol. Messenger RNA expression of mouse Ugt isozymes was determined by the branched DNA assay. In C57BL/6 mice, male-predominant expression of Ugt2b1 and Ugt2b38 was observed in liver and kidney, respectively. Female-predominant expression was observed for Ugt1a1 and Ugt1a5 in liver and Ugt1a2 in kidney. In liver, regulation of Ugt1a1 and Ugt1a5 expression was attributed to repression of Ugt mRNA by male-pattern GH secretion. Conversely, regulation of Ugt2b1 expression in liver was attributed to male-pattern GH secretion. In kidney, regulation of Ugt2b38 expression was attributed to inductive effects by testosterone. Conversely, Ugt1a2 expression in kidney was negatively regulated by testosterone. In conclusion, gender differences in mouse Ugt mRNA expression were influenced by male-pattern GH secretion in liver, whereas gender differences were regulated by the effects of androgens in kidney.
UDP-glucuronosyltransferases (UGTs) are a family of microsomal enzymes that catalyze the addition of the high-energy cosubstrate UDP-glucuronic acid to endogenous and exogenous substrates, increasing their hydrophilicity and eventual excretion. UGT genes are composed of two major drug-metabolizing subfamilies, UGT1 and UGT2 (Mackenzie et al., 2005). UGT1 genes are unique in that each consists of a unique first exon and 5′-regulatory region; however, each is spliced with common exons 2 through 5. UGT1 enzymes conjugate a variety of compounds, particularly prescribed drugs, such as acetaminophen, and the endogenous heme-byproduct, bilirubin. In contrast, UGT2 genes each contain six individual exons, and UGT2 isozymes conjugate many endogenous steroids and exogenous substrates, such as morphine (Burchell et al., 1995; Tephly et al., 1998; Radominska-Pandya et al., 1999).
In clinical studies, numerous glucuronidated drugs exhibit gender-related differences in metabolism. For example, the benzodiazepine S-oxazepam, a UGT2B15 substrate, is glucuronidated at a higher rate in human liver microsomes from males than females (Court et al., 2004). In addition, the plasma clearance of S-oxazepam is 40% higher in males versus females (Greenblatt et al., 1980). Conversely, the hyperlipidemic drug fenofibrate is glucuronidated at a higher rate in females (Liu et al., 1991). In rodents, particularly rats, several other chemicals exhibit gender differences in glucuronidation. Mycophenolic acid (MPA) area under the curve (AUC) is similar between sexes; however, MPA-glucuronide AUC is lower in female rats, suggesting gender differences in glucuronidation rates of MPA (Stern et al., 2007). In addition, bisphenol A (BPA) glucuronidation in female rat liver microsomes is higher than in male counterparts. Expression of rat UGT2B1 mRNA, a BPA-metabolizing enzyme, is higher in female rat liver than in males (Takeuchi et al., 2004). Lastly, bilirubin-UGT activity is higher in female rat liver preparations than in males (Muraca and Fevery, 1984).
Gender-divergent gene expression in liver has been characterized for many rodent genes whose proteins are involved in drug metabolism and disposition, such as cytochromes P450 (P450s), multidrug resistance-associated proteins (Mrps), and organic anion-transporting polypeptides (Oatps) (Waxman et al., 1991; Cheng et al., 2006; Maher et al., 2006). Likewise, previous studies report gender-divergent mRNA expression patterns for both rat and mouse UGTs (Shelby et al., 2003; Buckley and Klaassen, 2007). In kidney, organic anion transporters (Oats) show gender differences in mRNA expression, particularly Oat1 and Oat2 in mice (Buist and Klaassen, 2004). Furthermore, gender differences in Ugt gene expression were observed in mouse liver and kidney, notably Ugt1a1, Ugt1a5, and Ugt2b1 in liver and Ugt1a2 and Ugt2b5/37/38 in kidney (Buckley and Klaassen, 2007).
In liver, the biochemical processes regulating the gender-divergent gene expression patterns commonly stem from endogenous hormones, in particular, growth hormone (GH). Male-pattern (MP) GH secretion results in intermittent peaks of GH release into circulation, followed by troughs, on regular intervals. In contrast, female-pattern (FP) GH secretion is continuously low (Jansson et al., 1985; MacLeod et al., 1991; Veldhuis et al., 1995). MP-GH secretion promotes activity of GH-stimulated transcription factors, such as signal transducer and activator of transcription (STAT5)-b, and regulates male-specific rat CYP gene expression in concordance with the repression of liver-specific transcription factors, such as hepatocyte nuclear factors (HNFs) 3 and 6 (Waxman et al., 1995; Delesque-Touchard et al., 2000; Wiwi and Waxman, 2005; Waxman and O'Connor, 2006; Holloway et al., 2007). Female-specific expression of rat CYP2C12 is regulated by FP-GH secretion via continuously low levels of STAT5b and regulation by HNFs (Endo et al., 2005). More recently, regulation of several mouse Cyps and solute carriers by Stat5b were further characterized in Stat5b-deficient mice (Clodfelter et al., 2006). In kidney, gender-specific gene expression is primarily attributed to the actions of sex hormones (androgens and estrogens), as reported for rat Oats, mouse Oatps, and mouse Mrps (Buist et al., 2003; Ljubojevic et al., 2004; Cheng et al., 2006; Maher et al., 2006).
The purpose of the current study was to elucidate the hormones responsible for gender-divergent Ugt gene expression patterns in mice. To determine whether sex hormones and/or gender-specific GH secretion patterns regulate Ugt gene expression in liver and kidney, three mouse models were used: 1) hypophysectomized (HX) mice, 2) gonadectomized (GNX), and 3) GH releasing hormone receptor-deficient (lit/lit) mice. HX and GNX mice models enable determination of the roles of sex hormones, whereas HX and lit/lit mice contribute to the understanding of GH regulation.
Materials and Methods
Chemicals and Reagents. Recombinant rat GH was purchased from Dr. A. F. Parlow (National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA). Time-release hormone pellets (21-day) containing 0.5 mg of 17β-estradiol (Sigma-Aldrich, St. Louis, MO) and 1 mg of rat GH (National Hormone and Peptide Program) were purchased from Innovative Research of America (Sarasota, FL). Time-release hormone pellets containing 5 mg of testosterone propionate (Sigma-Aldrich) and cholesterol (Sigma-Aldrich) were purchased from Dr. Jonathan Li (Hormone Pellet Press, Leawood, KS). All the other chemicals were purchased from Sigma-Aldrich.
FAM dye-labeled TaqMan assays-on-demand, including gene-specific polymerase chain reaction (PCR) primers and dye-labeled probes, specific for mouse Ugt2b5 (Mm01623253_s1), Ugt2b37 (Mm00662960_mH), Ugt2b38 (Mm00836029_mH), and mouse glyceraldehyde-3-phosphate dehydrogenase (Mm99999915_g1), as well as TaqMan Universal PCR Master Mix were purchased from Applied Biosystems (Foster City, CA). Gene-specific sequences for forward and reverse PCR primers and dye-labeled probes are available from Applied Biosystems. Moloney murine leukemia virus (MMLV) reverse transcriptase and oligo(dT)s were purchased from Promega (Madison, WI).
Animals. Naive controls. Eight-week-old male and female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were housed according to the American Animal Association Laboratory Animal Care guidelines. Mice were allowed food (Teklad Rodent Diet 8064; Harlan Teklad, Madison, WI) and water ad libitum, and were acclimated to the housing facility for 1 week before treatment.
lit/lit mice. Seven- to 8-week-old male and female lit/lit mice (C57BL/6 background) were purchased from The Jackson Laboratory. The lit/lit mice were treated (n = 6/sex/treatment) with recombinant rat GH in male or female secretion patterns according to previously reported dosing regimens (Maher et al., 2006). Time-release FP-GH pellets (21-day) and placebo controls were inserted subcutaneously between the scapulas with a 10-gauge trochar while mice were under isoflurane anesthesia. Entrance incision was closed by a single stainless steel 9-mm wound clip. MP-GH secretion pattern was mimicked by twice-daily intraperitoneal injections of rat GH (2.5 mg/kg/day) in saline in a dosing volume of 5 ml/kg. Twice-daily injections of saline at the same dosing volume were used as controls. Liver and kidneys were removed 9 days after surgery, flash-frozen in liquid nitrogen, and stored at –80°C.
GNX mice. Eight-week-old surgically castrated male and ovariectomized female C57BL/6 mice were purchased from Charles River Laboratories, Inc. (Wilmington, MA). GNX mice were treated (n = 6/sex/treatment) with time-release 5 mg of testosterone, 0.5 mg of 17β-estradiol, and 1 mg of placebo pellets, as reported by Maher et al. (2006). Pellets were inserted as described above. Nine days after hormone pellet implantation, livers and kidneys were extracted from the mice, frozen in liquid nitrogen, and stored at –80°C.
HX mice. Eight-week-old HX male and female C57BL/6 mice were purchased from Charles River Laboratories, Inc. HX mice were given 5% sucrose-supplemented drinking water ad libitum on arrival and for the duration of the study. HX mice (n = 8/sex/treatment) were treated with placebo, FP-GH secretion, testosterone, and 17β-estradiol pellets, and MP-GH secretion-patterned injections were performed as described above. Nine days after hormone pellet implantation, livers and kidneys were removed from the mice, frozen in liquid nitrogen, and stored at –80°C. At necropsy, HX mice were examined for the presence of remaining pituitary tissue. Animals with intact pituitary tissue were excluded from the study. After examination, all the treatment groups contained between four and seven mice.
Total RNA Isolation. Total RNA was extracted from each tissue using RNA-Bee Reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. RNA was quantified by UV spectrophotometry at 260/280 nm and diluted to 1 μg/μl in diethyl pyrocarbamate-treated water. RNA samples were analyzed by formaldehyde-agarose gel electrophoresis, and integrity was confirmed by visualization of 18S and 28S rRNA bands.
Branched DNA Signal Amplification Assay. Individual mouse Ugt mRNA transcripts were detected using the QuantiGene branched DNA (bDNA) signal amplification assay (Panomics, Fremont, CA). Probe sets for mouse Ugt transcripts were described previously (Chen et al., 2003; Buckley and Klaassen, 2007). In brief, capture extender, label extender, and blocker probes were combined and diluted into lysis buffer. Total RNA (1 μg/μl; 10 μl) was added to each well of 96-well plates containing 50 μl of capture hybridization buffer and 50 μl of diluted probe set, and allowed to hybridize at 53°C overnight. Plates were cooled to 46°C and rinsed twice with wash buffer. Amplifier reagent (100 μl), diluted 1:1000 in amplifier/label probe buffer, was added to each well and incubated at 46°C for 1 h. Plates were rinsed again with wash buffer, and label reagent (100 μl), diluted 1:1000 in amplifier/label probe buffer, was added and incubated at 46°C for 1 h. Plates were rinsed in wash buffer, and substrate reagent (100 μl) was added to each well. Alkaline phosphatase luminescence was activated by the addition of dioxetane substrate reagent. Plates were incubated for 1 h at 37°C, and luminescence was quantified with a Quantiplex 320 bDNA luminometer (Bayer Corp., Diagnostics Div., Tarrytown, NY). Analysis of luminescence from the 96-well plates was performed by Quantiplex Data Management Software version 5.02 (Bayer Corp., Diagnostics Div.). Luminescence for each well is reported as relative light units per 10 μg of total RNA.
Real-Time PCR. Reverse transcription of liver and kidney mRNA was performed using MMLV reverse transcriptase (RT). In brief, 12.5-μl reactions containing 250 ng of total RNA were incubated with deoxynucleoside-5′-triphosphates, RT buffer, oligo(dT)s, rRNasin (Promega), and MMLV RT at 42°C for 15 min. The completed RT reactions were diluted 10× in water. Fifty-microliter real-time reactions were composed of 10 μl of cDNA, 2.5 μl of gene-specific 20× TaqMan probes, 25 μl of TaqMan Universal PCR Master Mix, and 12.5 μl of water. The 20× TaqMan mixtures contained gene-specific PCR primers and dye-labeled probe. Reaction conditions were as follows: denaturing at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min. RT-PCR reactions were performed on an Applied Biosystems 7300 Real-Time PCR System, and data were obtained as Ct values. Data were analyzed by the ΔΔCt method using glyceraldehyde-3-phosphate dehydrogenase as an internal control for each sample and Ugt2b5 mRNA expression in male liver as the reference.
Statistical Analysis. Data are presented as mean ± S.E.M. Statistical differences between genders in naive control mice were determined by a two-tailed Student's t test (p ≤ 0.05). Statistical differences between untreated, placebo, and hormone treatment groups were determined by a one-way analysis of variance followed by Duncan's multiple range post hoc test. Asterisks (*) represent statistical differences (p ≤ 0.05) in mRNA levels between control and treatment groups. Daggers (†) indicate statistical differences between naive controls and HX mice, and double-daggers (‡) indicate statistical differences between HX-placebo and treated groups (p ≤ 0.05).
Results
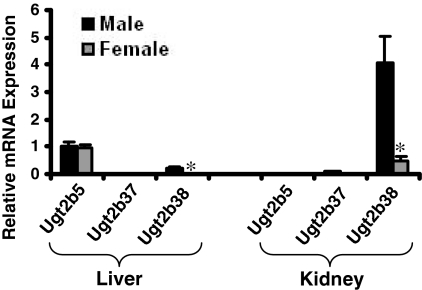
Real-Time PCR Analysis of Mouse Ugt2b5, Ugt2b37, and Ugt2b38 in Mouse Liver and Kidney. Previous studies indicated that, by the bDNA assay, mouse Ugt2b5, Ugt2b37, and Ugt2b38 mRNA are expressed in adult mouse liver and kidney, with male-predominant expression in kidney (Buckley and Klaassen, 2007). The aforementioned study used a single bDNA probe set to detect Ugt2b5, Ugt2b37, and Ugt2b38 because of the high sequence homology between the three transcripts. To elucidate the expression pattern of each of the three mRNAs, real-time PCR analysis was used to detect mRNA expression patterns in liver and kidney of untreated male and female mice. Figure 1 illustrates that Ugt2b5 mRNA was the predominant gene expressed in liver. However, in kidney, Ugt2b38 transcripts were almost solely responsible for expression previously detected by the bDNA assay. In addition, the male-predominant expression pattern in kidney observed by the bDNA assay was also attributed to Ugt2b38, as noted in Fig. 1.
Fig. 1.
Relative mRNA expression of Ugt2b5, Ugt2b37, and Ugt2b38 as determined by real-time PCR in untreated male and female C57BL/6 mouse livers (n = 5/gender/tissue). Data are relative to Ugt2b5 mRNA expression in male liver as determined by ΔΔCt analysis. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female mice (p ≤ 0.05).
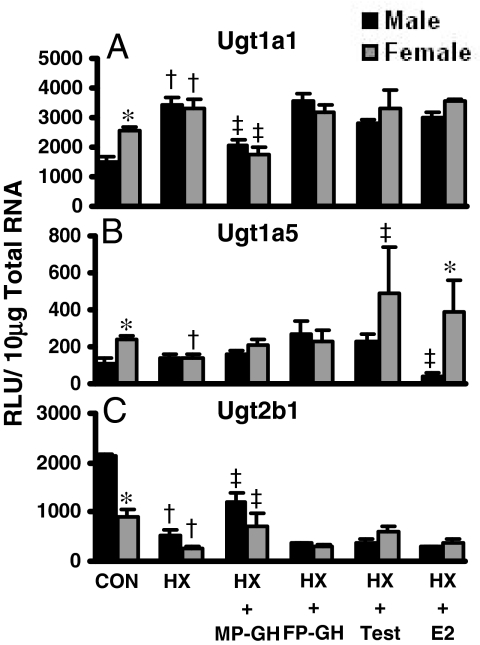
Regulation of Ugts by GH Secretion Patterns and Sex Hormones in HX Mice. Buckley and Klaassen (2007) previously reported female-predominant mRNA expression for Ugt1a1 and Ugt1a5 and male-predominant expression of Ugt2b1 in naive mouse livers. HX mice, pituitary surgically removed, were used to study the effects of MP- and FP-GH secretion and the effects of androgens and estrogens on Ugt mRNA expression in liver. Figure 2 illustrates the alterations in mRNA expression patterns following HX in liver for the three aforementioned mouse Ugts. In HX mice, female-predominant mRNA expression of Ugt1a1 observed in naive-control mice was ablated (Fig. 2A). However, in MP-GH secretion-treated HX male and female mice, Ugt1a1 mRNA decreased 40 to 47%. Neither FP-GH secretion nor treatment with sex hormones significantly altered Ugt1a1 mRNA in HX mice.
Fig. 2.
The mRNA expression of Ugt1a1 (A), Ugt1a5 (B), and Ugt2b1 (C) in livers of male and female HX mice treated with growth or sex hormones (n = 4–6/gender/treatment). Treatment groups include CON, naive controls; HX, placebo; HX-MP-GH, male-pattern GH; HX-FP-GH, female-pattern GH, HX-Test, testosterone; and HX-E2, 17β-estradiol. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and HX mice, and double-daggers (‡) indicate statistical differences between HX-placebo and treated groups (p ≤ 0.05).
In HX mice, Ugt1a5 mRNA levels in liver were not different between sexes, whereas Ugt1a5 mRNA was female-predominant in naive controls (Fig. 2B). Neither MP-GH nor FP-GH secretion-patterned treatment altered Ugt1a5 mRNA in liver. However, in female HX mice treated with testosterone, Ugt1a5 mRNA was increased. Conversely, in male HX mice treated with 17β-estradiol, hepatic Ugt1a5 mRNA decreased.
In contrast to Ugt1a1 and Ugt1a5, hepatic expression of Ugt2b1 mRNA was male-predominant. In HX mice, the gender difference of Ugt2b1 was ablated (Fig. 2C). MP-GH secretion-patterned treatment in HX mice resulted in a 224 to 260% increase in Ugt2b1 mRNA levels. FP-GH secretion or estradiol treatments had no effect on Ugt2b1 mRNA expression in liver.
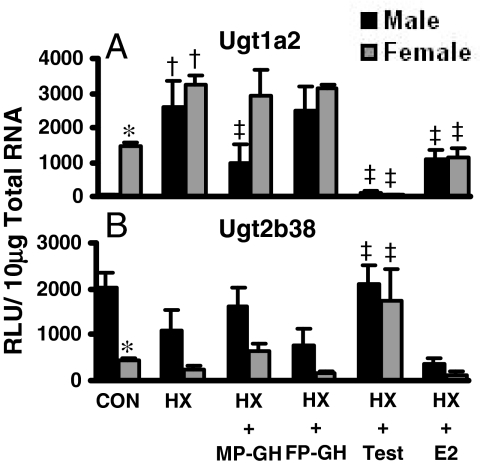
In kidney, previous reports indicate gender differences in Ugt mRNA expression in mice, specifically female-predominant Ugt1a2 and male-predominant Ugt2b38 (Chen et al., 2003; Buckley and Klaassen, 2007). Figure 3 illustrates the effects of hormone treatment on Ugt1a2 and Ugt2b38 mRNA in male HX and female HX mice. In HX mice, female-predominant mRNA expression of Ugt1a2 in kidney was ablated; HX markedly increased Ugt1a2 mRNA in kidneys of male mice and doubled Ugt1a2 mRNA in female mice (Fig. 3A). Treatment of HX mice with MP-GH secretion patterns decreased Ugt1a2 mRNA in kidneys of male mice by 60%; however, MP-GH secretion-patterned treatment of female HX mice did not alter Ugt1a2 mRNA in kidney, and FP-GH secretion had no effect on Ugt1a2 expression in kidney. In male HX and female HX mice, testosterone decreased Ugt1a2 in kidney by 97 to 99%. In addition, in male HX and female HX mice, 17β-estradiol decreased Ugt1a2 mRNA but only by 50%.
Fig. 3.
The mRNA expression of Ugt1a2 (A) and Ugt2b38 (B) in kidneys of male and female HX mice treated with growth or sex hormones (n = 4–6/gender/treatment). Treatment groups include CON, naive controls; HX, placebo; HX-MP-GH, male-pattern GH; HX-FP-GH, female-pattern GH, HX-Test, testosterone; and HX-E2, 17β-estradiol. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and HX mice, and double-daggers (‡) indicate statistical differences between HX-placebo and treated groups (p ≤ 0.05).
In kidneys of HX mice, male predominance of Ugt2b38 mRNA was not statistically significant as in naive mice, mainly because of decreased expression in male HX mice (Fig. 3B). In male HX and female HX mice, testosterone administration increased Ugt2b38 mRNA in kidney 2- and 7-fold, respectively. In HX mice, MP-GH or FG-GH secretion patterns and estradiol treatment had minimal effects on Ugt2b38 mRNA expression in kidney.
Regulation of Ugt mRNA by Testosterone and Estradiol in GNX Mice. The regulation of Ugts in mouse liver of GNX mice, followed by androgen or estrogen treatments, is shown in Fig. 4. In control mice, liver Ugt1a1 mRNA expression is female-predominant; however, in GNX mice, Ugt1a1 expression was similar between males and females because of increased expression in males (Fig. 4A). In GNX mice, testosterone treatment reduced Ugt1a1 mRNA in liver by 35 to 48%. In addition, 17β-estradiol-treated GNX mice reduced Ugt1a1 mRNA in liver by 24 to 40%.
Fig. 4.
The mRNA expression of Ugt1a1 (A), Ugt1a5 (B), and Ugt2b1 (C) in livers of male and female GNX mice treated with sex hormones (n = 6/gender/treatment). Treatment groups include CON, naive controls; GNX, placebo; GNX-Test, testosterone; and GNX-E2, 17β-estradiol. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and GNX mice, and double-daggers (‡) indicate statistical differences between GNX-placebo and treated groups (p ≤ 0.05).
Ugt1a5 mRNA in liver was female-predominant in control mice, whereas in GNX mice, the Ugt1a5 gender difference was eliminated because of increased expression in males (Fig. 4B). Testosterone treatment of GNX mice resulted in a decrease of Ugt1a5 mRNA only in female livers.
In control mice, male-predominant mRNA expression of Ugt2b1 in liver was observed; however, in GNX mice, Ugt2b1 levels in male mice decreased to those observed in livers of female GNX mice, eliminating the gender difference (Fig. 4C). Testosterone treatment of GNX mice increased Ugt2b1 mRNA in liver by more than 100%. Estradiol treatment of GNX mice did not alter Ugt2b1 expression in liver.
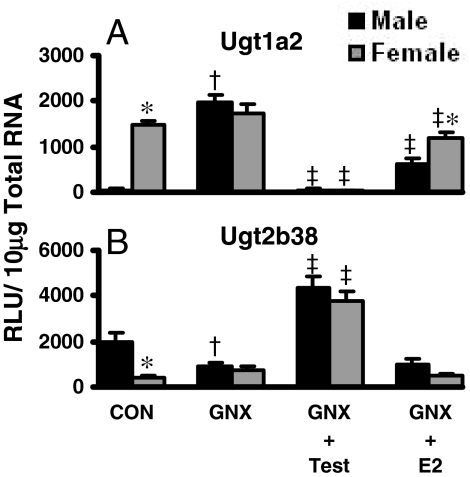
In kidney, Ugt1a2 mRNA is highly and almost exclusively expressed in female mice (Fig. 5). Castration resulted in an increase of Ugt1a2 to levels observed in female mice, abolishing the female predominance (Fig. 5A). Testosterone administration to GNX mice reduced kidney Ugt1a2 mRNA by 98%. Estradiol treatment reduced Ugt1a2 mRNA in kidneys of GNX mice by 30 to 60%.
Fig. 5.
The mRNA expression of Ugt1a2 (A) and Ugt2b38 (B) in kidneys of male and female GNX mice treated with sex hormones (n = 6/gender/treatment). Treatment groups include CON, naive controls; GNX, placebo; GNX-Test, testosterone; and GNX-E2, 17β-estradiol. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and GNX mice, and double-daggers (‡) indicate statistical differences between GNX-placebo and treated groups (p ≤ 0.05).
In GNX mice, male-predominant Ugt2b38 expression observed in control kidneys was eliminated because of decreased expression in male GNX mice (Fig. 5B). Testosterone treatment of GNX mice increased Ugt2b38 mRNA expression approximately 400%. However, estradiol treatment of GNX mice had no effect on Ugt2b38 expression in kidney.
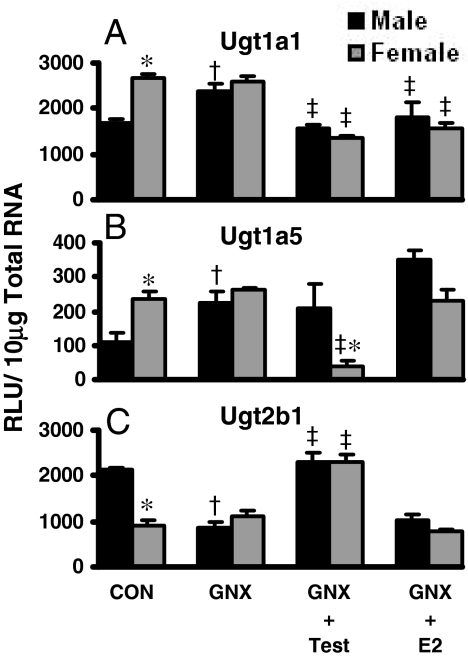
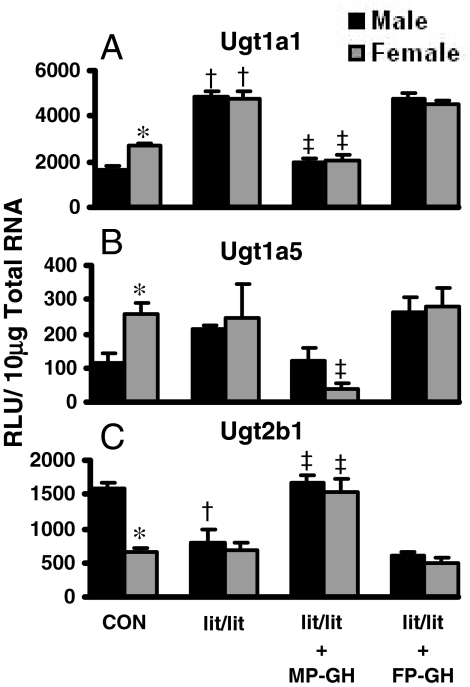
Regulation of Ugt mRNA by GH in lit/lit Mice. The regulation of Ugt1a1, Ugt1a5, and Ugt2b1 by MP- and FP-GH patterns in mouse liver is shown in Fig. 6. In lit/lit mice, female-predominant Ugt1a1 and Ugt1a5 mRNA expression in liver was eliminated (Fig. 6, A and B). MP-GH secretion-patterned treatment of male and female lit/lit mice decreased Ugt1a1 mRNA in liver by 60%. Likewise, MP-GH secretion-patterned treatment of lit/lit mice decreased Ugt1a5 mRNA by 40% in males and 85% in females. FP-GH treatment of lit/lit mice did not affect Ugt1a1 or Ugt1a5 expression in liver.
Fig. 6.
The mRNA expression of Ugt1a1 (A), Ugt1a5 (B), and Ugt2b1 (C) in livers of male and female lit/lit mice treated with GH (n = 6/gender/treatment). Treatment groups include CON, naive controls; lit/lit, placebo; lit/lit-MP-GH, male-pattern GH; and lit/lit-FP-GH, female-pattern GH. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and lit/lit mice, and double-daggers (‡) indicate statistical differences between lit/lit-placebo and treated groups (p ≤ 0.05).
In lit/lit mice, male-predominant expression of Ugt2b1 in livers of control mice was ablated because of decreased expression in male mice (Fig. 6C). MP-GH treatment of lit/lit mice increased Ugt2b1 mRNA by more than 100%. FP-GH secretion treatment of lit/lit did not affect Ugt2b1 expression in liver.
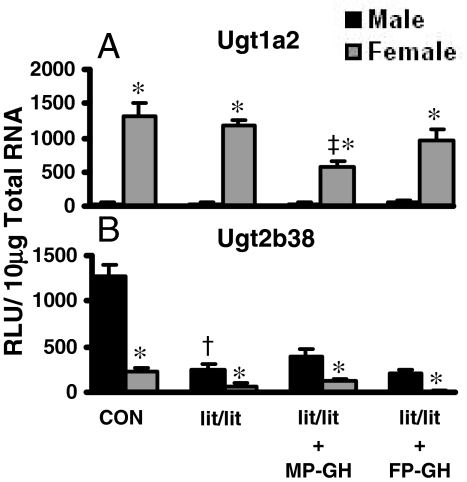
The effects of MP- and FP-GH secretion patterns on Ugt1a1 and Ugt2b38 mRNA expression in kidney are noted in Fig. 7. In lit/lit mice before or after treatment with MP-GH or FP-GH secretion patterns, the female predominance of Ugt1a2 mRNA expression was evident (Fig. 7A). However, MP-GH secretion treatment of the lit/lit mice decreased Ugt1a2 expression in the kidney. Ugt2b38 mRNA expression was male-predominant in the mouse kidney. In lit/lit mice, the expression of Ugt2b38 was much lower in the male mice, and MP-GH or FP-GH secretion-patterned treatments had little effect (Fig. 7B).
Fig. 7.
The mRNA expression of Ugt1a2 (A) and Ugt2b38 (B) in kidneys of male and female lit/lit mice treated with GH (n = 6/gender/treatment). Treatment groups include CON, naive controls; lit/lit, placebo; lit/lit-MP-GH, male-pattern GH; and lit/lit-FP-GH, female-pattern GH. Data are expressed as relative light units/10 μg of total RNA. Values are expressed as mean ± S.E.M. Asterisks (*) indicate statistically significant differences between male and female control mice (p ≤ 0.05). Daggers (†) indicate statistical differences between naive controls and lit/lit mice, and double-daggers (‡) indicate statistical differences between lit/lit-placebo and treated groups (p ≤ 0.05).
Discussion
Glucuronidation of drugs, environmental contaminants, and endogenous molecules serves as a detoxification process and termination of endogenous molecular signals. Among species, sexes, and populations, exposures to exogenous compounds and endogenous biosignaling molecules, such as sex steroids, are not identical. Therefore, laboratory animal species, as well as humans in some instances, exhibit numerous sex-based characteristics, including drug metabolism.
Differential metabolism of some clinically prescribed drugs occurs between male and female patients, including drugs metabolized by P450s and UGTs. A review of clinical studies reveals examples of CYP3A4 substrates that exhibit female-predominant clearance, including erythromycin, midazolam, verapamil, and others (Anderson, 2002). Alternatively, caffeine, a CYP1A2 substrate, is metabolized more rapidly in males than females (Jaffe et al., 2002). Clearance rates of drugs are determined by factors in addition to P450 metabolism, such as conjugation reactions and transport. Many drugs that are primarily glucuronidated show gender-divergent clearance, such as S-oxazepam (M > F) and fenofibrate (F > M) (Liu et al., 1991; Court et al., 2004). In rodents, there are examples of gender-divergent glucuronidation of drugs and exogenous chemicals, including MPA and BPA (Takeuchi et al., 2004; Stern et al., 2007).
Gender differences in drug conjugation are probably linked to gender-divergent expression of drug-metabolizing enzymes. Higher clearance of BPA in female versus male rats correlates with female-predominant expression of UGT2B1 mRNA, one of the isoforms primarily responsible for BPA glucuronidation (Takeuchi et al., 2004). Previous studies illustrate gender differences in basal mRNA expression levels of UGTs in rodent species, notably in rats and mice. However, rats and mice do not share identical gender differences (Strasser et al., 1997; Shelby et al., 2003; Buckley and Klaassen, 2007). Mice display gender-divergent mRNA expression patterns in liver and kidney. Ugt1a1 and Ugt1a5 expression in liver and Ugt1a2 in kidney are female-predominant, whereas Ugt2b1 expression in liver and Ugt2b38 in kidney are male-predominant (Buckley and Klaassen, 2007). The current study establishes the role of MP-GH excretion pattern and male sex hormones (testosterone) in the differential regulation of male- and female-specific Ugt mRNA expression in mice.
In the present study, gender differences in Ugt mRNA expression in liver were regulated by MP-GH secretion but were also influenced by testosterone (Table 1). Ugt1a1 mRNA expression, the major bilirubin-conjugating isozyme, was reduced by MP-GH secretion as shown in both HX and GH releasing hormone receptor-deficient (lit/lit) mouse models. However, testosterone administration reduced Ugt1a1 mRNA levels in GNX mice, suggesting regulation of MP-GH secretion by androgens as previously observed for mouse Oatps (Cheng et al., 2006).
TABLE 1.
Summary of MP-GH and testosterone on mouse Ugt mRNA expression in liver and kidney
HX mice were treated with either MP-GH or testosterone. GNX mice were treated with testosterone and lit/lit mice with MP-GH. Up arrow (↑) indicates up-regulation of Ugt mRNA expression as compared with placebo controls. Down arrow (↓) indicates decreased Ugt mRNA expression compared with placebo controls. Multiple arrows indicate amplitude of change. Side arrows (⇆) indicate no significant change in mRNA expression compared with placebo controls.
|
HX
|
GNX
|
lit/lit
|
||
|---|---|---|---|---|
| MP-GH | Test | Test | MP-GH | |
| Ugt1a1 (liver) | ↓ | ⇆ | ↓ | ↓ |
| Ugt1a5 (liver) | ⇆ | ⇆ | ⇆ | ↓ |
| Ugt2b1 (liver) | ↑ | ⇆ | ↑ | ↑ |
| Ugt1a2 (kidney) | ⇆ | ↓ ↓ | ↓ ↓ | ⇆ |
| Ugt2b38 (kidney) | ⇆ | ↑ | ↑ ↑ | ⇆ |
Ugt1a5 and Ugt2b1 in mouse liver were also regulated by MP-GH secretion. Hormonal regulation of Ugt1a5 expression in liver was comparable with Ugt1a1, in that both were down-regulated by MP-GH secretion. In contrast to Ugt1a1 and Ugt1a5, Ugt2b1 was expressed in liver in a male-predominant pattern. Also in contrast to Ugt1a1 and Ugt1a5, which are down-regulated by MP-GH secretion, Ugt2b1 was up-regulated by MP-GH secretion.
In mouse liver, Mrps, Oatps, and now Ugts have been identified as targets for MP-GH regulation (Cheng et al., 2006; Maher et al., 2006). It is presumed that MP-GH regulation of these liver genes occurs via STAT5b activation, which is strengthened by evidence of STAT5b binding sites in Mrp promoters (Maher et al., 2006). In addition, STAT5b regulates transcription of several hepatic drug-metabolizing enzymes, most notably Cyps, and transporters, particularly solute carriers via a network of gender-specific Stat5b gene regulation (Clodfelter et al., 2006).
In liver, male and female GH patterns regulate GH-responsive transcription factors to generate male- and female-specific gene expression patterns. Pulsatile peaks of GH produced by MP-GH secretion activates STAT5b in male liver, resulting in activation of male genes, such as rat Cyp2a2, by direct activation and repression of female-specific transcription factors, notably HNF3β and HNF6. HNF4α and HNF3γ also serve as positive regulators of male-specific Cyp2a2 expression. In females, continuously low levels of GH result in low STAT5b activity and allow repression of male-specific genes by HNF3β and HNF6, yielding expression of female-specific rat Cyp2c12 (Delesque-Touchard et al., 2000; Endo et al., 2005; Wiwi and Waxman, 2005; Waxman and O'Connor, 2006; Holloway et al., 2007).
In rats, glucuronidation of bilirubin, a UGT1A1 substrate, was increased 100% by HX and returned to control levels after administration of GH mimicking male patterns (Gueraud et al., 1997). This supports data observed for Ugt1a1 in HX mice, where mRNA levels increased in male HX mice to nearly twice of controls and returned to control male levels after MP-GH administration to HX mice. These data suggest mice may have higher bilirubin-UGT activity in females than in males, similar to rats (Muraca and Fevery, 1984). In addition, a comprehensive analysis of bilirubin levels in U.S. human subjects revealed higher levels in males (Zucker et al., 2004), suggesting gender-dimorphic UGT1A1 expression in humans, leading to differential bilirubin conjugation and circulating levels.
In kidney, sex hormones directly regulate gene expression of transporters and drug-metabolizing enzymes. Rat OAT1 mRNA is male-predominant and HX decreases OAT1 to levels observed in females, whereas androgen treatment increases expression in kidney (Buist et al., 2003; Ljubojevic et al., 2004). Female-predominant mouse Mrp3 mRNA in kidney results from stimulatory effects of estrogens, as observed in GNX and HX mouse models. However, female-predominant expression of Mrp4 in mouse kidney results from down-regulation of Mrp4 in males by androgen and MP-GH secretion (Maher et al., 2006). Oatp1a1 and Oatp3a1 mRNAs are up-regulated by androgens in mouse kidneys as shown in GNX and HX mice (Cheng et al., 2006). Likewise, mouse Ugt gender differences in kidney mRNA expression are regulated by androgens, not GH (Table 1). Ugt1a2 and Ugt2b38 mRNA expression patterns in kidney are initiated by the repressive and inductive effects of androgens, respectively.
Taken together, MP-GH pattern secretion shapes gender-specific gene expression in liver, whereas sex hormones are the dominant factor influencing gender-dependent transporter and Ugt gene expression in kidney. However, the cumulative physiological effects of such gender patterns in liver and kidney depend on a variety of factors, such as cellular localization of membrane transporters, direction of transport, and transporter and enzyme substrate specificity. In clinical studies, countless drugs are taken up, excreted, reabsorbed, and metabolized in liver and kidney, many by transporters and enzymes that are sexually dimorphic. In addition to genetic factors, some cases of polymorphic variation in drug disposition could involve gender-specific gene expression patterns, especially with drugs that are primarily transported or metabolized by a single gender-regulated protein.
In summary, mouse gender-specific Ugt mRNA expression patterns in liver are attributable to inductive or repressive effects of MP-GH secretion, and secondarily to androgens influencing GH secretion in animals containing a functioning pituitary. In contrast, gender-specific Ugt mRNA expression in kidney is chiefly regulated by positive or negative effects of androgens. Results observed for hormonal regulators of mouse Ugt gender-divergent gene expression are common among drug-metabolizing enzymes and transporters involved in hepatic and renal disposition of numerous chemicals. These gender-specific patterns may be crucial in understanding the mechanisms by which many drugs display variations in clearance.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grants ES09649, ES09716, ES07079].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024224.
ABBREVIATIONS: UGT, UDP-glucuronosyltransferase; MPA, mycophenolic acid; AUC, area under the curve; BPA, bisphenol A; P450, cytochrome P450; MRP, multidrug resistance-associated protein; OATP, organic anion-transporting polypeptide; OAT, organic anion transporter; GH, growth hormone; MP, male pattern; FP, female pattern; STAT, signal transducer and activator of transcription; HNF, hepatocyte nuclear factor; HX, hypophysectomy; GNX, gonadectomy; PCR, polymerase chain reaction; MMLV, Moloney murine leukemia virus; bDNA, branched DNA; RT, reverse transcriptase; Ct, cycle threshold.
References
- Anderson GD (2002) Sex differences in drug metabolism: cytochrome P-450 and uridine diphosphate glucuronosyltransferase. J Gend Specif Med 5 25–33. [PubMed] [Google Scholar]
- Buckley DB and Klaassen CD (2007) Tissue- and gender-specific mRNA expression of UDP-glucuronosyltransferases (UGTs) in mice. Drug Metab Dispos 35 121–127. [DOI] [PubMed] [Google Scholar]
- Buist SC, Cherrington NJ, and Klaassen CD (2003) Endocrine regulation of rat organic anion transporters. Drug Metab Dispos 31 559–564. [DOI] [PubMed] [Google Scholar]
- Buist SC and Klaassen CD (2004) Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1–3; Slc22a6–8) mRNA levels. Drug Metab Dispos 32 620–625. [DOI] [PubMed] [Google Scholar]
- Burchell B, Brierley CH, and Rance D (1995) Specificity of human UDP-glucuronosyltransferases and xenobiotic glucuronidation. Life Sci 57 1819–1831. [DOI] [PubMed] [Google Scholar]
- Chen C, Staudinger JL, and Klaassen CD (2003) Nuclear receptor, pregnane X receptor, is required for induction of UDP-glucuronosyltranferases in mouse liver by pregnenolone-16 alpha-carbonitrile. Drug Metab Dispos 31 908–915. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Lu H, and Klaassen CD (2006) Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol 70 1291–1297. [DOI] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, and Waxman DJ (2006) Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mol Endocrinol 20 1333–1351. [DOI] [PubMed] [Google Scholar]
- Court MH, Hao Q, Krishnaswamy S, Bekaii-Saab T, Al-Rohaimi A, von Moltke LL, and Greenblatt DJ (2004) UDP-glucuronosyltransferase (UGT) 2B15 pharmacogenetics: UGT2B15 D85Y genotype and gender are major determinants of oxazepam glucuronidation by human liver. J Pharmacol Exp Ther 310 656–665. [DOI] [PubMed] [Google Scholar]
- Delesque-Touchard N, Park SH, and Waxman DJ (2000) Synergistic action of hepatocyte nuclear factors 3 and 6 on CYP2C12 gene expression and suppression by growth hormone-activated STAT5b. Proposed model for female specific expression of CYP2C12 in adult rat liver. J Biol Chem 275 34173–34182. [DOI] [PubMed] [Google Scholar]
- Endo M, Takahashi Y, Sasaki Y, Saito T, and Kamataki T (2005) Novel gender-related regulation of CYP2C12 gene expression in rats. Mol Endocrinol 19 1181–1190. [DOI] [PubMed] [Google Scholar]
- Greenblatt DJ, Divoll M, Harmatz JS, and Shader RI (1980) Oxazepam kinetics: effects of age and sex. J Pharmacol Exp Ther 215 86–91. [PubMed] [Google Scholar]
- Gueraud F, Masmoudi T, Goudonnet H, and Paris A (1997) Differential effect of hypophysectomy and growth hormone treatment on hepatic glucuronosyltransferases in male rats: evidence for an action at a pretranslational level for isoforms glucuronidating bilirubin. Biochem Pharmacol 53 1637–1647. [DOI] [PubMed] [Google Scholar]
- Holloway MG, Cui Y, Laz EV, Hosui A, Hennighausen L, and Waxman DJ (2007) Loss of sexually dimorphic liver gene expression upon hepatocyte-specific deletion of Stat5a-Stat5b locus. Endocrinology 148 1977–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, and Watkins PB (2002) Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol Endocrinol Metab 283 E1008–E1015. [DOI] [PubMed] [Google Scholar]
- Jansson JO, Edén S, and Isaksson O (1985) Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6 128–150. [DOI] [PubMed] [Google Scholar]
- Liu HF, Vincent-Viry M, Galteau MM, Guéguen R, Magdalou J, Nicolas A, Leroy P, and Siest G (1991) Urinary glucuronide excretion of fenofibric and clofibric acid glucuronides in man. Is it polymorphic? Eur J Clin Pharmacol 41 153–159. [DOI] [PubMed] [Google Scholar]
- Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, and Sabolic I (2004) Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol 287 F124–F138. [DOI] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, and Nebert DW (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15 677–685. [DOI] [PubMed] [Google Scholar]
- MacLeod JN, Pampori NA, and Shapiro BH (1991) Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol 131 395–399. [DOI] [PubMed] [Google Scholar]
- Maher JM, Cheng X, Tanaka Y, Scheffer GL, and Klaassen CD (2006) Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol 71 1470–1478. [DOI] [PubMed] [Google Scholar]
- Muraca M and Fevery J (1984) Influence of sex and sex steroids on bilirubin uridine diphosphate-glucuronosyltransferase activity of rat liver. Gastroenterology 87 308–313. [PubMed] [Google Scholar]
- Radominska-Pandya A, Czernik PJ, Little JM, Battaglia E, and Mackenzie PI (1999) Structural and functional studies of UDP-glucuronosyltransferases. Drug Metab Rev 31 817–899. [DOI] [PubMed] [Google Scholar]
- Shelby MK, Cherrington NJ, Vansell NR, and Klaassen CD (2003) Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab Dispos 31 326–333. [DOI] [PubMed] [Google Scholar]
- Stern ST, Tallman MN, Miles KK, Ritter JK, Dupuis RE, and Smith PC (2007) Gender-related differences in mycophenolate mofetil-induced gastrointestinal toxicity in rats. Drug Metab Dispos 35 449–454. [DOI] [PubMed] [Google Scholar]
- Strasser SI, Smid SA, Mashford ML, and Desmond PV (1997) Sex hormones differentially regulate isoforms of UDP-glucuronosyltransferase. Pharm Res 14 1115–1121. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsutsumi O, Nakamura N, Ikezuki Y, Takai Y, Yano T, and Taketani Y (2004) Gender difference in serum bisphenol A levels may be caused by liver UDP-glucuronosyltransferase activity in rats. Biochem Biophys Res Commun 325 549–554. [DOI] [PubMed] [Google Scholar]
- Tephly TR, Green MD, Coffman BL, King C, Cheng Z, and Rios G (1998) Metabolism of endobiotics and xenobiotics by UDP-glucuronosyltransferase. Adv Pharmacol 42 343–346. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liem AY, South S, Weltman A, Weltman J, Clemmons DA, Abbott R, Mulligan T, Johnson ML, and Pincus S (1995) Differential impact of age, sex steroid hormones, and obesity on basal versus pulsatile growth hormone secretion in men as assessed in an ultra-sensitive chemiluminescence assay. J Clin Endocrinol Metab 80 3209–3222. [DOI] [PubMed] [Google Scholar]
- Waxman DJ and O'Connor C (2006) Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol 20 2613–2629. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Pampori NA, Ram PA, Agrawal AK, and Shapiro BH (1991) Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A 88 6868–6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Ram PA, Park SH, and Choi HK (1995) Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J Biol Chem 270 13262–13270. [DOI] [PubMed] [Google Scholar]
- Wiwi CA and Waxman DJ (2005) Role of hepatocyte nuclear factors in transcriptional regulation of male-specific CYP2A2. J Biol Chem 280 3259–3268. [DOI] [PubMed] [Google Scholar]
- Zucker SD, Horn PS, and Sherman KE (2004) Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology 40 827–835. [DOI] [PubMed] [Google Scholar]