Abstract
The effects of [4′-(6-allyl-methyl-amino-hexyloxy)-2′-fluoro-phenyl]-(4-bromophenyl)-methanone fumarate (Ro 48-8071), an inhibitor of 2,3-oxidosqualene:lanosterol cyclase (cyclase), were evaluated on CYP3A4 and CYP2B6 mRNA content in primary cultured human hepatocytes. In seven hepatocyte culture preparations, 24-h treatment with 3, 10, or 30 μM Ro 48-8071 produced median increases in CYP3A4 mRNA content that were 2.2-, 7.1-, and 8.5-fold greater than untreated control, respectively, and produced increases in CYP2B6 mRNA content that were 3.0-, 4.6-, and 3.4-fold greater than control, respectively. Increases in CYP3A4 immunoreactive protein content were also measured in Ro 48-8071-treated hepatocytes. To evaluate the effects of cyclase inhibitor treatments further, a pregnane X receptor (PXR)-responsive transactivation assay in HepG2 cells was used. Ro 48-8071, trans-N-(4-chlorobenzoyl)-N-methyl-(4-dimethylaminomethylphenyl)-cyclohexylamine (BIBX 79), and 3β-(2-diethylaminoethoxy)androst-5-en-17-one HCl (U18666A) induced luciferase expression from a PXR-responsive reporter with EC50s of 0.113, 0.916, and 0.294 μM, respectively. Treatment of the HepG2 system with (E)N-ethyl-N-(6,6-dimethyl-2-hepten-4-ynyl)-3-[(3,3′-bithiophen-5-yl)methoxy]benzenemethanamine (NB-598), an inhibitor of squalene monooxygenase, at concentrations sufficient to achieve cholesterol biosynthesis inhibition significantly inhibited cyclase inhibitor-mediated, but not rifampicin-mediated, reporter induction. Direct treatment of the HepG2 system with 1 to 10 μM squalene 2,3:22,23-dioxide, but not squalene 2,3-oxide, significantly activated PXR-responsive reporter expression. Also, squalene 2,3:22,23-dioxide bound to human PXR in vitro with an IC50 of 3.35 μM. These data indicate that cyclase inhibitors are capable of producing CYP3A4 and CYP2B6 induction in primary cultured human hepatocytes, and that an endogenous squalene metabolite is a conserved intracrine activator of PXR.
A primary mode of defense that is used by animals against their chemical environments involves recognition by a “xenobiotic-sensing” receptor followed by the induction of phase I and phase II xenobiotic-metabolizing enzymes, as well as “phase III” transporters. As the archetype of this mechanism, many xenobiotics bind to the pregnane X receptor (PXR), owing to the receptor's unusually accommodating ligand-binding pocket (Watkins et al., 2001, 2003). On ligand binding, PXR, in partnership with the retinoic X receptor, is transformed into an active transcription factor that increases the expression of target genes, which include members of the CYP3A family (e.g., CYP3A23 in rat, CYP3A11 in mouse, and CYP3A4 in human). These CYP3A enzymes catalyze the phase I metabolism of numerous xenobiotic substrates, including a large number of clinically used drugs (Quattrochi and Guzelian, 2001).
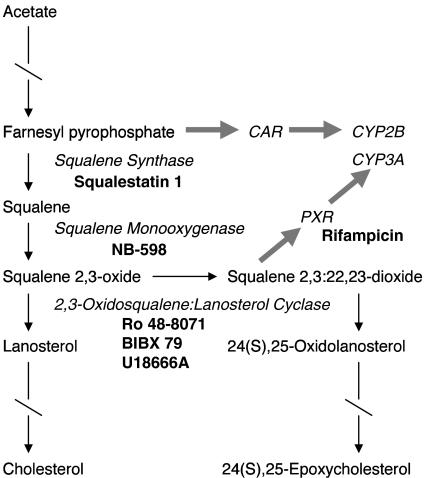
In addition to serving as a xenobiotic recognition and metabolizing system, PXR and CYP3A enzymes are increasingly perceived to function in the metabolism of endogenous molecules. For example, the cholestatic secondary bile acid, lithocholate, both activates PXR and is a substrate for CYP3A (Staudinger et al., 2001; Xie et al., 2001). We have used chemical inhibitors of various steps of the cholesterol biosynthetic pathway as an approach for identifying endogenous modulators of hepatic cytochrome P450 expression (Fig. 1). In this regard, we have reported that inhibitors of squalene synthase (e.g., squalestatin 1), the first committed step in cholesterol biosynthesis, selectively induce CYP2B expression in primary cultured rat hepatocytes and rat liver through a mechanism that requires the biosynthesis of one or more endogenous isoprenoids and activation of constitutive androstane receptor (CAR) (Kocarek and Mercer-Haines, 2002). By contrast, treatment of primary cultured rat or mouse hepatocytes with an inhibitor of 2,3-oxidosqualene:lanosterol cyclase (cyclase; e.g., Ro 48-8071), which catalyzes the second step downstream from squalene synthase, causes the selective induction of CYP3A (Shenoy et al., 2004a). This latter effect is mediated by PXR, as illustrated by the loss of cyclase inhibitor-mediated CYP3A induction in cultured hepatocytes prepared from PXR-null mice (Shenoy et al., 2004a). In addition, cyclase inhibitor-inducible CYP3A expression requires cyclase blockade and the ongoing synthesis of an endogenous squalene metabolite, most likely squalene 2,3-oxide and/or squalene 2,3:22,23-dioxide, as indicated by the loss of induction when hepatocytes are cotreated with an inhibitor of an upstream step in the cholesterol biosynthetic pathway (Shenoy et al., 2004a). For example, cyclase inhibitor-inducible CYP3A expression was suppressed when rat hepatocyte cultures were cotreated with NB-598, a potent inhibitor of squalene monooxygenase, which catalyzes the step immediately upstream of cyclase (Fig. 1).
Fig. 1.
Cholesterol biosynthesis pathway, abridged to highlight the metabolites (regular type), enzymes or receptors (italicized type), and drugs (boldface type) that are featured in this study. Arrows represent metabolic reactions; broken arrows indicate multiple steps; and thick shaded arrows represent receptor activation and induction processes.
There are considerable differences among species in the regulation of xenobiotic-metabolizing enzyme expression. A substantial portion of this variability can be attributed to interspecies differences in the amino acid sequences of the ligand-binding domains of PXR, with consequent differences in the dimensions of the large ligand-binding pockets (Jones et al., 2000). As a classic example, the catatoxic steroid pregnenolone 16α-carbonitrile is an efficacious activator of rodent PXR and inducer of rodent CYP3A, while having little activity toward the rabbit or human orthologs (Kocarek et al., 1995; Jones et al., 2000). By contrast, the macrocyclic antibiotic rifampicin displays the opposite species dependence for PXR activation and CYP3A induction (Kocarek et al., 1995; Jones et al., 2000). Therefore, it seems possible that interspecies differences apply not only to xenobiotics but also to endogenous molecules. Indeed, this is clearly illustrated by recent findings involving the bile acid precursor sterol 5β-cholestanoic acid-3α,7α,12α-triol. This sterol, which accumulates in the absence of functional CYP27, is an activator of murine PXR and a substrate for CYP3A (Furster and Wikvall, 1999; Honda et al., 2001; Dussault et al., 2003; Goodwin et al., 2003). CYP3A metabolism initiates an alternative pathway of sterol side-chain shortening, permitting the formation of cholic acid in hepatocytes lacking CYP27 activity (Honda et al., 2001; Goodwin et al., 2003). By contrast, bile acid precursor sterols do not activate human PXR, thereby explaining why humans with the genetic disease cerebrotendinous xanthomatosis, attributable to CYP27 deficiency, produce reduced levels of normal bile acids, accumulate sterols in various tissues, and exhibit a host of severe pathologies, whereas mice that have been genetically engineered to lack CYP27 do not (Dussault et al., 2003; Goodwin et al., 2003). This study was undertaken to investigate whether the PXR activation and CYP3A induction that we previously reported to occur in rodent hepatocytes following cyclase inhibition are conserved in human liver cell culture systems.
Materials and Methods
Materials. Ro 48-8071 and U18666A were purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). BIBX 79 was a gift from Boehringer Ingelheim USA (Ridgefield, CT). NB-598 was a gift from Banyu Pharmaceutical Co., Ltd. (Tokyo, Japan). Rifampicin, cholesterol, squalene, and T0901317 were purchased from Sigma-Aldrich (St. Louis, MO). Squalene 2,3-oxide (2,3-oxidosqualene, racemic) and squalene 2,3:22,23-dioxide (2,3,22,23-dioxidosqualene, mixture of diastereomers) were purchased from Echelon Biosciences (Salt Lake City, UT). Cell culture media, fetal bovine serum, and antibiotics were purchased from Invitrogen (Carlsbad, CA). Recombinant human insulin (Novolin R) was purchased from Novo Nordisk Pharmaceuticals, Inc. (Princeton, NJ). Other materials were obtained from the sources indicated below.
Primary Cultured Human Hepatocytes. Plated primary cultures of human hepatocytes were obtained through the Liver Tissue and Cell Distribution System (N01-DK-7-0004; University of Minnesota, Minneapolis, MN). Following hepatocyte preparation and overnight culture at the University of Pittsburgh, the hepatocytes, in T25 flasks, were express-shipped to Wayne State University and maintained as described previously (Duniec-Dmuchowski et al., 2007). The day following Matrigel treatment, the hepatocytes were incubated with fresh medium alone (untreated) or containing 3 to 30 μMRo 48-8071, 0.1% dimethyl sulfoxide (DMSO), or 50 μM rifampicin (in DMSO). The cultures were either harvested after 24-h incubation or retreated at 24 h and harvested after a total of 48 h of incubation.
TaqMan Real-Time Reverse Transcription-Polymerase Chain Reaction Analysis. Total RNA was prepared from individual T25 flasks of human hepatocytes, and levels of CYP3A4 and CYP2B6 mRNA were measured using TaqMan Gene Expression Assays Hs00430021_m1 and Hs00167937_g1, respectively (Applied Biosystems, Foster City, CA), as described previously (Duniec-Dmuchowski et al., 2007). Normalized mRNA contents were expressed relative to the untreated or DMSO-treated control group. The Wilcoxon sign-ranked test was used to compare median -fold changes against the hypothetical value of 1 (GraphPad Prism, version 5.0; GraphPad Software Inc., San Diego, CA). This test requires a sample size of at least six and therefore was applied only to the data shown in Fig. 2.
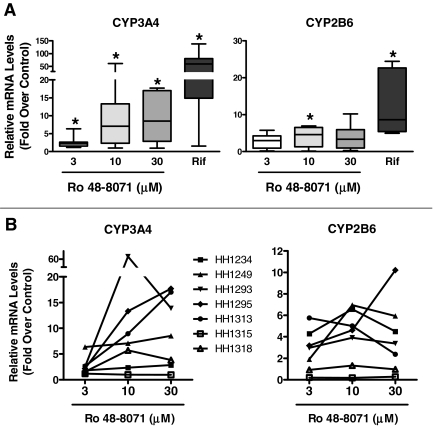
Fig. 2.
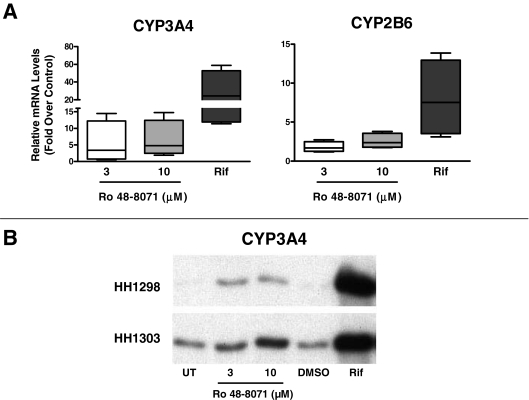
Effects of Ro 48-8071 treatment on CYP3A4 and CYP2B6 mRNA levels in primary cultured human hepatocytes. Seven preparations of primary cultured human hepatocytes were incubated for 24 h with standard medium alone or containing 3, 10, or 30 μM Ro 48-8071, 0.1% DMSO, or 50 μM rifampicin (Rif). After treatment, hepatocytes were harvested for preparation of total RNA, and CYP3A4 and CYP2B6 mRNA levels were measured as described under Materials and Methods. The Ro 48-8071 data are presented as -fold changes relative to untreated controls, whereas the Rif data are presented as -fold changes relative to DMSO-treated controls. A, data are presented as box and whisker plots, in which the horizontal lines within the boxes represent the median values, the tops and bottoms of the boxes represent the 25th and 75th percentile values, and the upper and lower error bars represent the maximum and minimum values. *, significantly different from the hypothetical value of 1, p < 0.05. B, Ro 48-8071 data from each individual hepatocyte preparation are plotted.
Western Blot Analysis. Microsomes were isolated from primary cultures of human hepatocytes (two pooled T25 flasks of hepatocytes per treatment group) as described previously (Kocarek and Reddy, 1996; Kocarek et al., 2002). Protein concentrations were determined by the bicinchoninic acid assay (Smith et al., 1985) using bovine serum albumin as standard. One microgram of microsomal protein of each sample was resolved by SDS-polyacrylamide gel electrophoresis on a Criterion precast 10% acrylamide Tris-HCl gel (Bio-Rad, Hercules, CA) and electrophoretically transferred to a polyvinylidene difluoride membrane (Bio-Rad). Blots were blocked overnight in 5% nonfat dry milk in Tris-buffered saline/Tween 20 (TBST) in a cold room. After brief washing in TBST, blots were incubated with a 1:20,000 dilution of monoclonal antibody WB-MAB-3A (reported to detect CYP3A4 and CYP3A5; BD Gentest, Woburn, MA) in blocking buffer overnight in a cold room. After extensive washing in TBST, blots were incubated with horseradish peroxidase-conjugated goat anti-mouse antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:20,000 in blocking buffer for 1 h at room temperature. Immunoreactive bands were visualized using ECL Plus Western Blotting Detection Reagents (GE Healthcare, Piscataway, NJ) according to the manufacturer's instructions.
Transient Transfection Analysis. HepG2 cells were cultured and transfected with the PXR-responsive reporter plasmid XREM-CYP3A4-Luc (provided by Dr. Bryan Goodwin, GlaxoSmithKline, Research Triangle Park, NC), the human PXR expression plasmid pSG5-hPXR1 (provided by Dr. Steven Kliewer, University of Texas Southwestern, Dallas, TX), and pRL-CMV (Promega, Madison, WI) as described previously (Duniec-Dmuchowski et al., 2007). The following day, the cells were treated as described in the legends to Figs. 4, 5, and 7. Twenty-four hours later, the cells were harvested for measurement of firefly and Renilla luciferase activities using the Dual-Luciferase Reporter Assay System (Promega). Luciferase data were analyzed by one-way analysis of variance followed by the Newman-Keuls or Dunnett's multiple comparison test and by fitting of concentration-response relationships with sigmoidal curves (variable slopes) using Prism version 5.0 (GraphPad Software Inc.).
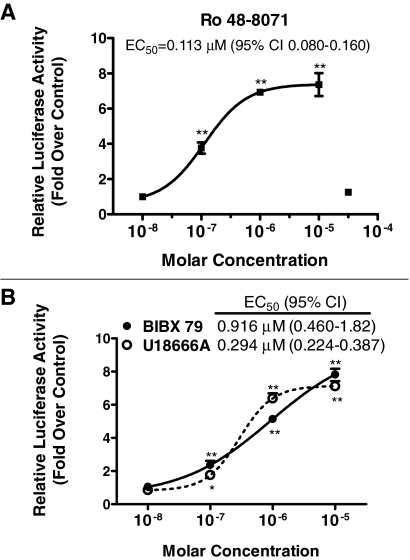
Fig. 4.
Concentration-dependent effects of cyclase inhibitor treatments on PXR-responsive reporter expression in HepG2 cells. HepG2 cells were transiently transfected with pSG5-hPXR1, XREM-CYP3A4-Luc, and pRL-CMV and then incubated for 24 h with medium alone or containing 10–8 to 3 × 10–5 M Ro 48-8071 (A) or with medium containing 0.1% DMSO or 10–8 to 10–5 M BIBX 79 or U18666A (B). After treatment, cells were harvested for measurement of firefly and Renilla luciferase activities. Normalized (firefly/Renilla) values are expressed as -fold changes ± S.D. (n = 3 wells/treatment group) relative to the appropriate negative control group (i.e., medium alone for Ro 48-8071; DMSO-treated for BIBX 79 and U18666A). Concentration-response data were fit to sigmoid relationships, and EC50 values with 95% confidence intervals (CIs) are shown. *, p < 0.05 and **, p < 0.01 versus negative control.
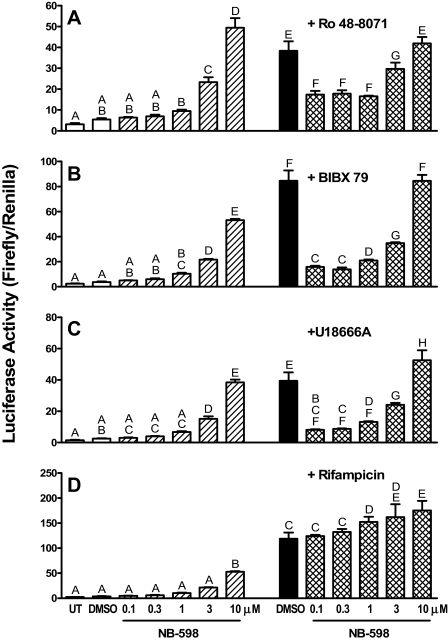
Fig. 5.
Effects of NB-598 cotreatments on cyclase inhibitor-mediated induction of PXR-responsive reporter expression in HepG2 cells. HepG2 cells were transiently transfected with pSG5-PXR, XREM-CYP3A4-Luc, and pRL-CMV and then incubated for 24 h with medium alone or containing 0.1% DMSO or 0.1 to 10 μM NB-598, alone or in combination with 10 μMRo 48-8071 (A), 10 μM BIBX 79 (B), 10 μM U18666A (C), or 50 μM rifampicin (D). After treatment, cells were harvested for measurement of firefly and Renilla luciferase activities. Data are expressed as mean normalized (firefly/Renilla) luciferase values ± S.D. (n = 3 wells/treatment group). Groups not sharing a letter are significantly different from each other, p < 0.05.
Fig. 7.
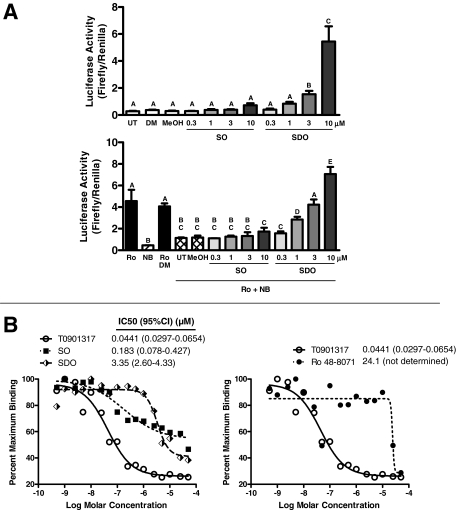
Concentration-dependent abilities of squalene 2,3-oxide and squalene 2,3:22,23-dioxide treatments to activate PXR-responsive reporter expression in HepG2 cells and to bind to PXR in vitro. A, HepG2 cells were transiently transfected with pSG5-hPXR1, XREM-CYP3A4-Luc, and pRL-CMV and then incubated for 24 h with (top) medium alone (UT) or containing 0.1% DMSO (DM), 0.1% methanol (MeOH), 0.3 to 10 μM squalene 2,3-oxide (SO), or 0.3 to 10 μM squalene 2,3:22,23-dioxide (SDO) or (bottom) 10 μM Ro 48-8071 (Ro), 0.1 μM NB-598 (NB), Ro and DM, or Ro and NB alone (UT) or in combination with MeOH, SO, or SDO. After treatment, cells were harvested for measurement of firefly and Renilla luciferase activities. Normalized (firefly/Renilla) values are expressed as mean ± S.D. (n = 3 wells/treatment group). Groups not sharing a letter are significantly different from each other, p < 0.05. B, the LanthaScreen TR-FRET PXR (SXR) competitive binding assay was used to evaluate the abilities of SO and SDO (left) and Ro 48-8071 (right) to interact with PXR in vitro. T0901317 was included as a positive control PXR ligand (the same binding data for T0901317 are replicated in each panel). IC50 values and 95% confidence intervals (CIs) are shown. For the Ro 48-8071 data, the IC50 value is an estimate, and CI could not be calculated.
Thin-Layer Chromatographic Detection of Metabolically Labeled Lipids. Metabolic labeling and detection of nonsaponifiable lipids was performed by modification of methods described by Pill et al. (1987) and Boogaard et al. (1987). In brief, 1.5 million HepG2 cells were plated into 60-mm dishes in Dulbecco's modified Eagle's medium supplemented with nonessential amino acids, 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin and incubated for 48 h. The cells were then treated with 0.1% DMSO, rifampicin, NB-598, and/or Ro 48-8071, as described in the legend to Fig. 6. One hour later, 2 μCi RS-[2-14C]mevalonate (MVA) (specific activity 55 mCi/mmol; GE Healthcare) was added to the culture medium. After 24 h, cells were washed with and scraped into cold phosphate-buffered saline and pelleted. The cell pellets were saponified in 15% potassium hydroxide in ethanol for 1 h at 80 °C. Nonsaponifiable lipids were extracted twice with n-hexane, and extracts were combined and evaporated under nitrogen. The residues were dissolved in 60 μl of hexane-chloroform (70:30, v/v) and spotted onto reverse-phase thin-layer chromatography plates (KC18; Whatman, Clifton, NJ), which were then developed twice with acetonitrile-chloroform (2:1, v/v). Authentic samples of cholesterol, squalene, squalene 2,3-oxide, and squalene 2,3:22,23-dioxide served as markers. These standards were spotted onto separate lanes and detected by spraying with 10% sulfuric acid in ethanol and heating. Radiolabeled lipids were then detected by exposing the plate to Eastman Kodak (Rochester, NY) BioMax XAR film for 3 days at room temperature.
Fig. 6.
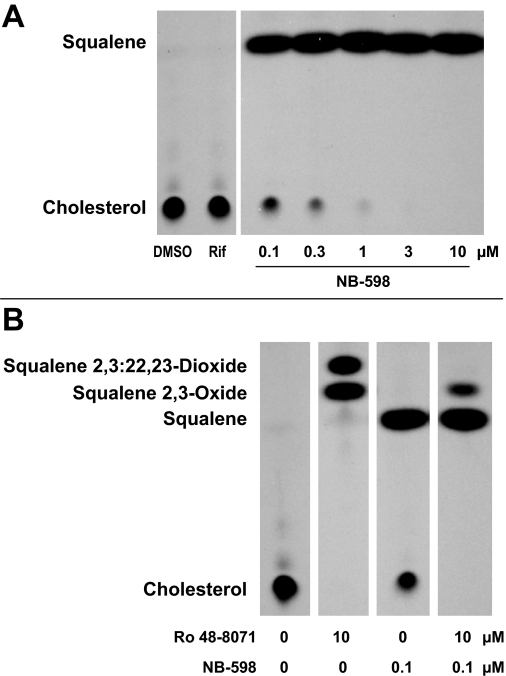
Effects of NB-598 and Ro 48-8071 treatments on sterol biosynthesis in HepG2 cells. A and B, HepG2 cells were incubated for 24 h with medium containing 0.1% DMSO, 30 μM rifampicin (Rif), or 0.1 to 10 μM NB-598 (A) or with 0.1% DMSO (0, 0), 10 μM Ro 48-8071, 0.1 μM NB-598, or Ro 48-8071 and NB-598 in combination (B). After treatment and metabolic labeling with [14C]MVA, cells were harvested for evaluation of nonsaponifiable lipid contents by thin-layer chromatography and autoradiography as described under Materials and Methods.
PXR in Vitro Binding Assay. The LanthaScreen time-resolved fluorescence resonance energy transfer (TR-FRET) PXR [steroid and xenobiotic receptor (SXR)] competitive binding assay (Invitrogen) was performed essentially according to the manufacturer's instructions except that the assay volume was reduced to 20 μl. All of the steps were carried out at room temperature. A serial dilution series of each test compound (squalene 2,3-oxide, squalene 2,3:22,23-dioxide, and T0901317) was prepared in the appropriate solvent (ethanol for the squalene metabolites, DMSO for T0901317). The test compounds were then diluted to 2 times their final concentrations in TR-FRET PXR (SXR) assay buffer, and 10 μl of each dilution was aliquoted in triplicate into the wells of a black 384-well assay plate (MatriCal Bioscience, Spokane, WA). Then, 5 μl of 4× Fluormone PXR (SXR) Green (fluorescent PXR ligand) was added to each well, followed by addition of 5 μl of 4× PXR–ligand-binding domain (glutathione-S-transferase fusion)/dithiothreitol/terbium-labeled anti-glutathione-S-transferase antibody solution in assay buffer. The plate was protected from light and incubated with shaking for 1 h, after which TR-FRET was measured using a Victor3 multilabel reader (PerkinElmer Life and Analytical Sciences, Waltham, MA) at the following settings: excitation wavelength 340 nm, emission wavelengths 520 and 490 nm, delay time 100 μs, and integration time 200 μs. The TR-FRET ratio for each sample was calculated by dividing the emission signal at 520 nm by the emission signal at 490 nm. Binding curves with IC50 values were generated by fitting emission ratio versus log molar ligand concentration data with sigmoid curves [log(inhibitor) versus response – variable slope] using Prism version 5.0 (GraphPad Software Inc.).
Results
Hepatocytes isolated from seven human livers were incubated for 24 h with the prototypical PXR ligand, rifampicin (at 50 μM), or with the cyclase inhibitor, Ro 48-8071, at concentrations of 3, 10, and 30 μM, and effects on CYP3A4 and CYP2B6 mRNA levels were measured (Fig. 2). Figure 2A shows the data plotted as -fold increases over control (i.e., Ro 48-8071 relative to untreated and rifampicin relative to DMSO-treated) in box and whisker format. Ro 48-8071 concentrations of 3, 10, and 30 μM produced significant median increases in CYP3A4 mRNA levels of 2.2-, 7.1-, and 8.5-fold, respectively, and increased CYP2B6 mRNA levels by 3.0-, 4.6-, and 3.4-fold, respectively (although only the effect at 10 μM was significant). For comparison, rifampicin treatment increased CYP3A4 and CYP2B6 mRNA levels by 59- and 8.7-fold in these hepatocyte preparations. There was considerable variability in the effects of Ro 48-8071 among hepatocyte preparations, as indicated both by the box and whisker plots (Fig. 2A) and the individual concentration-response plots shown in Fig. 2B. In most of the preparations, the Ro 48-8071-mediated increase in CYP3A4 mRNA content was maximal or near-maximal at 10 μM, although in two of the preparations the increase was at least 30% greater at 30 μM than it was at 10 μM. For CYP2B6, the Ro 48-8071-mediated increase in mRNA content was greatest at 10 μM in all but one preparation.
In four additional human hepatocyte preparations, 48-h treatment with Ro 48-8071 increased CYP3A4 mRNA content 3.4- to 4.8-fold and increased CYP2B6 mRNA content 1.7- to 2.4-fold at 3 and 10 μM, respectively (Fig. 3A). Although the sample size was insufficient to show statistical significance, in two of these hepatocyte preparations, microsomal CYP3A immunoreactive protein levels were also evaluated and found to be increased in Ro 48-8071-treated hepatocytes relative to untreated controls (Fig. 3B).
Fig. 3.
Effects of 48-h Ro 48-8071 treatment on CYP3A4 and CYP2B6 expression in primary cultured human hepatocytes. A, four preparations of primary cultured human hepatocytes were treated for 48 h with standard medium alone or containing 3 or 10 μM Ro 48-8071, 0.1% DMSO, or 50 μM rifampicin (Rif). After treatment, hepatocytes were harvested for preparation of total RNA, and CYP3A4 and CYP2B6 mRNA levels were measured as described under Materials and Methods. The Ro 48-8071 data are presented as -fold changes relative to untreated controls, whereas the Rif data are presented as -fold changes relative to DMSO-treated controls. Data are presented as box and whisker plots, in which the horizontal lines within the boxes represent the median values, the tops and bottoms of the boxes represent the 25th and 75th percentile values, and the upper and lower error bars represent the maximum and minimum values. B, hepatocytes from two of the preparations described in A were harvested for preparation of microsomes, and CYP3A immunoreactive protein levels were measured by Western blot hybridization.
The above-described experiments showed that treatment of primary cultured human hepatocytes with a cyclase inhibitor, Ro 48-8071, was capable of increasing CYP3A4 and CYP2B6 expression. To investigate the underlying mechanism, we used a HepG2-based platform, in which the cells were transiently cotransfected with a plasmid expressing human PXR and a PXR-responsive reporter plasmid (Duniec-Dmuchowski et al., 2007). When the transfected HepG2 cells were treated with Ro 48-8071, luciferase expression increased with an EC50 of 0.113 μM (Fig. 4A). Ro 48-8071-mediated induction was maximal at 1 μM and maintained at 10 μM but was completely attenuated at 30 μM (Fig. 4A). Treatment with either of two additional cyclase inhibitors, BIBX-79 or U18666A, also potently increased luciferase expression (EC50s of 0.916 and 0.294 μM, respectively) (Fig. 4B). In these experiments, Ro 48-8071, BIBX-79, and U18666A all produced maximal increases over control of ∼7- to 8-fold (Fig. 4, A and B) by comparison with rifampicin, which increased reporter activity ∼12-fold (data not shown).
To determine whether cyclase inhibitor treatments activated human PXR as a consequence of cyclase inhibition, rather than by functioning as direct PXR ligands, experiments were performed in which the step in the cholesterol biosynthetic pathway immediately preceding cyclase-catalyzed conversion of 2,3-oxidosqualene to lanosterol was inhibited (Fig. 1). Thus, NB-598 was used to inhibit squalene monooxygenase, which catalyzes the conversion of squalene to 2,3-oxidosqualene. In our previous study, NB-598 treatment inhibited Ro 48-8071-inducible CYP3A expression in primary cultured rat hepatocytes, but the inhibitory effect was critically dependent on the NB-598 concentration (Shenoy et al., 2004a). Therefore, transfected HepG2 cells were treated with a range of NB-598 concentrations, alone or in combination with a cyclase inhibitor (Fig. 5, A–C) or rifampicin, as a direct PXR ligand control (Fig. 5D). Treatment with NB-598 alone at concentrations of 0.1 or 0.3 μM had no significant effect on luciferase expression (Fig. 5, A–D) but markedly inhibited cholesterol biosynthesis as indicated by reduction of [14C]MVA incorporation into cholesterol with corresponding accumulation of [14C]squalene (Fig. 6A). Higher concentrations of NB-598, which produced essentially complete blockade of cholesterol biosynthesis (Fig. 6A), caused a concentration-dependent increase in luciferase activity that attained, at 10 μM NB-598, ∼45% of the increase produced by rifampicin treatment (Fig. 5D). As expected, rifampicin treatment had no effect on [14C]MVA incorporation into cholesterol (Fig. 6A). When transfected HepG2 cells were treated with one of the cyclase inhibitors (at 10 μM), cotreatment with NB-598 at those concentrations that alone had no effect on PXR-responsive reporter expression (0.1 or 0.3 μM) significantly attenuated cyclase inhibitor-inducible expression, whereas higher NB-598 concentrations again produced concentration-dependent increases in luciferase activity (Fig. 5, A–C). In correspondence with these findings, treatment with 10 μM Ro 48-8071 produced complete inhibition of cholesterol biosynthesis with accumulation of squalene 2,3-oxide and squalene 2,3:22,23-dioxide, whereas treatment with 0.1 μM NB-598 again produced marked inhibition of cholesterol biosynthesis with squalene accumulation (Fig. 6B). Combination treatment with 10 μM Ro 48-8071 and 0.1 μM NB-598 caused complete inhibition of cholesterol biosynthesis with accumulation of squalene and a small amount of squalene 2,3-oxide attributable to the residual squalene monooxygenase activity in the presence of 0.1 μM NB-598 (Fig. 6B). The significant reduction of cyclase inhibitor-mediated PXR activation by cotreatment with a low concentration of NB-598 that was capable of effectively inhibiting squalene monooxygenase but incapable of causing PXR activation strongly supports the conclusion that cyclase inhibitor-inducible activation of human PXR is mediated through the accumulation of squalene 2,3-oxide and/or squalene 2,3:22,23-dioxide. Cotreatment with NB-598 did not suppress rifampicin-inducible luciferase activity at any concentration tested (Fig. 5D). Rather, cotreatment with NB-598 concentrations of 1 μM or greater significantly enhanced the reporter induction that was produced by rifampicin treatment (Fig. 5D).
The abilities of squalene 2,3-oxide and squalene 2,3:22,23-dioxide to activate human PXR directly were evaluated next. Transfected HepG2 cells were treated with 0.3 to 10 μM squalene 2,3-oxide or squalene 2,3:22,23-dioxide, either alone (Fig. 7A, top) or in combination with Ro 48-8071 and NB-598, to prevent both endogenous formation and cyclase-mediated conversion of the squalene metabolites (Fig. 7A, bottom). Whereas treatment with squalene 2,3-oxide alone did not significantly increase PXR-responsive reporter expression (∼2.5-fold increase at 10 μM), treatment with squalene 2,3: 22,23-dioxide produced a marked concentration-dependent increase in reporter expression (∼18.4-fold increase at 10 μM) (Fig. 7A, top). As described above, treatment with Ro 48-8071 increased PXR-responsive reporter expression, and this increase was significantly attenuated by NB-598 cotreatment (Fig. 7A, bottom). Additional cotreatment with squalene 2,3:22,23-dioxide, but not squalene 2,3-oxide, significantly increased reporter activity relative to the level measured in Ro 48-8071/NB-598-cotreated cells (Fig. 7A, bottom).
Finally, a TR-FRET-based in vitro competitive binding assay was used to assess the abilities of squalene 2,3-oxide and squalene 2,3: 22,23 to bind directly to human PXR. T0901317 was used as a positive control PXR ligand in this assay, as we and others have recently reported that this compound is a potent activator of human PXR (Duniec-Dmuchowski et al., 2007; Mitro et al., 2007; Xue et al., 2007). T0901317 bound to PXR with an IC50 of 44.1 nM (Fig. 7B), consistent with the published PXR-activating and binding affinity of this compound (Duniec-Dmuchowski et al., 2007; Mitro et al., 2007; Xue et al., 2007). The competition curve for squalene 2,3:22,23-dioxide resembled that for T0901317, both in terms of slope and maximal displacement of the fluorescent PXR ligand (Fig. 7B, left). Squalene 2,3:22,23-dioxide bound to PXR with an IC50 of 3.35 μM (Fig. 7B, left), which was in accord with the concentration range that produced effective PXR transactivation in the HepG2 system. By contrast, although squalene 2,3-oxide bound to PXR with a calculated IC50 of 0.183 μM, the competition curve for this squalene metabolite was shallow and showed less maximal fluorescent ligand displacement relative to those for T0901317 and squalene 2,3:22,23-dioxide (Fig. 7B, left). For comparison, Ro 48-8071 showed little evidence of PXR binding at concentrations up to 10 μM. Higher concentrations caused fluorescent ligand displacement with a steep slope that permitted only estimation of an IC50 value (24.1 μM) without confidence intervals (Fig. 7B, right).
Discussion
Treatment of primary cultures of human hepatocytes with the cyclase inhibitor Ro 48-8071 increased the expression of CYP3A4, as well as expression of CYP2B6, which like CYP3A4 is a target gene for both PXR and CAR (Goodwin et al., 2001). Although there was considerable variability in the magnitude of Ro 48-8071-mediated CYP3A4/CYP2B6 induction among hepatocyte culture preparations, this variability was consistent with that observed with other inducers. For example, when 23 preparations of primary cultured human hepatocytes were treated with rifampicin, we observed CYP3A4 mRNA levels to increase from 1.6- to 436-fold (Fang et al., 2007). In most preparations, the magnitude of CYP3A4 mRNA induction that was produced by Ro 48-8071 treatment was lower than that produced by rifampicin treatment. For example, after 24-h treatment with 10 μM Ro 48-8071, the median increase in CYP3A4 mRNA content was 7.1-fold, whereas the median increase produced by rifampicin treatment was 59-fold.
The Ro 48-8071 concentrations required to produce effective CYP3A4 induction in primary cultured human hepatocytes were in the low micromolar range (3–30 μM), whereas PXR-responsive reporter activation in transfected HepG2 cells was maximal at 1 μMRo 48-8071. Thus, the Ro 48-8071 concentrations that produced CYP3A4 induction in primary cultured human hepatocytes were comparable with those we previously reported to cause CYP3A induction in primary cultured rat hepatocytes (Shenoy et al., 2004a) but were higher than those required to produce PXR activation in HepG2 cells. We speculate that these differences in Ro 48-8071 concentration-mediated effects between the primary hepatocyte and HepG2 systems are probably attributable to differences in the xenobiotic metabolism/transport capabilities of the cells.
One facet of cyclase inhibitor pharmacology that has been repeatedly observed is the ability of concentrations that produce submaximal inhibition of cyclase activity to cause accumulation of 24(S),25-epoxycholesterol, a potent endogenous LXR agonist, whereas maximally effective concentrations of cyclase inhibitors prevent 24(S),25-epoxycholesterol production (Mark et al., 1996; Morand et al., 1997; Janowski et al., 1999). We and others have reported that 24(S),25-epoxycholesterol is an effective activator of rodent PXR (Shenoy et al., 2004b; Gnerre et al., 2005). Therefore, in determining the mechanism by which cyclase inhibitor treatment causes PXR activation, it is necessary to consider whether effects are caused by 1) direct actions of the drugs as PXR ligand agonists, 2) effects of endogenous oxysterols that are formed downstream of cyclase, such as 24(S),25-epoxycholesterol, or 3) effects of endogenous metabolites that accumulate upstream of cyclase blockade. In this regard, we previously reported, through the use of various upstream pathway inhibitors and estimation of cellular squalene/sterol metabolite contents, that cyclase inhibition activated rodent PXR through accumulation of one or both of the squalene metabolites, squalene 2,3-oxide and squalene 2,3:22,23-dioxide (Shenoy et al., 2004a). The concentration-dependent effects of cyclase inhibitor treatments on squalene/sterol metabolite production in HepG2 cells have been investigated previously. Ro 48-8071 concentrations of 3 to 10 nM caused accumulation of 24(S),25-epoxycholesterol, whereas 100 nM Ro 48-8071 produced almost complete blockade of cholesterol biosynthesis, prevented 24(S),25-epoxycholesterol accumulation, and promoted accumulation of squalene 2,3-oxide and squalene 2,3:22,23-dioxide (Morand et al., 1997). BIBX 79 and U18666A produced their greatest effects on PXR-responsive reporter expression at concentrations of 10 and 1 μM, respectively. Short-term incubation of HepG2 cells with BIBX 79 was reported to inhibit cholesterol biosynthesis by greater than 98% at 1 μM, a concentration that completely blocked 24(S),25-epoxycholesterol formation but caused substantial accumulation of squalene 2,3-oxide and squalene 2,3:22,23-dioxide (Mark et al., 1996). In the same study, incubation with 0.1 μM U18666A effectively inhibited cholesterol biosynthesis, although 24(S),25-epoxycholesterol continued to accumulate at this concentration (Mark et al., 1996). At 1 μM U18666A, biosynthesis of cholesterol and 24(S),25-epoxycholesterol was abolished, whereas squalene 2,3-oxide and squalene 2,3:22,23-dioxide accumulated substantially (Mark et al., 1996). Therefore, the concentrations of the various cyclase inhibitors that caused PXR activation in HepG2 cells were those that promote the accumulation of squalene 2,3-oxide and squalene 2,3:22,23-dioxide, but not 24(S),25-epoxycholesterol. Consistent with these findings, we have observed that direct incubation of human PXR/reporter-transfected HepG2 cells with 24(S),25-epoxycholesterol does not cause reporter induction (Duniec-Dmuchowski et al., 2007). These findings suggest that 24(S),25-epoxycholesterol, like the bile acid precursor sterol, 5β-cholestanoic acid-3α,7α,12α-triol, activates rodent but not human PXR.
Our findings using the squalene monooxygenase inhibitor NB-598 in combination with the various cyclase inhibitors strongly implicate either or both of the squalene metabolites, squalene 2,3-oxide and squalene 2,3:22,23-dioxide, as endogenous activators of human PXR. NB-598 concentrations of 0.1 to 0.3 μM are sufficient to produce effective inhibition of cholesterol biosynthesis in HepG2 cells (Horie et al., 1990) (Fig. 6A). These NB-598 concentrations caused significant inhibition of the PXR-responsive reporter induction that was produced by all the cyclase inhibitors, while having no inhibitory effect on rifampicin-mediated induction. Higher concentrations of NB-598 produced concentration-dependent increases in PXR-responsive reporter expression, either when used alone or in combination with a cyclase inhibitor, an effect we speculate is attributable to direct PXR ligand activity. However, coincubation with NB-598, at concentrations of 1 μM and higher, also produced significant increases in reporter induction above that produced by a maximally effective concentration of the direct-acting PXR ligand rifampicin. The mechanism underlying this drug interaction is under investigation.
Direct treatments of PXR/reporter-transfected HepG2 cells with squalene 2,3-oxide or squalene 2,3:22,23-dioxide revealed that the latter compound was capable of producing marked PXR activation. This effect was observed either when squalene 2,3:22,23-dioxide was applied as the only treatment or when the squalene 2,3:22,23-dioxide was used together with NB-598 and Ro 48-8071, the combination of which served both to prevent the endogenous formation of squalene oxide metabolites and their further conversion to lanosterol or 24(S),25-oxidolanosterol and downstream sterols. Use of an in vitro TR-FRET-based assay confirmed the ability of squalene 2,3:22,23-dioxide to bind directly to human PXR with an affinity that was consistent with the ability of this metabolite to transactivate PXR in the HepG2 system. These results indicate that squalene 2,3:22,23-dioxide is capable of activating human PXR and therefore may mediate the PXR activation that occurs following treatment with a cyclase inhibitor. Although the HepG2 transfection data do not support a role for squalene 2,3-oxide in PXR activation, it is not possible to conclude definitively that squalene 2,3-oxide is inactive in this regard because the degree of cellular uptake of this metabolite following exogenous treatment is unknown. Squalene 2,3-oxide did show some ability to bind to human PXR in vitro, although the binding curve did not reveal the same efficacy of fluorescent ligand displacement that was seen for squalene 2,3:22,23-dioxide. It is also important to emphasize that the squalene oxide compounds that were used for these studies were mixtures of optical isomers rather than the pure enantiomers that are formed by endogenous metabolism. As a final point, although NB-598 treatment significantly inhibited cyclase-mediated PXR activation, the PXR activity was not reduced to the control level occurring in untreated HepG2 cells. This residual PXR activation is probably attributable to some ability of the cyclase inhibitors to function as direct PXR ligands. In this regard, Ro 48-8071 did exhibit ability to bind to human PXR in vitro at high concentrations.
In summary, our results indicate that cyclase inhibitors are capable of producing CYP3A4 and CYP2B6 induction in primary cultures of human hepatocytes and of activating human PXR. As in rodent systems, these effects are mediated through the actions of endogenous squalene metabolites, the likeliest candidate being squalene 2,3:22,23-dioxide. Unlike the endogenous sterols that have been found capable of activating rodent but not human PXR, the PXR-activating abilities of the squalene metabolites are conserved from rodent to human. Of the other known endogenous inducers of CYP3A4, vitamin D3 exerts its effect through the vitamin D receptor (Schmiedlin-Ren et al., 2001). In addition, although the primary bile acid chenodeoxycholic acid has been reported to be capable of activating PXR (Xie et al., 2001), recent findings indicate that primary bile acids can also induce CYP3A as a consequence of farnesoid X receptor activation, both directly through the transactivation of CYP3A4 as an farnesoid X receptor target gene (Gnerre et al., 2004) and indirectly through the transcriptional up-regulation of PXR (Jung et al., 2006). Certain secondary bile acids, such as lithocholic acid and ursodeoxycholic acid, are known to activate murine and human PXR, consistent with a conserved role for PXR in protection from cholestasis (Uppal et al., 2007). Our results show that squalene metabolites are conserved intracrine activators of PXR and that cyclase may be considered to be a “receptor” that regulates the expression of CYP3A4 and other PXR target genes.
This work was supported in part by the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL50710]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES05823]; the National Institutes of Health National Institute of Environmental Health Sciences Center [Grant P30-ES06639] (Cell Culture and Gene Transfer Technologies, Imaging and Cytometry, and Microarray and Bioinformatics Facility Cores); and the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Contract N01-DK70004 HHSN267200700004C].
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.025130.
ABBREVIATIONS: PXR, pregnane X receptor; CAR, constitutive androstane receptor; cyclase, 2,3-oxidosqualene:lanosterol cyclase; Ro 48-8071, [4′-(6-allyl-methyl-amino-hexyloxy)-2′-fluoro-phenyl]-(4-bromophenyl)-methanone fumarate; NB-598, (E)N-ethyl-N-(6,6-dimethyl-2-hepten-4-ynyl)-3-[(3,3′-bithiophen-5-yl)methoxy]benzenemethanamine; U18666A, 3β-(2-diethylaminoethoxy)androst-5-en-17-one HCl; BIBX 79, trans-N-(4-chlorobenzoyl)-N-methyl-(4-dimethylaminomethylphenyl)-cyclohexylamine; DMSO, dimethyl sulfoxide; TBST, Tris-buffered saline/Tween 20; MVA, mevalonate; TR-FRET, time-resolved fluorescence resonance energy transfer; SXR, steroid and xenobiotic receptor; T0901317, N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide.
References
- Boogaard A, Griffioen M, and Cohen LH (1987) Regulation of 3-hydroxy-3-methylglutarylcoenzyme A reductase in human hepatoma cell line Hep G2. Effects of inhibitors of cholesterol synthesis on enzyme activity. Biochem J 241 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duniec-Dmuchowski Z, Ellis E, Strom SC, and Kocarek TA (2007) Regulation of CYP3A4 and CYP2B6 expression by liver X receptor agonists. Biochem Pharmacol 74 1535–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussault I, Yoo HD, Lin M, Wang E, Fan M, Batta AK, Salen G, Erickson SK, and Forman BM (2003) Identification of an endogenous ligand that activates pregnane X receptor-mediated sterol clearance. Proc Natl Acad Sci U S A 100 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang HL, Strom SC, Ellis E, Duanmu Z, Fu J, Duniec-Dmuchowski Z, Falany CN, Falany JL, Kocarek TA, and Runge-Morris M (2007) Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4alpha and pregnane X receptor. J Pharmacol Exp Ther 323 586–598. [DOI] [PubMed] [Google Scholar]
- Furster C and Wikvall K (1999) Identification of CYP3A4 as the major enzyme responsible for 25-hydroxylation of 5β-cholestane-3α,7α,12α-triol in human liver microsomes. Biochim Biophys Acta 1437 46–52. [DOI] [PubMed] [Google Scholar]
- Gnerre C, Blättler S, Kaufmann MR, Looser R, and Meyer UA (2004) Regulation of CYP3A4 by the bile acid receptor FXR: evidence for functional binding sites in the CYP3A4 gene. Pharmacogenetics 14 635–645. [DOI] [PubMed] [Google Scholar]
- Gnerre C, Schuster GU, Roth A, Handschin C, Johansson L, Looser R, Parini P, Podvinec M, Robertsson K, Gustafsson JA, et al. (2005) LXR deficiency and cholesterol feeding affect the expression and phenobarbital-mediated induction of cytochromes P450 in mouse liver. J Lipid Res 46 1633–1642. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Gauthier KC, Umetani M, Watson MA, Lochansky MI, Collins JL, Leitersdorf E, Mangelsdorf DJ, Kliewer SA, and Repa JJ (2003) Identification of bile acid precursors as endogenous ligands for the nuclear xenobiotic pregnane X receptor. Proc Natl Acad Sci U S A 100 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Moore LB, Stoltz CM, McKee DD, and Kliewer SA (2001) Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol 60 427–431. [PubMed] [Google Scholar]
- Honda A, Salen G, Matsuzaki Y, Batta AK, Xu G, Leitersdorf E, Tint GS, Erickson SK, Tanaka N, and Shefer S (2001) Side chain hydroxylations in bile acid biosynthesis catalyzed by CYP3A are markedly up-regulated in Cyp27–/– mice but not in cerebrotendinous xanthomatosis. J Biol Chem 276 34579–34585. [DOI] [PubMed] [Google Scholar]
- Horie M, Tsuchiya Y, Hayashi M, Iida Y, Iwasawa Y, Nagata Y, Sawasaki Y, Fukuzumi H, Kitani K, and Kamei T (1990) NB-598: a potent competitive inhibitor of squalene epoxidase. J Biol Chem 265 18075–18078. [PubMed] [Google Scholar]
- Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, and Mangelsdorf DJ (1999) Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc Natl Acad Sci U S A 96 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14 27–39. [DOI] [PubMed] [Google Scholar]
- Jung D, Mangelsdorf DJ, and Meyer UA (2006) Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem 281 19081–19091. [DOI] [PubMed] [Google Scholar]
- Kocarek TA, Dahn MS, Cai H, Strom SC, and Mercer-Haines NA (2002) Regulation of CYP2B6 and CYP3A expression by hydroxymethylglutaryl coenzyme A inhibitors in primary cultured human hepatocytes. Drug Metab Dispos 30 1400–1405. [DOI] [PubMed] [Google Scholar]
- Kocarek TA and Mercer-Haines NA (2002) Squalestatin 1-inducible expression of rat CYP2B: evidence that an endogenous isoprenoid is an activator of the constitutive androstane receptor. Mol Pharmacol 62 1177–1186. [DOI] [PubMed] [Google Scholar]
- Kocarek TA and Reddy AB (1996) Regulation of cytochrome P450 expression by inhibitors of hydroxymethylglutaryl-coenzyme A reductase in primary cultured rat hepatocytes and in rat liver. Drug Metab Dispos 24 1197–1204. [PubMed] [Google Scholar]
- Kocarek TA, Schuetz EG, Strom SC, Fisher RA, and Guzelian PS (1995) Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab Dispos 23 415–421. [PubMed] [Google Scholar]
- Mark M, Muller P, Maier R, and Eisele B (1996) Effects of a novel 2,3-oxidosqualene cyclase inhibitor on the regulation of cholesterol biosynthesis in HepG2 cells. J Lipid Res 37 148–158. [PubMed] [Google Scholar]
- Mitro N, Vargas L, Romeo R, Koder A, and Saez E (2007) T0901317 is a potent PXR ligand: implications for the biology ascribed to LXR. FEBS Lett 581 1721–1726. [DOI] [PubMed] [Google Scholar]
- Morand OH, Aebi JD, Dehmlow H, Ji YH, Gains N, Lengsfeld H, and Himber J (1997) Ro 48-8071, a new 2,3-oxidosqualene:lanosterol cyclase inhibitor lowering plasma cholesterol in hamsters, squirrel monkeys, and minipigs: comparison to simvastatin. J Lipid Res 38 373–390. [PubMed] [Google Scholar]
- Pill J, Aufenanger J, Stegmeier K, Schmidt FH, and Muller D (1987) Thin-layer chromatography of radioactively labeled cholesterol and precursors from biological material. A simple and sensitive method for investigating actions on the sterol pathway. Fresenius Z Anal Chem 327 558–560. [Google Scholar]
- Quattrochi LC and Guzelian PS (2001) CYP3A regulation: from pharmacology to nuclear receptors. Drug Metab Dispos 29 615–622. [PubMed] [Google Scholar]
- Schmiedlin-Ren P, Thummel KE, Fisher JM, Paine MF, and Watkins PB (2001) Induction of CYP3A4 by 1α,25-dihydroxyvitamin D3 is human cell line-specific and is unlikely to involve pregnane X receptor. Drug Metab Dispos 29 1446–1453. [PubMed] [Google Scholar]
- Shenoy SD, Spencer TA, Mercer-Haines NA, Abdolalipour M, Wurster WL, Runge-Morris M, and Kocarek TA (2004a) Induction of CYP3A by 2,3-oxidosqualene:lanosterol cyclase inhibitors is mediated by an endogenous squalene metabolite in primary cultured rat hepatocytes. Mol Pharmacol 65 1302–1312. [DOI] [PubMed] [Google Scholar]
- Shenoy SD, Spencer TA, Mercer-Haines NA, Alipour M, Gargano MD, Runge-Morris M, and Kocarek TA (2004b) CYP3A induction by liver X receptor ligands in primary cultured rat and mouse hepatocytes is mediated by the pregnane X receptor. Drug Metab Dispos 32 66–71. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, and Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150 76–85. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 98 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal H, Saini SP, Moschetta A, Mu Y, Zhou J, Gong H, Zhai Y, Ren S, Michalopoulos GK, Mangelsdorf DJ, et al. (2007) Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology 45 422–432. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Maglich JM, Moore LB, Wisely GB, Noble SM, Davis-Searles PR, Lambert MH, Kliewer SA, and Redinbo MR (2003) 2.1 A crystal structure of human PXR in complex with the St. John's wort compound hyperforin. Biochemistry 42 1430–1438. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, and Redinbo MR (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292 2329–2333. [DOI] [PubMed] [Google Scholar]
- Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, Waxman DJ, and Evans RM (2001) An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A 98 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Chao E, Zuercher WJ, Willson TM, Collins JL, and Redinbo MR (2007) Crystal structure of the PXR-T1317 complex provides a scaffold to examine the potential for receptor antagonism. Bioorg Med Chem 15 2156–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]