Abstract
The objective of this study was to determine the pharmacokinetic parameters of clonidine during pregnancy compared with previously published data in nonpregnant subjects. Serial blood and urine samples were collected in 17 women during mid to late pregnancy over one steady-state dosing interval to determine clonidine noncompartmental pharmacokinetic parameters (n = 17) and creatinine clearance. In six of these pregnant subjects, maternal and umbilical cord (venous and arterial) plasma samples were collected at the time of delivery for measurement of clonidine concentrations. Clonidine apparent oral clearance was found to be 440 ± 168 ml/min during pregnancy compared with 245 ± 72 ml/min as previously reported in nonpregnant subjects (p < 0.0001) (Cunningham et al., 1994). There was a strong correlation (r = 0.82, p < 0.001) between clonidine renal clearance, adjusted for variation in glomerular filtration rate, and urine pH. Umbilical cord to maternal plasma clonidine concentration ratios were 1.0 ± 0.1 (arterial) and 1.0 ± 0.1 (venous). In conclusion, clonidine is cleared more rapidly in pregnant women than in nonpregnant subjects. At the time of delivery, the fetus is exposed to similar plasma clonidine concentrations as the mother.
Chronic hypertension during pregnancy is associated with complications, including fetal growth restriction, premature birth, preeclampsia, placental abruption, hypertensive crisis, and in some cases fetal demise. Very little information is available on the pharmacokinetic changes that occur for antihypertensive medications during pregnancy. Clonidine, a centrally acting, α-2 adrenergic agonist, has been used for the treatment of hypertension during pregnancy (Kobinger, 1978; Hartikainen-Sorri et al., 1987). In the nonpregnant population, approximately 60% of clonidine is eliminated unchanged by the kidneys (Davies et al., 1977). There are significant changes in kidney function during pregnancy, with creatinine clearance reported to increase by 50 to 60% (Davison and Noble, 1981). The pharmacokinetics of clonidine have not been studied during pregnancy. The objective of this study was to characterize the pharmacokinetics of clonidine during pregnancy.
Materials and Methods
After receiving written informed consent from patients, we examined steady-state pharmacokinetics of orally administered clonidine in the plasma of 17 pregnant women receiving clonidine during mid pregnancy (22–26 weeks gestation) or late pregnancy (34–38 weeks gestation). Six of these women participated in maternal and umbilical cord (arterial and venous) plasma sample collections at the time of delivery for measurement of clonidine concentrations. Gestational age was based on the last menstrual period or early ultrasound dating.
Subject Selection. This study was approved by the University of Washington Investigational Review Board. Subjects were eligible to participate if they were pregnant, 18 years of age, had a hematocrit ≥28%, and were receiving oral clonidine for maternal hypertension.
Dosing Regimen. Oral clonidine therapy was not altered for study purposes. Dosages ranged from 0.15 to 0.30 mg per day, given in divided doses. The duration of the pharmacokinetic study was dependent on the subject's dosage interval, which ranged from 6 to 12 h. For 3 days before the study, oral clonidine tablets, from the same manufacturing lot, were provided. Subjects recorded the time of each dose on a study calendar. Subjects took their morning clonidine dose within 30 min of 8:00 AM. Subsequent doses were taken within 30 min of scheduled administration times. On the morning of the pharmacokinetic study, subjects were asked to fast, with the exception of clear liquids, for 5 h before clonidine administration until 1 h after dosing.
Sample Collection. Serial plasma samples were collected at the following times: 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h postdosing, or truncated to correspond to the subject's dosing interval. Urine was collected over one dosing interval to determine clonidine renal clearance and creatinine clearance. Plasma samples, maternal and umbilical cord (arterial and venous), were collected at the time of delivery for quantifying clonidine concentrations. All samples were stored at –80°C until sample analysis.
Clonidine Concentrations. Plasma clonidine concentrations were quantified using a validated liquid chromatography/mass spectrometry (LC/MS) assay. In brief, 250 μl of plasma samples was mixed with 500 pg of internal standard (clonidine-d4) in 500 μl of sodium carbonate (0.1 M) (pH = 9.3) and 3 ml of heptane/isoamyl alcohol (98.5:1.5). Standards were prepared by spiking blank plasma with 0, 12.5, 25, 50, 100, 200, 250, 375, 500, and 750 pg of clonidine. Samples were shaken on a horizontal shaker for 15 min at room temperature. After centrifugation (1500g for 10 min), the samples were frozen on dry ice and the organic layer decanted to a clean culture tube. Two milliliters of sulfuric acid aliquots (0.05 M) was added to the organic layer and again shaken for 15 min. After centrifugation (1500g for 10 min), the samples were frozen on dry ice and the organic layer was discarded. A 200-μl aliquot of 29.29% aqueous ammonium hydroxide was added to the aqueous layer along with 3 ml of heptane/isoamyl alcohol (98.5:1.5) and shaken for 15 min. After centrifugation (1500g for 10 min), the samples were frozen on dry ice and the organic layer decanted to a clean culture tube. The organic layer was evaporated under a stream of nitrogen at 40°C. The residue was reconstituted with 75 μl of formic acid (12 mM)/methanol (60:40), 10 μl of which was injected onto the LC/MS system.
Urine samples of 250 μl were mixed with 250 μl of formic acid (12 mM) and 50 ng of internal standard (clonidine-d4). Standards were prepared by spiking blank urine with 0, 1.25, 2.5, 6.25, 12.5, 18.75, and 25 ng of clonidine, respectively. The samples were vortexed, and 1 μl was injected onto the LC/MS.
The LC/MS system was an Agilent 1100 MSD (Agilent Technologies, Santa Clara, CA) consisting of binary pump, autosampler, column compartment, and mass spectrometer. The high-performance liquid chromatography column was a Phenomenex Synergi Polar RP80A, 250 mm × 2.0 mm, 4 μm (Phenomenex, Torrance, CA). The column was maintained at 40°C. Mobile phase components were 12 mM formic acid (A) and methanol (B), with an initial flow rate of 0.25 ml/min. The initial mobile phase was maintained for 1 min and consisted of 40% B for plasma samples and 35% B for urine samples. The percentage of B was increased linearly over the next 4 min to 90% for plasma and 60% for urine and then maintained constant for 1 min with a linear flow increase to 0.3 ml/min for urine and plasma. For the return gradient, the percentage of B was then decreased linearly over 1 min to 40% (plasma) or 35% (urine), which was then maintained constant. The flow rate was maintained at 0.3 ml/min for 1 min and then linearly decreased over 1 min to 0.25 ml/min and then maintained constant. The total run time was 12.5 min. For both plasma and urine assays, the mass spectrometer was operated in the positive electrospray mode and set for selective ion monitoring of m/z 230 (clonidine) and m/z 236 (clonidine-d4) with dwell time of 289 msec for each ion.
Pharmacokinetic Parameters. Steady-state pharmacokinetic parameters were determined using standard noncompartmental techniques. The area under the plasma clonidine concentration-time curve over one dosing interval (AUCτ) was calculated using the linear trapezoidal rule. Apparent oral clearance (CL/F) was calculated by CL/F = Dose/AUCτ. Clonidine renal clearance (CLrenal) was calculated by CLrenal = Ae,τ/AUCτ, where Ae,τ = the amount of clonidine excreted unchanged in the urine over one dosing interval. Creatinine clearance was calculated by CrCl = (VolumeUrine × CreatinineUrine)/(CreatinineSerum× τ). Clonidine renal clearance adjusted for glomerular filtration rate (GFR) was calculated by CLrenal adjusted for GFR = CLrenal – fu × CrCl, where the fraction unbound in plasma (fu) was assumed to be 0.80 (Hulter et al., 1979). In all our subjects, the relatively short dosing interval did not allow accurate estimation of clonidine half-life, which has previously been reported to be 11.1 ± 3.3 and 10.8 ± 2.2 h in nonpregnant healthy adults (Cunningham et al., 1994; Fujimura et al., 1994).
Statistics. Our clonidine pharmacokinetic parameters in pregnancy were compared with previously published parameters in healthy, nonpregnant volunteers (CL/F, Porchet et al., 1992; Cunningham et al., 1994) (CLrenal, Fujimura et al., 1994). Linear regression was used to determine correlation between creatinine clearance and clonidine renal clearance. Unpaired Student's t test was used to compare the pregnant and nonpregnant groups. A p value <0.05 was considered to be significant. All results are reported as mean ± S.D.
Results
Subjects. Seventeen subjects (10 white, 4 black, 1 Hispanic/Latina, 1 Hispanic/Latina/Native American, and 1 Native American/white) completed the pregnancy phase of the study. The average age was 32.1 ± 8.0 years old, body weight 97.6 ± 21.5 kg (range 71.0–153.6 kg), and height 163.9 ± 8.0 cm. Five subjects were studied during mid pregnancy (22–26 weeks gestation) and 12 subjects were studied during late pregnancy (34–38 weeks gestation). One subject completed both a mid- and late-pregnancy study day. The pharmacokinetic parameters were similar for each study day, and thus, to eliminate bias, we only included her late-pregnancy study day results. Six of these pregnant women were studied at the time of delivery.
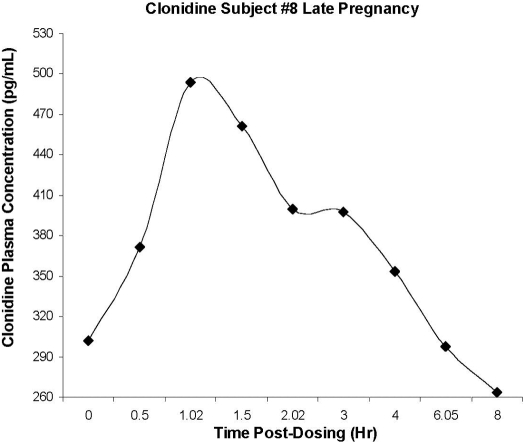
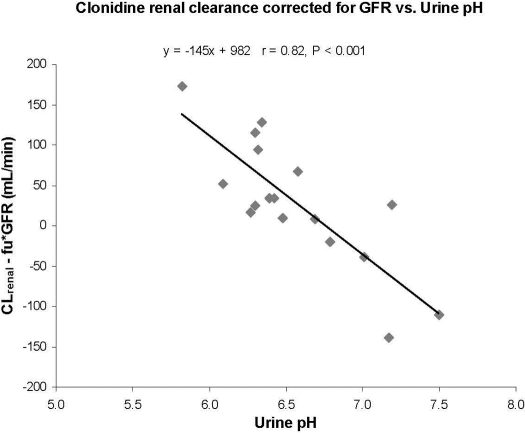
Pharmacokinetics. Figure 1 is a representative clonidine plasma concentration-time curve. The average maternal peak clonidine concentration was 574 ± 279 pg/ml. Mean apparent oral clearance of clonidine was significantly higher in pregnant women (440 ± 168 ml/min) compared with the literature reported for the nonpregnant population (245 ± 72 ml/min, p < 0.0001; 233 ± 38 ml/min, p < 0.0001) [Cunningham et al., 1994 (male n = 4, female n = 6, 20–42 years, radioimmunoassay); Porchet et al., 1992 (healthy volunteers n = 10, 23–34 years, radioimmunoassay)]. Creatinine clearance was 166 ± 75 ml/min in the pregnant women. It is interesting to note that clonidine renal clearance in pregnancy (153 ± 67 ml/min) was not significantly different from that seen in the nonpregnant population (183 ± 55 ml/min, p = 0.3) [Fujimura et al., 1994 (male n = 8, 26–38 years, 61–81 kg, gas chromatography/mass spectrometry and gas chromatography/electron capture detection assays)]. Only 36 ± 11% of the clonidine was excreted unchanged in the urine in pregnancy compared with 59 ± 18% in the nonpregnant population (p < 0.0001) [Arndts, 1983 (n = 19, radioimmunoassay)]. There was a poor correlation (r = 0.26) between clonidine renal clearance and creatinine clearance (data not shown). However, a strong correlation (r = 0.82, p < 0.001) existed between clonidine renal clearance, corrected for GFR, and urine pH (range 5.8–7.5; Fig. 2).
Fig. 1.
Representative steady-state oral clonidine plasma concentration time profile during late pregnancy (34.1 weeks gestation).
Fig. 2.
Correlation between clonidine renal clearance, corrected for GFR, and urine pH during mid pregnancy (22–26 weeks gestation, n = 5) and late pregnancy (34–38 weeks gestation, n = 12).
At parturition, the mean umbilical cord to maternal plasma clonidine concentration ratios were 1.0 ± 0.1 (venous) and 1.0 ± 0.1 (arterial), which remained fairly constant between 4 and 10 h after the last maternal clonidine dose (data not shown). The average maternal clonidine plasma concentration at the time of delivery was 404 ± 164 pg/ml (range 164–603 pg/ml). The average venous and arterial umbilical cord concentrations at the time of delivery were 394 ± 142 pg/ml (range 191–586 pg/ml) and 402 ± 147 pg/ml (range 204–614 pg/ml), respectively.
Discussion
The obstetricians at the University of Washington have frequently observed a diminished response to clonidine in pregnant patients near the end of a standard 12-h dosing interval. As a result, they often shorten the dosing interval to 6 to 8 h (i.e., prescribe more frequent dosing) when treating maternal hypertension. Because kidney function, as measured by creatinine clearance, is known to increase during pregnancy and clonidine is predominantly eliminated by the kidneys in nonpregnant subjects, we hypothesized that pregnancy may alter the pharmacokinetics of clonidine. Indeed, the pregnant subjects in our study have a significantly higher apparent oral clearance of clonidine than previously reported in nonpregnant healthy male and female subjects with similar age ranges. It is possible that the difference may in part reflect clonidine assay specificity in that we used an LC/MS assay and the previously published studies used radioimmunoassays. However, both radioimmunoassays demonstrate cross-reactivity with only one of clonidine's metabolites. The cross-reacting metabolite is present in very low concentrations in human plasma, making it very unlikely to be the explanation for the differences seen in this study (Darda et al., 1978). The question then becomes what is the mechanism underlying the increase in apparent oral clearance of clonidine. Possible mechanisms include changes in renal clearance, metabolic clearance, plasma protein binding, and/or bioavailability.
Despite the increase in creatinine clearance seen during pregnancy, clonidine renal clearance was not significantly different from that previously reported in healthy male volunteers. The situation may be complicated by the dependence of net secretion and/or reabsorption of clonidine upon urine pH. Clonidine is a basic drug with a pKa reported to be 8.05 (Dollery, 1991). At low urine pH, clonidine is ionized (protonated) resulting in net renal secretion, whereas at high urine pH, net renal reabsorption occurs. Thus, the rather large variation in urine pH observed in this study might have masked the impact of increased renal filtration seen during pregnancy resulting in no apparent change in clonidine renal clearance. In any case, clonidine renal clearance appears not to be the principal reason for the increase in clonidine apparent oral clearance.
Clonidine is a low hepatic extraction ratio drug (Davies et al., 1977). Change in hepatic blood flow is not a likely reason for the increased clonidine apparent oral clearance. For orally administered medications, changes in hepatic blood flow should not change the oral area under the curve for either high or low extraction ratio drugs. Although we expect that intestinal blood flow may be increased as a result of the increased cardiac output during pregnancy, this would probably result in increased bioavailability and higher plasma concentrations rather than the lower concentrations seen during pregnancy. Plasma protein binding is unlikely to be the reason for the 1.8-fold increase in clonidine apparent oral clearance because it is only 20% bound in the plasma. Even if the percentage bound for clonidine decreased to zero during pregnancy, it would not explain the changes seen in clonidine apparent oral clearance. Although not expected for clonidine, a drug with presumably good oral absorption, it is possible that the extent of absorption could be decreased in pregnancy.
Accordingly, alteration in hepatic and/or intestinal intrinsic clearances are the most likely reasons for the higher apparent oral clearance of clonidine during pregnancy. A lower recovery of unchanged drug in the urine was observed in our pregnant women compared with data reported for nonpregnant subjects (36% versus 59%), which is entirely consistent with the hypothesis that pregnancy shifts the major route of elimination for clonidine from the kidneys to organs involved in drug metabolism (Davies et al., 1977; Arndts, 1983). The enzymes involved in clonidine metabolism are currently unknown; however, pregnancy does increase the activities of CYP3A, CYP2D6, and CYP2C9 (Yerby et al., 1990; Tracy et al., 2005; Hebert et al., 2008). Clonidine is metabolized to five metabolites including the following: p-hydroxy-clonidine; dichlorophenylguanidine; 1-(2,6-dichloro-4-hydroxyphenyl)-guanidine; 2-[(2,6-dichlorophenyl)-imino]-imidazolidine-4-one; and 2-[2,6-dichloro-4-hydroxyphenyl)-imino]-imidazolidine-4-one (Darda et al., 1978). The primary metabolite (p-hydroxyclonidine) of clonidine is <10% of unchanged drug in the urine from nonpregnant subjects (Darda et al., 1978; Arndts et al., 1979). The metabolites of clonidine have been reported to be inactive (Lowenthal, 1980). Further research is underway to characterize which drug-metabolizing enzymes are involved in the nonrenal clearance of clonidine.
In our study, clonidine plasma concentrations in the umbilical cord were approximately the same as that in the maternal samples. This is similar to data reported by others (Hartikainen-Sorri et al., 1987; Boutroy et al., 1988). Boutroy et al. (1988) reported umbilical to maternal concentration ratio to be 0.87 ± 0.14 ng/ml, and three out of five newborns exposed in utero to clonidine (maternal dosage 0.3–0.45 mg/day) experienced hypertension during the first 48 h of life. Hypertension in these infants may in part be related to genetic predisposition, although it could also be attributed to a paradoxical neonatal reaction to clonidine. Rebound hypertension can be ruled out because clonidine concentrations in the infants were similar at the time of birth and at 24 h of age.
In conclusion, the clinical observation of decreased clonidine effect at the end of a 12-h dosing interval probably reflects the increased apparent oral clearance during pregnancy, which may necessitate higher or more frequent clonidine dosing. The increase in apparent oral clearance of clonidine during pregnancy is attributable to a change in its nonrenal clearance. Renal clearance of clonidine did not show any change despite the increase in creatinine clearance. The apparent lack of change in clonidine renal clearance may be due to the high variability in urine pH, subsequently altering tubular reabsorption of clonidine. Nonrenal clearance plays a much more significant role in overall clonidine clearance during pregnancy than in the nonpregnant population. Further research is needed to characterize clonidine pharmacodynamics and metabolism during pregnancy. Moreover, clonidine does cross the placenta, and similar concentrations are measured in maternal and umbilical cord plasma. Fetal and neonatal safety requires further investigation.
This work was supported in part by the National Institutes of Health National Institute of Child Health and Human Development [Grant 5U10-HD047892] (Unit Network Obstetric-Fetal Pharmacology Research); the National Institutes of Health National Center for Research Resources [Grants T32-RR023256, M01-RR00037, RR023256]; and the University of Washington Multidisciplinary Predoctoral Research Training Program.
Parts of this work were previously presented as a poster at the following conference: Buchanan ML, Easterling TR, Carr DB, Pon V, Shen DD, and Hebert MF (2007) Clonidine in pregnancy: an OPRU network study. American College of Clinical Pharmacy Annual Conference. 2007 Oct 14–17; Denver, CO. American College of Clinical Pharmacy, Lenexa, KS.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024984.
ABBREVIATIONS: LC/MS, liquid chromatography/mass spectrometry; AUCτ, area under the plasma concentration-time curve over one dosing interval; CL/F, apparent oral clearance; CLrenal, renal clearance; Ae,τ, amount of clonidine excreted unchanged in the urine over one dosing interval; GFR, glomerular filtration rate; CrCl, creatinine clearance; fu, fraction unbound in plasma.
References
- Arndts D, Stähle H, and Struck CJ (1979) A newly developed precise and sensitive radioimmunoassay for clonidine. Arzneimittelforschung 29 532–538. [PubMed] [Google Scholar]
- Arndts D (1983) New aspects of clinical pharmacology of clonidine. Chest 83 397–400. [DOI] [PubMed] [Google Scholar]
- Boutroy MJ, Gisonna CR, and Legagneur M (1988) Clonidine: placental transfer and neonatal adaptation. Early Hum Dev 17 275–286. [PubMed] [Google Scholar]
- Cunningham FE, Baughman VL, Peters J, and Laurito CE (1994) Comparative pharmacokinetics of oral versus sublingual clonidine. J Clin Anesth 6 430–433. [DOI] [PubMed] [Google Scholar]
- Darda S, Förster HJ, and Sthle H (1978) Metabolic degradation of clonidine. Arzneimittelforschung 28 255–259. [PubMed] [Google Scholar]
- Davies DS, Wing AM, Reid JL, Neill DM, Tippett P, and Dollery CT (1977) Pharmacokinetics and concentration-effect relationships of intravenous and oral clonidine. Clin Pharmacol Ther 21 593–601. [DOI] [PubMed] [Google Scholar]
- Davison JM and Noble MC (1981) Serial changes in 24 h creatinine clearance during normal menstrual cycles and the first trimester. Br J Obstet Gynaecol 88 10–17. [DOI] [PubMed] [Google Scholar]
- Dollery SC (1991) Clonidine (hydrochloride), in Therapeutic Drugs (Dollery SC, ed) pp C305–C311, Churchill Livingstone, New York.
- Fujimura A, Ebihara A, Ohashi K, Shiga T, Kumagai Y, Nakashima H, and Kotegawa T (1994) Comparison of the pharmacokinetics, pharmacodynamics, and safety of oral (Catapres) and transdermal (M-5041T) clonidine in healthy subjects. J Clin Pharmacol 34 260–265. [DOI] [PubMed] [Google Scholar]
- Hartikainen-Sorri AL, Heikkinen JE, and Koivisto M (1987) Pharmacokinetics of clonidine during pregnancy and nursing. Obstet Gynecol 69 598–600. [PubMed] [Google Scholar]
- Hebert MF, Easterling TR, Kirby B, Carr D, Buchanan M, Rutherford T, Thummel K, Fishbein D, and Unadkat J (2008) Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther 84 248–253. [DOI] [PubMed] [Google Scholar]
- Hulter HN, Licht JH, Ilnicki LP, and Singh S (1979) Clinical efficacy and pharmacokinetics of clonidine in hemodialysis and renal insufficiency. J Lab Clin Med 94 223–231. [PubMed] [Google Scholar]
- Kobinger W (1978) Central alpha-adrenergic systems as targets for hypotensive drugs. Rev Physiol Biochem Pharmacol 81 40–100. [DOI] [PubMed] [Google Scholar]
- Lowenthal DT (1980) Pharmacokinetics of clonidine. J Cardiovasc Pharmacol 2 S29–S37. [DOI] [PubMed] [Google Scholar]
- Porchet HC, Piletta P, and Dayer P (1992) Pharmacokinetic-pharmacodynamic modeling of the effects of clonidine on pain threshold, blood pressure, and salivary flow. Eur J Clin Pharmacol 42 655–661. [DOI] [PubMed] [Google Scholar]
- Tracy TS, Venkataramanan R, Glover DD, and Caritis SN (2005) for the National Institute for Child Health and Human Development Network of Maternal-Fetal Medicine Units. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol 192 633–639. [DOI] [PubMed] [Google Scholar]
- Yerby MS, Friel PN, McCormick K, Koerner M, Van Allen M, Leavitt AM, Sells CJ, and Yerby JA (1990) Pharmacokinetics of anticonvulsants in pregnancy: alterations in plasma protein binding. Epilepsy Res 5 223–228. [DOI] [PubMed] [Google Scholar]