Abstract
The purpose of this work was to identify human UDP-glucuronosyltransferases (UGTs) capable of glucuronidating dopamine. Using a sensitive liquid chromatography-tandem mass spectrometry method, we screened all 19 known human UGTs and found that only one enzyme, UGT1A10, catalyzed dopamine glucuronidation at substantial rates, yielding both dopamine-4-O-glucuronide (37.1 pmol/min/mg) and dopamine-3-O-glucuronide (32.7 pmol/min/mg). Much lower (<2 pmol/min/mg) or no dopamine glucuronidation activity was found for all other UGTs tested at 1 mM dopamine. Evaluation of the UGT1A10 expression pattern in human tissues by quantitative reverse transcription-polymerase chain reaction confirmed that it is mainly expressed in small intestine, colon, and adipose tissue, whereas only low levels were found in trachea, stomach, liver, testis, and prostate but not in brain. Dopamine glucuronidation assays using microsomes from human liver and intestine corroborated these findings because activity in intestinal microsomes was markedly higher than that in liver microsomes. Moreover, the glucuronidation regioselectivity in intestinal microsomes was similar to that of recombinant UGT1A10, and both enzyme sources exhibited sigmoidal kinetics with substrate affinity (KA) values in the range of 2 to 3 mM. Examination of four UGT1A10 mutants, F90A, F90L, F93A, and F93L, revealed lower dopamine glucuronidation in all of them, particularly in F90A and F93A. Nonetheless, the substrate affinities of the four mutants were similar to that of UGT1A10. It is interesting to note that mutant F93L exhibited regioselectivity, conjugating dopamine at the 4-hydroxyl (OH) position approximately 3 times more efficiently than at the 3-OH position. These results shed new light on the structure and function of UGT1A10 and indicate that dopamine may be a useful probe substrate for this enzyme.
UDP-glucuronosyltransferases (EC 2.4.1.17) (UGTs) catalyze the glucuronidation reaction, involving the transfer of the glucuronic acid moiety from UDP-glucuronic acid (UDPGA) to an aglycone substrate. There are 19 distinct human UGT isoforms, and they are divided into three subfamilies: UGT1A, UGT2A, and UGT2B (Mackenzie et al., 2005). The aglycone substrates of the UGTs are both endogenous compounds such as bilirubin and steroid hormones, as well as xenobiotics, including many drugs. Most of these aglycones are very lipophilic, and the conjugation with glucuronic acid stimulates their excretion from the cell and from the body through bile or urine. In most cases glucuronidation also renders the aglycone substrate biologically inactive (King et al., 2000; Tukey and Strassburg, 2000; Wells et al., 2004).
The aglycone substrate specificity of individual UGTs is very complex, and one of the major objectives in UGT research is gaining a detailed understanding of the substrate specificity of all the human UGTs. Most of them can glucuronidate many different substrates with variable chemical structures. This, together with high sequence homology among the UGTs, also means there is frequently partial overlap of the substrate specificity of individual isoforms. However, there are compounds that are mainly glucuronidated by a single or very few UGTs (Court, 2005), demonstrating that alongside similarities, there are also significant differences in their substrate specificity. Such specific substrates, or probe substrates, are important tools for the study of glucuronidation in microsomes from different tissues and in cell lines because nearly all of them contain several different UGTs, and currently we have no good way to determine the amount of each UGT in a given sample of this kind.
Dopamine (Fig. 1A) is an important neurotransmitter and its homeostasis in central and peripheral nervous system is important for many physiological processes as well as diseases (Eisenhofer et al., 2004). Dopamine is also found in the circulation, where the sulfated form is more abundant than the free form (Johnson et al., 1980). Its sulfonation is catalyzed by sulfotransferase (SULT) 1A3, an enzyme with a strong preference for catecholamines (Eisenhofer et al., 1999). SULT1A3 is mainly found in the gastrointestinal tract, where almost half of the endogenous dopamine is formed (Eisenhofer et al., 1997, 1999), but it is also found in the brain (Whittemore et al., 1985). Dopamine glucuronides were previously found in the human cerebrospinal fluid (Tyce et al., 1986), and one of the aims of the current study was to find out which UGTs are responsible for dopamine glucuronidation in humans.
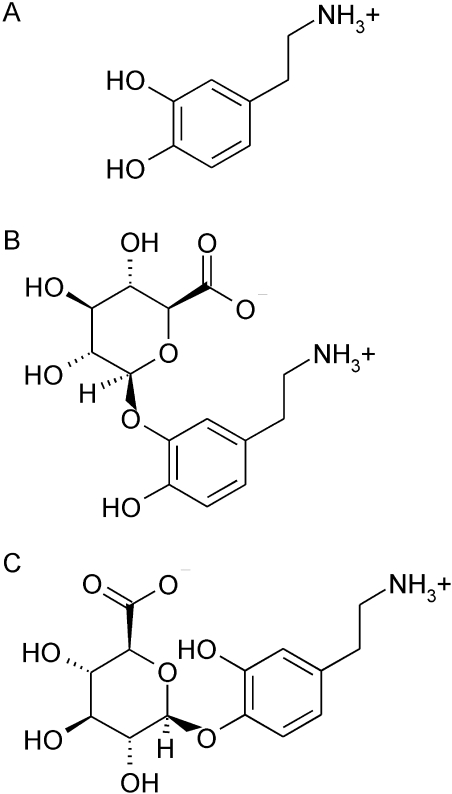
Fig. 1.
The structures of dopamine (A), dopamine-3-glucuronide (B), and dopamine-4-glucuronide (C).
The majority of UGTs are expressed in the liver, but they are also found in various other tissues. UGT1A10 was considered an extrahepatic UGT that is mainly expressed in the intestine (Strassburg et al., 1997, 1998, 1999; Mojarrabi and Mackenzie, 1998; Cheng et al., 1999; Zheng et al., 2002). However, in a recent study, low expression levels of UGT1A10 were detected in liver and hepatocytes (Li et al., 2007; Nakamura et al., 2008). Based on the latter, it might be speculated that low expression levels of UGT1A10 that were not detected previously could also occur in the brain. Such a finding, for a highly active enzyme like UGT1A10 (Xiong et al., 2006; Starlard-Davenport et al., 2007; Itäaho et al., 2008), would certainly have functional implications. Therefore, in the present study we have reexamined the expression of UGT1A10 in various human tissues, including the brain.
In previous works focusing on structure-function relationships of the UGTs, we have investigated the activities of UGT1A10 toward phenol compounds (Xiong et al., 2006) and different estrogens (Starlard-Davenport et al., 2007; Itäaho et al., 2008). In particular, it was suggested that Phe90 and Phe93 of UGT1A10 participated in the binding of phenolic substrates through ring stacking (Xiong et al., 2006). Consequently, it would be interesting to study how Ala and Leu mutations at positions 90 and 93 of UGT1A10 affect its activity toward dopamine.
It is often assumed that among the two major systems of phase II drug metabolism, sulfonation is a high-affinity but low-capacity process, whereas glucuronidation offers high capacity but low affinity. We have previously analyzed dopamine sulfonation by SULT1A3 (Itäaho et al., 2007), and in the present study we have analyzed the glucuronidation of this neurotransmitter. The results yield a new possible probe for UGT1A10 and shed new light on the activity of this important, primarily intestinal, enzyme.
Materials and Methods
Reagents and Enzymes. Dopamine hydrochloride, uridine 5′-diphosphoglucuronic acid trisodium salt, and d-saccharic acid 1,4-lactone were purchased from Sigma-Aldrich (St. Louis, MO). Sodium dihydrogen phosphate dihydrate and disodium hydrogen phosphate were from Fluka (Buchs, Switzerland and Steinheim, Germany). Radiolabeled [14C]UDPGA was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Dopamine-4-O-glucuronide was synthesized in our laboratory as described elsewhere (Uutela et al., 2009). Pooled human liver microsomes (HLM) and intestinal microsomes, as well as recombinant UGT2B15, were purchased from BD Gentest (Woburn, MA). The other recombinant UGTs were produced in our laboratory as His-tagged proteins in baculovirus-infected insect cells (Kurkela et al., 2003, 2007). The activity of UGT2B10 was studied using freshly harvested cells because of possible partial inactivation upon membranes/microsomes preparation (Kaivosaari et al., 2007). The other recombinant UGTs, as well as four UGT1A10 mutants that were described recently (Xiong et al., 2006; Starlard-Davenport et al., 2007), were studied as membranes that had been stored at –70°C until used. HPLC-grade acetonitrile was from Rathburn Chemicals (Walkerburn, UK), and formic acid (98–100%) was from Riedel-de Haën (Seelze, Germany).
HPLC and Mass Spectrometric Methods and Equipment. The samples were analyzed by LC-MS/MS as described elsewhere (Uutela et al., 2009) with minor modifications in HPLC conditions. Agilent 1100 series HPLC equipment (Agilent Technologies, Waldbronn, Germany) was used with a Supelco Discovery HS F5 (4 × 150 mm, 3 μm) column (Supelco, Bellafonte, PA). A mixture of acetonitrile and 0.1% aqueous formic acid was used as the mobile phase. The gradient was started after 1 min of isocratic flow of 5% acetonitrile. The acetonitrile proportion was raised up to 20% in 10 min and then decreased again to 5% for the rest of the run. The flow rate was 0.9 ml/min, and a 5-min equilibration was used between samples. Retention times for dopamine-3-glucuronide (Fig. 1B) and dopamine-4-glucuronide (Fig. 1C) were (mean ± S.D.) 5.3 ± 0.18 and 4.7 ± 0.13 min, respectively, and the total run time was 13 min.
An API3000 triple-quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Concord, ON, Canada) with a TurboIonSpray source was used for detection, as described elsewhere (Uutela et al., 2009). Purified air (Atlas Copco CD 2; Atlas Copco, Wilrijk, Belgium) was used as a nebulizing gas, and nitrogen generated with Whatman 75-72 generator (Haverhill, MA) was used as a turbo, curtain, and collision gas. The turbo gas flow rate was 6 l/min, and its temperature was 280°C. Analyses were carried out in the positive ion mode, and the ion spray (5500 V) and orifice (declustering) voltages (24 V) were optimized. The collision energies were 28 and 18 V, and the collision cell exit potentials were 11.1 and 12.5 V for the selected reaction monitoring pairs 330.1/137.1 and 330.1/154.1, respectively. The data were collected and processed by Analyst 1.4.2 software (Applied Biosystems/MDS Analytical Technologies, Concord, Canada).
Quantification of Glucuronides. Dopamine-4-O-glucuronide was used as an external standard to quantify both glucuronide isomers, and standards were analyzed with every sample set. The correlation coefficient was >0.99 in all cases, and the linear range was from 10 nM to 1.0 μM. The limit of detection was 1 nM, and the lowest enzymatic activity detected was 0.02 pmol/min/mg. To verify that the signals of the two dopamine glucuronides were similar, the ratio of the peak areas of both glucuronides, dopamine-4-glucuronide and dopamine-3-glucuronide, was determined using HPLC (Agilent Technologies) coupled with a radiochemical detector (model 9701; Reeve Analytical, Glasgow, UK). To produce both dopamine glucuronide isomers as radioactive compounds, the samples containing dopamine (2 mM) and recombinant UGT1A10 (1.2 mg/ml) were incubated in the presence of 6 μM radiolabeled [14C]UDPGA and 50 μM unlabeled UDPGA at 37°C for 3 h. The other conditions were as described below. The same amounts of the same incubated samples were injected onto two separate HPLC systems, one coupled with a radiochemical detector and the other coupled with a mass spectrometer. The peak areas for each of the two glucuronides were found to be of substantially the same size in both systems (data not shown).
Screening of Microsomes, Recombinant UGTs, and Mutants of UGT1A10. The activities of human liver and intestinal microsomes, 19 human recombinant UGTs, and four mutants of UGT1A10 toward dopamine were screened. The reaction mixtures contained 0.4 to 1.6 mg/ml protein, 1 mM UDPGA, 5 mM saccharolactone, 2% dimethyl sulfoxide, and 5 mM MgCl2 in 50 mM phosphate buffer (pH 7.4). The total incubation volume was 250 μl, and the dopamine concentration was 1 mM. The activities of the four mutants of UGT1A10 were screened in the presence of both 1 and 5 mM dopamine. The duplicated samples were incubated at 37°C for 60 min. The reactions were terminated by adding 25 μl of chilled 4 M perchloric acid and cooling the tubes in a cold block, followed by centrifugation for 5 min at 16,100g to remove the precipitated proteins. Then 100 μl of the supernatant were injected for LC-MS/MS.
Enzyme Kinetic Studies. Kinetic constants were determined for dopamine glucuronidation by UGT1A10 and the four mutants as well as by human liver and intestinal microsomes. The samples were prepared in triplicate similarly to the screening samples, except that the protein concentrations were 100 to 400 μg/ml and the incubation times were 30 to 45 min, which were within the linear range of the glucuronide formation. Two concentrations of the cosubstrate UDPGA were tested with the most active sample, recombinant UGT1A10. The dopamine glucuronidation rates were essentially identical under both conditions, indicating that 1 mM was a saturating UDPGA concentration in this experimental system (data not shown). Hence, 1 mM UDPGA was used for all the other UGT assays. Eight dopamine concentrations, ranging from 0.1 to 10 mM, were used in the kinetic analyses.
Normalization of Enzymatic Activities. The expression levels of all recombinant UGT enzymes were determined by immunodetection as described previously (Kurkela et al., 2007). The commercial UGT2B15 was the only enzyme for which the expression level could not be normalized because of the absence of a His-tag. The other enzymatic activities presented in this study were normalized with respect to the expression level of UGT1A10. The relative expression levels per milligram of protein in the tested membrane batch of the recombinant UGTs that catalyzed dopamine glucuronidation were as follows: 1A1 (4.0), 1A3 (1.1), 1A6 (1.7), 1A7 (12), 1A8 (10), 1A9 (2.0), 1A10 (1.0), 2A1 (4.7), 2A3 (1.3), 2B7 (5.7), 2B11 (3.2), 2B17 (0.53), 10F90A (2.4), 10F90L (0.48), 10F93A (3.7), and 10F93L (2.1).
Enzyme Kinetic Analyses. The normalized initial velocity data from the enzyme kinetic studies were fitted either to the Michaelis-Menten equation [v = Vmax × S/(Km+ S), where v is the initial velocity of the reaction, S is the substrate concentration, Vmax is the maximal velocity, and Km is the substrate concentration at 0.5 Vmax], to the substrate inhibition equation [v = Vmax × S/(Ks + S + S2/Ki), where Ki is the constant describing the substrate inhibition interaction], or to the Hill equation [v = Vmax × Sn/(KAn + Sn), where KA is the substrate concentration at 0.5 Vmax and n is the Hill coefficient] by nonlinear regression using GraphPad Prism (version 4.03 for Windows; GraphPad Software Inc., San Diego, CA). The best kinetic model was selected, considering the randomness of the residuals, the standard errors of the estimates, and the correlation coefficients. None of the reactions followed the substrate inhibition equation.
UGT1A9 and UGT1A10 mRNA Quantitation in Human Tissues. Total RNA was extracted using TRIzol reagent (Sigma-Aldrich) from 47 human livers and pooled. Liver donors were all of European-American ancestry and included both males (n = 36) and females (n = 11). Use of the tissue was approved by the institutional review board of Tufts University School of Medicine. Total RNAs from human adrenal gland (n = 62), whole brain, cerebellum (n = 24), fetal brain (n = 59), colon (n = 3), kidney, lung, placenta, prostate (n = 47), small intestine (n = 5), testis (n = 19), thyroid (n = 65), trachea, and uterus (n = 10) were purchased from Clontech (Mountain View, CA), whereas total RNAs from human adipose tissue, stomach, pancreas, and ovary were from Ambion (Austin TX). To generate cDNA, 1 μg of total RNA was treated with DNase (Promega, Madison, WI) and reverse transcribed (Superscript II; Invitrogen, Carlsbad, CA) with random hexamer primer (0.1 μg). Quantitative PCR reactions (25 μl) included SYBR Green 2× Master Mix (Applied Biosystems, Foster City, CA), 10 μl of 1:10 diluted cDNA (except for a 1:30 dilution for liver cDNA and a 1:500 dilution for cDNA assayed with 18S rRNA primers), and 200 nM of each primer. Primer pair sequences were as follows: CCC CTC GAT GCT CTT AGC TGA GTG T (18S-rRNA forward), CGC CGG TCC AAG AAT TTC ACC TCT (18S-rRNA reverse), GGA GCC ACT GGT TCA CCA TGA G (UGT1A9 forward), AGA TCC TCC AGG GTA TAT GAA GTT GAA (UGT1A9 reverse), TTG ATA CCT GTG GCT TAA TTG TTG CT (UGT1A10 forward), and GGC ATC TGA GAA CCC TAA GAG ATC AT (UGT1A10 reverse). Real-time PCR analysis (model 7300; Applied Biosystems) was performed with the following PCR method: 95°C for 10 min and 40 to 45 cycles of 95°C for 30 s and 60°C for 60 s. Amplification specificity was ensured initially by sequencing of representative PCR products and in each run by PCR product duplex melting temperature analysis. Negative controls included exclusion of cDNA template and reverse transcription enzyme. mRNA concentrations were calculated using standard curves of PCR threshold cycle number versus concentration of template derived from serial dilutions of purified PCR product. Curves were linear (R2 > 0.99) over the concentration ranges 10–9 to 10–14 M for 18S rRNA and 10–14 to 10–18 M for other gene products, which defined the upper and lower quantitation limits of the respective assays. For each tissue, cDNA reactions were performed in triplicate, and quantitative PCR reactions were performed at least in duplicate. Results are expressed as the mean ± S.E. number of mRNA copies per 109 copies of 18S rRNA. Assay precision as reflected by the coefficient of variation of replicates averaged 26% and 31% for UGT1A9 and UGT1A10, respectively.
Results
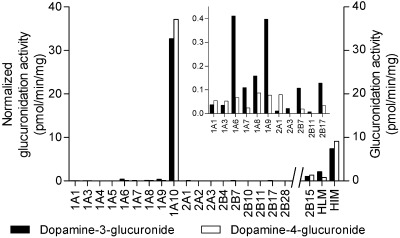
Screening of Recombinant UGTs. The 19 human UGTs of subfamilies 1A, 2A, and 2B were screened for dopamine glucuronidation activity. The substrate concentration in the screening studies was somewhat high (1 mM), and we used a sensitive detection method (LC-MS/MS). Under these conditions, a few UGTs exhibited detectable activity but only one, UGT1A10, glucuronidated dopamine at significant rates (Fig. 2). The regioselectivity of dopamine glucuronidation was also studied, and in the case of UGT1A10 there was only a minor difference between the two hydroxyls of the dopamine molecule; dopamine-4-glucuronide was produced slightly more than dopamine-3-glucuronide. No dopamine-N-glucuronide was detected in these assays.
Fig. 2.
Dopamine glucuronidation by different UGT enzymes. Glucuronidation activities were determined with 1 mM dopamine and 1 mM UDPGA, and the samples were incubated at 37°C for 60 min. Normalized glucuronidation rates (with respect to the expression level of UGT1A10) of the enzymes for which the relative expression level could be determined are shown on the left side of the figure. The reaction rates in the samples for which normalization was not possible or relevant [UGT2B15 Supersomes, HLM, and HIM] are on right side of the figure, and they could be directly compared with UGT1A10. The insert shows an enlargement of the normalized rates for UGTs that exhibited very low activity. All values are means of two samples.
Very low dopamine glucuronidation activity was detected with UGTs 1A1, 1A3, 1A6, 1A7, 1A8, 1A9, 2A1, 2A3, 2B7, 2B11, 2B15, and 2B17 (Fig. 2). The regioselectivity of these UGTs mostly differed from that of UGT1A10, and some exhibited a strong preference for the 3-OH (Fig. 2). UGT2A3 and UGT2B11 even seemed to be totally selective for the 3-OH, but because their activity was so low, barely above the detection limit, it is possible that the dopamine-4-glucuronide they might have produced remained undetected. In addition, we evaluated possible formation of dopamine N-glucuronide by recombinant UGTs, particularly UGT1A4 and UGT2B10, but none was detected (data not shown).
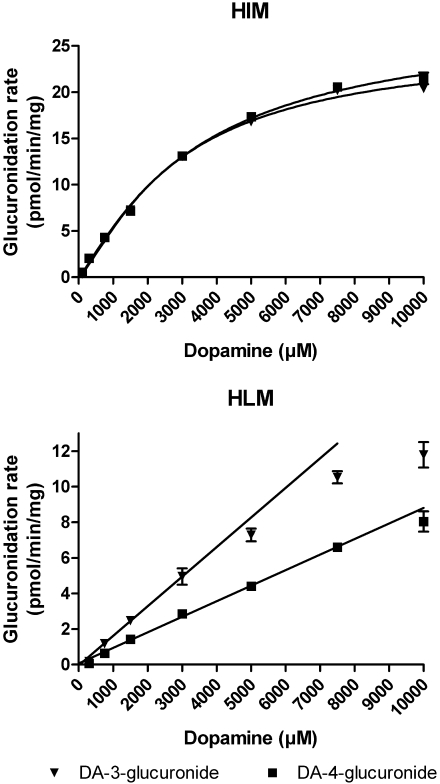
We also measured dopamine glucuronidation activity using microsomes from human liver and human intestine (Fig. 2). The activity per milligram of protein in the assay in intestinal microsomes was 5 to 10 times higher than that in liver microsomes, depending on which dopamine glucuronide was compared. Interestingly HLM exhibited a clear preference for the 3-OH glucuronidation of dopamine, which is a very similar regioselectivity to that of the very low activity of liver UGT1A6, 1A9, 2B7, and 2B17 (Fig. 2). On the other hand, human intestinal microsomes (HIM) exhibited very little preference for the 4-OH glucuronidation of dopamine and had a regioselectivity nearly identical to that of recombinant UGT1A10 (Fig. 2).
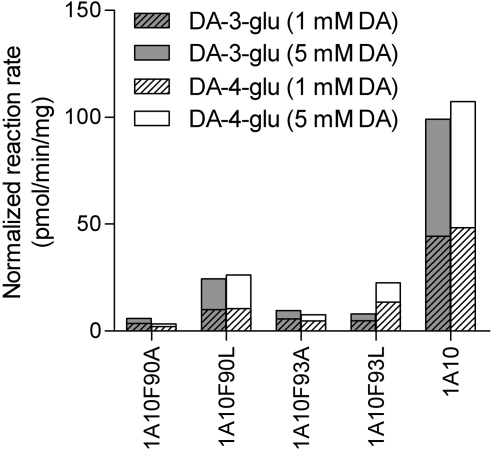
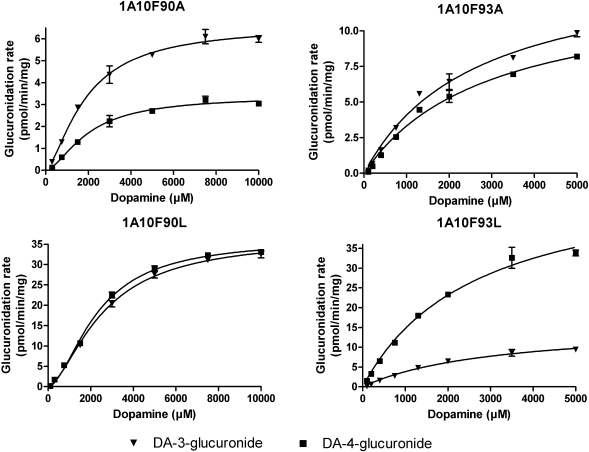
After discovering the dopamine glucuronidation activity of UGT1A10, we used this substrate to study four active-site mutants of this enzyme, F90A, F90L, F93A, and F93L, that we have recently evaluated using different substrates (Xiong et al., 2006; Starlard-Davenport et al., 2007). The mutants were assayed in the presence of both 1 and 5 mM dopamine and revealed relatively low dopamine glucuronidation activity in comparison with wild-type UGT1A10 (Fig. 3). Nevertheless, substitution of either Phe90 or Phe93 with alanine, as in F90A and F93A, lowered the activity toward the 4-OH of dopamine much more than replacement of either of these two phenylalanine residues with leucine. The two F to L mutants, F90L and F93L, differed sharply from each other with respect to dopamine glucuronidation at the 3-OH, however (Fig. 3). Whereas mutant 10F90L, like wild-type UGT1A10, exhibited similar glucuronidation activity toward both hydroxyls of dopamine, mutant 10F93L exhibited approximately 3 times higher glucuronidation activity toward the 4-OH than toward the 3-OH of dopamine.
Fig. 3.
Dopamine (DA) glucuronidation by four mutants of human UGT1A10. Glucuronidation rates were determined with 1 and 5 mM dopamine in the presence of 1 mM UDPGA. The samples were incubated in duplicate at 37°C for 60 min, and mean values are shown. Reaction rates were normalized for the expression levels with respect UGT1A10.
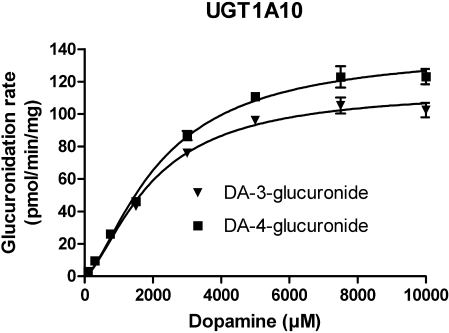
Enzyme Kinetic Studies. The enzyme kinetics of dopamine glucuronidation by UGT1A10, mutants 10F90A, 10F90L, 10F93A, 10F93L, and human liver and intestinal microsomes were analyzed with various dopamine concentrations. Dopamine glucuronidation by UGT1A10 was best described by the Hill equation (Fig. 4; Table 1) and the same type of sigmoidal kinetics was exhibited by the two mutants at phenylalanine 90, whereas mutants 10F93A and 10F93L exhibited Michaelis-Menten kinetics (Fig. 5; Table 1). For the native samples, the activity of HIM followed the Hill equation (Fig. 6; Table 1). Unfortunately, enzyme kinetic parameters could not be determined for HLM because enzyme saturation could not be achieved at the highest substrate concentration tested (10 mM).
Fig. 4.
Glucuronidation of dopamine (DA) by recombinant human UGT1A10. Samples were incubated in the presence of 1 mM UDPGA and 200 μg/ml protein at 37°C for 30 min. All samples were prepared in triplicate. The initial rate data were fitted to the Hill equation, and the kinetic constants are shown in Table 1.
TABLE 1.
The estimated kinetic parameters for glucuronidation of dopamine
The Km and KA values and the Vmax values of UGT1A10 and human intestinal microsomes are presented as best-fit values ± S.E. The Vmax values of the UGT1A10 mutants are normalized for their expression levels compared with wild-type recombinant UGT1A10. Enzyme kinetic parameters for human liver microsomes could not be determined because enzyme saturation could not be achieved at the highest substrate concentration tested.
|
Dopamine-3-O-Glucuronide
|
Dopamine-4-O-Glucuronide
|
|||||
|---|---|---|---|---|---|---|
| Km or KA | Vmax | n | Km or KA | Vmax | n | |
| μM | pmol/min/mg | μM | pmol/min/mg | |||
| 1A10a | 1950 ± 171 | 116 ± 4.89 | 1.52 | 2190 ± 205 | 140 ± 6.50 | 1.49 |
| 10F90Aa | 1890 ± 211 | 6.62 | 1.50 | 1990 ± 240 | 3.38 | 1.65 |
| 10F90La | 2590 ± 150 | 36.7 | 1.60 | 2310 ± 103 | 36.2 | 1.74 |
| 10F93Ab | 2630 ± 353 | 14.9 | 2910 ± 352 | 13.0 | ||
| 10F93Lb | 3110 ± 479 | 15.8 | 2670 ± 344 | 54.1 | ||
| HIMa | 2870 ± 267 | 25.3 ± 1.13 | 1.26 | 3410 ± 398 | 28.1 ± 1.56 | 1.17 |
| HLM | Could not be determined. | Could not be determined. | ||||
The data were fitted to the Hill equation.
The data were fitted to the Michaelis-Menten equation.
Fig. 5.
Enzyme kinetics of dopamine (DA) glucuronidation by four mutants of human UGT1A10. Samples were incubated in the presence of 1 mM UDPGA and 100 to 400 μg/ml protein at 37°C for 45 min. All samples were prepared in triplicate. The normalized initial rate data were fitted to the Michaelis-Menten (10F93A and 10F93L) or Hill equation (10F90A and 10F90L). The enzyme kinetic constants are listed in Table 1.
Fig. 6.
Glucuronidation of dopamine (DA) by HIM and HLM. Samples were incubated in the presence of 1 mM UDPGA and 200 μg/ml protein at 37°C for 45 min. All samples were prepared in triplicate. The initial rate data of HIM were fitted to the Hill equation (see Table 1 for kinetic constants). The kinetic constants for HLM could not be determined, but the Clint values were determined as the slopes of the regression lines fitted to the initial rates at low dopamine concentrations. Clint values for the formation of dopamine-3-glucuronide and dopamine-4-glucuronide were 1.7 and 0.9 nl/min/mg, respectively.
The kinetic analyses revealed that the dopamine affinity for all these UGTs was low. Nonetheless, it is noteworthy that the KA values of recombinant UGT1A10 and HIM were in the same range, 2 to 3 mM (Table 1). Moreover, the mutagenesis of Phe90 and Phe93 to either Ala or Leu did not affect the affinity of the enzyme for dopamine, even if all of them lowered the Vmax values (Table 1).
UGT1A9 and UGT1A10 mRNA Quantitation in Human Tissues. Because UGT1A10 seemed to be the most active human enzyme in dopamine glucuronidation, we evaluated the tissue specificity of expression of this isoform. Although studies with some tissues were done previously, there were inconsistencies in results, particularly regarding whether this enzyme is expressed in liver (Strassburg et al., 1997, 1998, 1999; Mojarrabi and Mackenzie, 1998; Cheng et al., 1999; Zheng et al., 2002; Li et al., 2007; Nakamura et al., 2008). In addition, because dopamine is an important neurotransmitter in the brain, we wanted to find out whether UGT1A10 is expressed in the brain. In the same tissues, we also analyzed the expression pattern of UGT1A9, an enzyme that is highly homologous to UGT1A10 in its nucleotides and amino acid sequence, but differs from it markedly in tissue distribution. Results presented in Table 2 confirm the previous findings of Li et al. (2007) that although UGT1A10 is largely an extrahepatic enzyme, this UGT is also expressed in the liver at a very low level. Nonetheless, UGT1A10 is expressed mainly in the intestine and in adipose tissue. In addition to very low expression in liver, very low expression of UGT1A10 was detected in testis and prostate and slightly higher expression in trachea (Table 2). These findings of low expression levels of UGT1A10 outside the intestine highlight the absence of any detectable mRNA for UGT1A10 (or UGT1A9) in the brain. UGT1A9, in contrast to UGT1A10, is expressed mainly in the liver and in the kidneys. It is noteworthy that UGT1A9 is also expressed at considerable levels in the adipose tissue, small intestine, and colon (Table 2).
TABLE 2.
UDP-glucuronosyltransferase 1A9 and 1A10 mRNA expression in human tissues
The values are mean ± S.E.
| Tissue | 1A9 | 1A10 |
|---|---|---|
| copies per 109 copies of 18S rRNA | ||
| Adipose | 86 ± 15 | 313 ± 57 |
| Adrenal gland (n = 62) | 3 ± 0 | — |
| Brain | — | — |
| Brain, cerebellum (n = 24) | — | — |
| Brain, fetal (n = 59) | — | — |
| Colon (n = 3) | 188 ± 55 | 1210 ± 299 |
| Kidney | 2157 ± 269 | — |
| Liver, adult (n = 47) | 3239 ± 42 | 6 ± 0 |
| Lung | — | — |
| Ovary | — | — |
| Pancreas | — | — |
| Placenta | 3 ± 0 | — |
| Prostate (n = 47) | 8 ± 1 | 6 ± 1 |
| Small intestine (n = 5) | 319 ± 109 | 660 ± 254 |
| Stomach | 4 ± 1 | 21 ± 3 |
| Testis (n = 19) | 13 ± 3 | 6 ± 1 |
| Thyroid (n = 65) | 1 ± 0 | — |
| Trachea | 32 ± 7 | 27 ± 8 |
| Uterus (n = 10) | — | — |
—, not detected.
Discussion
After the discovery of dopamine glucuronide in rat brain (Uutela et al., 2009), we have examined the dopamine glucuronidation activity of the 19 human UGTs of subfamilies 1A, 2A, and 2B. The results demonstrated that UGT1A10 catalyzes this reaction at a much higher rate than that for any other human UGT (Fig. 2). The relatively high activity of UGT1A10 was not reported in a previous study by Taskinen et al. (2003), simply because UGT1A10 was not included in that study. In addition, we have detected very low dopamine glucuronidation activity in many more UGTs than previously, probably because of the improved detection method that was used in the present study. More importantly, our results with the recombinant human UGTs are in good agreement with the results from human liver and intestinal microsomes (Table 1; Fig. 6) because the expression of UGT1A10 in the intestine is much higher than its expression in the liver (Table 2) (Mojarrabi and Mackenzie, 1998; Cheng et al., 1999).
The dopamine glucuronidation activity we have found in HIM is in apparent contrast with the results of an earlier study on catechol glucuronidation (Antonio et al., 2003). No detectable dopamine glucuronidation activity by human intestinal microsomes was reported in that work, and we suggest that the reason(s) for that finding may lie in the experimental conditions, particularly a lower substrate concentration during the assays (500 μM versus 1000 μM in the present work) and lower detection sensitivity. In addition, it is possible that variability in the quality of intestinal microsomes also contributed to the differences between the studies.
Although our original aim was to expose the possible contribution of glucuronidation to dopamine metabolism in different tissues, the results steered the focus of this research to a new direction. Dopamine seems to be a useful probe substrate for UGT1A10. This compound largely follows the two criteria for a good probe compound, namely that it is selective for one isoform and exhibits an affinity for the individual enzyme similar to that for human tissues or microsomes (Court, 2005). The fact that we have discovered that many other isoforms glucuronidated dopamine at a detectable rate tells more about the high sensitivity of the assay system (LC-MS/MS) than about the activity of these UGTs. For example, the normalized activity of the second-best UGT after UGT1A10, UGT1A6 glucuronidating dopamine at the 3-OH, was less than 1.3% of the respective activity of UGT1A10 (Fig. 2). Moreover, kinetics and regioselectivity of dopamine glucuronidation by recombinant UGT1A10 is very similar to that of human intestinal microsomes (Figs. 4 and 6), strongly suggesting that UGT1A10 is by far the main contributor to this activity in the native sample as well.
The kinetic studies revealed that the dopamine affinity for both recombinant UGT1A10 and human intestinal microsomes was low. This finding may suggest that glucuronidation does not play a significant role in dopamine metabolism in humans. Nonetheless, the rather low substrate affinity should not prevent the use of dopamine as a probe substrate for UGT1A10, as the low affinity to serotonin did not prevent the latter compound from being a useful probe substrate for UGT1A6 (Krishnaswamy et al., 2003). The availability of such a probe substrate for UGT1A6 has provided a means to study this enzyme and its interactions with other UGTs (Fujiwara et al., 2007; Kurkela et al., 2007) and is likely to be instrumental in future studies as well.
Dopamine glucuronidation by UGT1A10 was not appreciably regioselective, but there was a slight preference for 4-OH. Although one should be cautious when discussing the regioselectivity of enzymes that exhibit very low dopamine glucuronidation rates, it is tempting to draw attention to some of our observations concerning these UGTs. UGT1A7, UGT1A8, and UGT1A9 are the most homologous to UGT1A10, and these three UGTs strongly favored the 3-OH over the 4-OH (Fig. 2). These observations are in line with a previous study on the regioselectivity of 11 human UGTs in dobutamine glucuronidation (Alonen et al., 2005). Overall, the regioselectivity of individual UGTs in the case of dopamine was similar to the corresponding findings for the two catecholic hydroxyls of dobutamine. It is interesting to note that there are only 16 amino acid residues in UGT1A10 that differ from the residues at the corresponding positions in UGT1A7, UGT1A8, or UGT1A9. Hence, dopamine glucuronidation could be used to identify the amino acids that are responsible for the differences in the activity and regioselectivity among these closely homologous UGTs.
Of the hepatic UGTs that catalyze dopamine glucuronidation at low but detectable rates, 1A1, 1A3 and 2B15 exhibited no clear regioselectivity or very low preference for the 4-OH, whereas 1A6, 1A9, 2B7, and 2B17 exhibited strong preference for the 3-OH (Fig. 2). Based on these results, dopamine glucuronidation by HLM was expected to yield more dopamine-3-glucuronide than dopamine-4-glucuronide. This expectation was in full agreement with the experimental results concerning the regioselectivity as well as the low dopamine glucuronidation activity in human liver microsomes (Fig. 6).
UGT1A10 is expressed mainly along the gastrointestinal tract (Table 2) (Mojarrabi and Mackenzie, 1998; Strassburg et al., 1998). The most active enzyme in dopamine sulfonation, SULT1A3, is also expressed mainly in the intestine (Sundaram et al., 1989; Eisenhofer et al., 1999). In addition, almost half of the endogenous dopamine is produced in the mesenteric organs, where most of the dopamine sulfates are produced (Eisenhofer et al., 1997). UGT1A10 is probably much less important for dopamine metabolism than SULT1A3, whose affinity to dopamine is approximately 1000 times higher (Km at the 2–3 μM range) (Itäaho et al., 2007). In rat, the glucuronidation is a more important metabolic pathway for dopamine than in human (Wang et al., 1983), most likely because sulfotransferase corresponding to human SULT1A3 does not exist in rodents (Eisenhofer et al., 1999; Honma et al., 2001).
Recent studies have shown that two phenylalanine residues in UGT1A10, Phe90 and Phe93, may be directly involved in substrate binding (Xiong et al., 2006). The effect of mutating these Phe residues into Ala (Xiong et al., 2006) or Ala and Leu (Starlard-Davenport et al., 2007) on several enzymatic activities have been examined. The results were quite complex, revealing effects on either the Vmax or the Km, depending on the substrate. In the present study all of the four mutations lowered the normalized Vmax of the reaction, without significantly affecting its Km values (Table 1; Fig. 5). We were surprised to find that the extent of Vmax reduction, at least for the formation of dopamine-4-glucuronide, seemed to depend more on the type of Phe substitution than on its position. Replacing either Phe90 or Phe93 with Ala decreased Vmax much more than a Leu substitution at either position (Table 1; Fig. 5). A possible explanation for these findings is that leucine is more similar than alanine to phenylalanine when it comes to hydrophobicity and size. In any case, the situation is even more complex when one is looking at the regioselectivity of the mutants. Whereas at position 90 the regioselectivity of the F to L mutant was similar to that of the wild type, mutant 10F93L exhibited very different regioselectivity, glucuronidating dopamine at the 4-OH approximately 3 times faster than at the 3-OH (Table 1; Fig. 5). Thus, it seems that more studies should be conducted to fully elucidate the significance of the residues at position 90 and 93 on binding different substrates to UGT1A10.
In summary, the results of this study highlight the activity of the human UGT1A10 and, thereby, the contribution of intestinal UGTs to first-pass metabolism of drugs and other xenobiotics. We have identified a new possible probe substrate for UGT1A10 and used it to study mutants of this enzyme. The results revealed distinct effects that are likely to be dependent on differences in the size of the side chain as much as on differences in their positions within the protein. These findings should facilitate better understanding of the substrate specificity of the human UGTs and the factors that determine it.
Acknowledgments
We are grateful to Johanna Mosorin for skillful technical assistance.
This study was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grants GM61834, GM075893]; the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK60109]; and the Sigrid Juselius Foundation.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.025692.
ABBREVIATIONS: UGT, UDP-glucuronosyltransferase; OH, hydroxyl; UDPGA, UDP-glucuronic acid; SULT, sulfotransferase; HPLC, high-performance liquid chromatography; LC, liquid chromatography; MS/MS, tandem mass spectrometry; PCR, polymerase chain reaction; HLM, human liver microsomes; HIM, human intestinal microsomes.
References
- Alonen A, Aitio O, Hakala K, Luukkanen L, Finel M, and Kostiainen R (2005) Biosynthesis of dobutamine monoglucuronides and glucuronidation of dobutamine by recombinant human UDP-glucuronosyltransferases. Drug Metab Dispos 33 657–663. [DOI] [PubMed] [Google Scholar]
- Antonio L, Xu J, Little JM, Burchell B, Magdalou J, and Radominska-Pandya A (2003) Glucuronidation of catechols by human hepatic, gastric, and intestinal microsomal UDP-glucuronosyltransferases (UGT) and recombinant UGT1A6, UGT1A9, and UGT2B7. Arch Biochem Biophys 411 251–261. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Radominska-Pandya A, and Tephly TR (1999) Studies on the substrate specificity of human intestinal UDP-glucuronosyltransferases 1A8 and 1A10. Drug Metab Dispos 27 1165–1170. [PubMed] [Google Scholar]
- Court MH (2005) Isoform-selective probe substrates for in vitro studies of human UDP-glucuronosyltransferases. Methods Enzymol 400 104–116. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Aneman A, Friberg P, Hooper D, Fåndriks L, Lonroth H, Hunyady B, and Mezey E (1997) Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab 82 3864–3871. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Coughtrie MWH, and Goldstein DS (1999) Dopamine sulphate: an enigma resolved. Clin Exp Pharmacol Physiol 26 S41–S53. [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, and Goldstein DS (2004) Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharm Rev 56 331–349. [DOI] [PubMed] [Google Scholar]
- Fujiwara R, Nakajima M, Yamanaka H, Katoh M, and Yokoi T (2007) Interactions between human UGT1A1, UGT1A4, and UGT1A6 affect their enzymatic activities. Drug Metab Dispos 35 1781–1787. [DOI] [PubMed] [Google Scholar]
- Honma W, Kamiyama Y, Yoshinari K, Sasano H, Shimada M, Nagata K, and Yamazoe Y (2001) Enzymatic characterization and interspecies difference of phenol sulfotransferases, ST1A forms. Drug Metab Dispos 29 274–281. [PubMed] [Google Scholar]
- Itäaho K, Alakurtti S, Yli-Kauhaluoma J, Taskinen J, Coughtrie MW, and Kostiainen R (2007) Regio-selective sulfonation of dopamine by SULT1A3 in vitro provides a molecular explanation for the preponderance of dopamine-3-O-sulfate in human blood circulation. Biochem Pharmacol 74 504–510. [DOI] [PubMed] [Google Scholar]
- Itäaho K, Mackenzie PI, Ikushiro S, Miners JO, and Finel M (2008) The configuration of the 17-hydroxy group variably influences the glucuronidation of β-estradiol and epiestradiol by human UDP-glucuronosyltransferases. Drug Metab Dispos 36 2307–2315. [DOI] [PubMed] [Google Scholar]
- Johnson GA, Baker CA, and Smith RT (1980) Radioenzymatic assay of sulfate conjugates of catecholamines and DOPA in plasma. Life Sci 26 1591–1598. [DOI] [PubMed] [Google Scholar]
- Kaivosaari S, Toivonen P, Hesse LM, Koskinen M, Court MH, and Finel M (2007) Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol Pharmacol 72 761–768. [DOI] [PubMed] [Google Scholar]
- King CD, Rios GR, Green MD, and Tephly TR (2000) UDP-glucuronosyltransferases. Curr Drug Metab 1 143–161. [DOI] [PubMed] [Google Scholar]
- Krishnaswamy S, Duan SX, Von Moltke LL, Greenblatt DJ, and Court MH (2003) Validation of serotonin (5-hydroxtryptamine) as an in vitro substrate probe for human UDP-glucuronosyltransferase (UGT) 1A6. Drug Metab Dispos 31 133–139. [DOI] [PubMed] [Google Scholar]
- Kurkela M, García-Horsman JA, Luukkanen L, Mörsky S, Taskinen J, Baumann M, Kostiainen R, Hirvonen J, and Finel M (2003) Expression and characterization of recombinant human UDP-glucuronosyltransferases (UGTs); UGT1A9 is more resistant to detergent inhibition than the other UGTs, and was purified as an active dimeric enzyme. J Biol Chem 278 3536–3544. [DOI] [PubMed] [Google Scholar]
- Kurkela M, Patana AS, Mackenzie PI, Court MH, Tate CG, Hirvonen J, Goldman A, and Finel M (2007) Interactions with other human UDP-glucuronosyltransferases attenuate the consequences of the Y485D mutation on the activity and substrate affinity of UGT1A6. Pharmacogenet Genomics 17 115–126. [DOI] [PubMed] [Google Scholar]
- Li X, Bratton S, and Radominska-Pandya A (2007) Human UGT1A8 and UGT1A10 mRNA are expressed in primary human hepatocytes. Drug Metab Pharmacokinet 22 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, and Nebert DW (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15 677–685. [DOI] [PubMed] [Google Scholar]
- Mojarrabi B and Mackenzie PI (1998) Characterization of two UDP glucuronosyltransferases that are predominantly expressed in human colon. Biochem Biophys Res Commun 247 704–709. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Nakajima M, Yamanaka H, Fujiwara R, and Yokoi T (2008). Expression of UGT1A and UGT2B mRNA in human normal tissues and various cell lines. Drug Metab Dispos 36 1461–1464. [DOI] [PubMed] [Google Scholar]
- Starlard-Davenport A, Xiong Y, Bratton S, Gallus-Zawada A, Finel M, and Radominska-Pandya A (2007) Phenylalanine90 and phenylalanine93 are crucial amino acids within the estrogen binding site of the human UDP-glucuronosyltransferase 1A10. Steroids 72 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburg CP, Manns MP, and Tukey RH (1998) Expression of the UDP-glucuronosyltransferase 1A locus in human colon: identification and characterization of the novel extrahepatic UGT1A8. J Biol Chem 273 8719–8726. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, and Tukey RH (1997) Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol 52 212–220. [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Strassburg A, Nguyen N, Li Q, Manns MP, and Tukey RH (1999) Regulation and function of family 1 and family 2 UDP-glucuronosyltransferase genes (UGT1A, UGT2B) in human oesophagus. Biochem J 338 489–498. [PMC free article] [PubMed] [Google Scholar]
- Sundaram RS, Szumlanski C, Otterness D, van Loon JA, and Weinshilboum RM (1989) Human intestinal phenol sulfotransferase: assay conditions, activity levels and partial purification of the thermolabile form. Drug Metab Dispos 17 255–264. [PubMed] [Google Scholar]
- Taskinen J, Ethell BT, Pihlavisto P, Hood AM, Burchell B, and Coughtrie MW (2003) Conjugation of catechols by recombinant human sulfotransferases, UDP-glucuronosyltranserases and soluble catechol O-methyltransferase: structure-conjugation relationships and predictive models. Drug Metab Dispos 31 1187–1197. [DOI] [PubMed] [Google Scholar]
- Tukey RH and Strassburg CP (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40 581–616. [DOI] [PubMed] [Google Scholar]
- Tyce GM, Messick JM, Yaksh TL, Byer DE, Danielson DR, and Rorie DK (1986) Amine sulfate formation in the central nervous system. Fed Proc 45 2247–2253. [PubMed] [Google Scholar]
- Uutela P, Karhu L, Piepponen P, Käenmäki M, Ketola RA, and Kostiainen R (2009) Discovery of dopamine glucuronide in rat and mouse brain microdialysis samples using liquid chromatography tandem mass spectrometry. Anal Chem 81 427–434. [DOI] [PubMed] [Google Scholar]
- Wang PC, Kuchel O, Buu NT, and Genest J (1983) Catecholamine glucuronidation: an important metabolic pathway for dopamine in the rat. J Neurochem 40 1435–1440. [DOI] [PubMed] [Google Scholar]
- Wells PG, Mackenzie PI, Chowdhury JR, Guillemette C, Gregory PA, Ishii Y, Hansen AJ, Kessler FK, Kim PM, Chowdhury NR, et al. (2004) Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab Dispos 32 281–290. [DOI] [PubMed] [Google Scholar]
- Whittemore RM, Pearce LB, and Roth JA (1985) Purification and kinetic characterization of a dopamine-sulfating form of phenol sulfotransferase from human brain. Biochemistry 24 2477–2482. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Bernardi D, Bratton S, Ward MD, Battaglia E, Finel M, Drake RR, and Radominska-Pandya A (2006) Phenylalanine 90 and 93 are localized within the phenol binding site of human UDP-glucuronosyltransferase 1A10 as determined by photoaffinity labeling, mass spectrometry, and site-directed mutagenesis. Biochemistry 45 2322–2332. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Fang JL, and Lazarus P (2002) Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab Dispos 30 397–403. [DOI] [PubMed] [Google Scholar]