Abstract
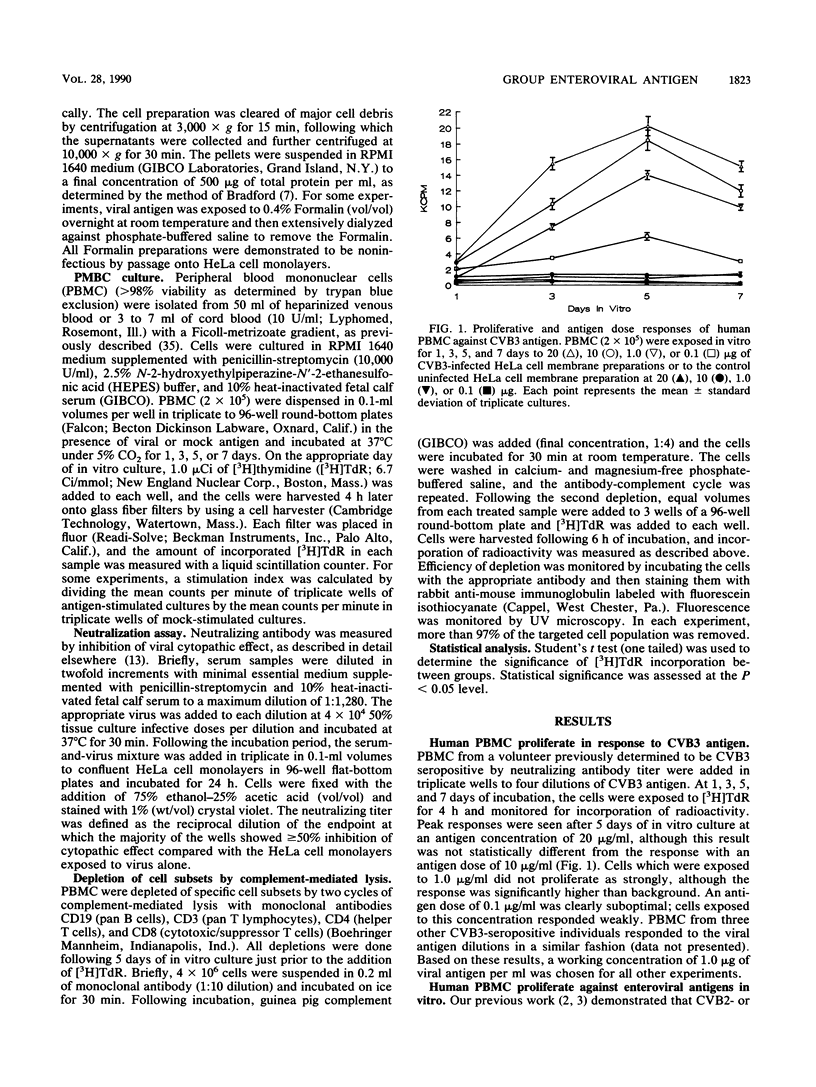
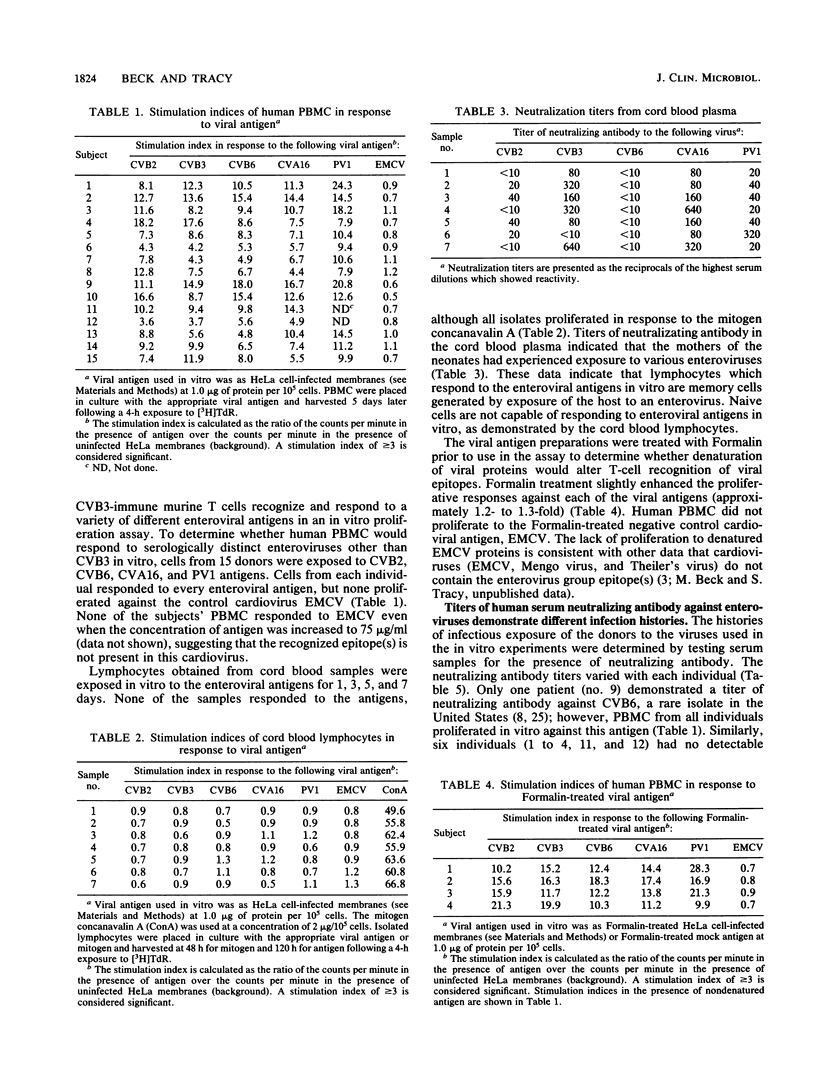
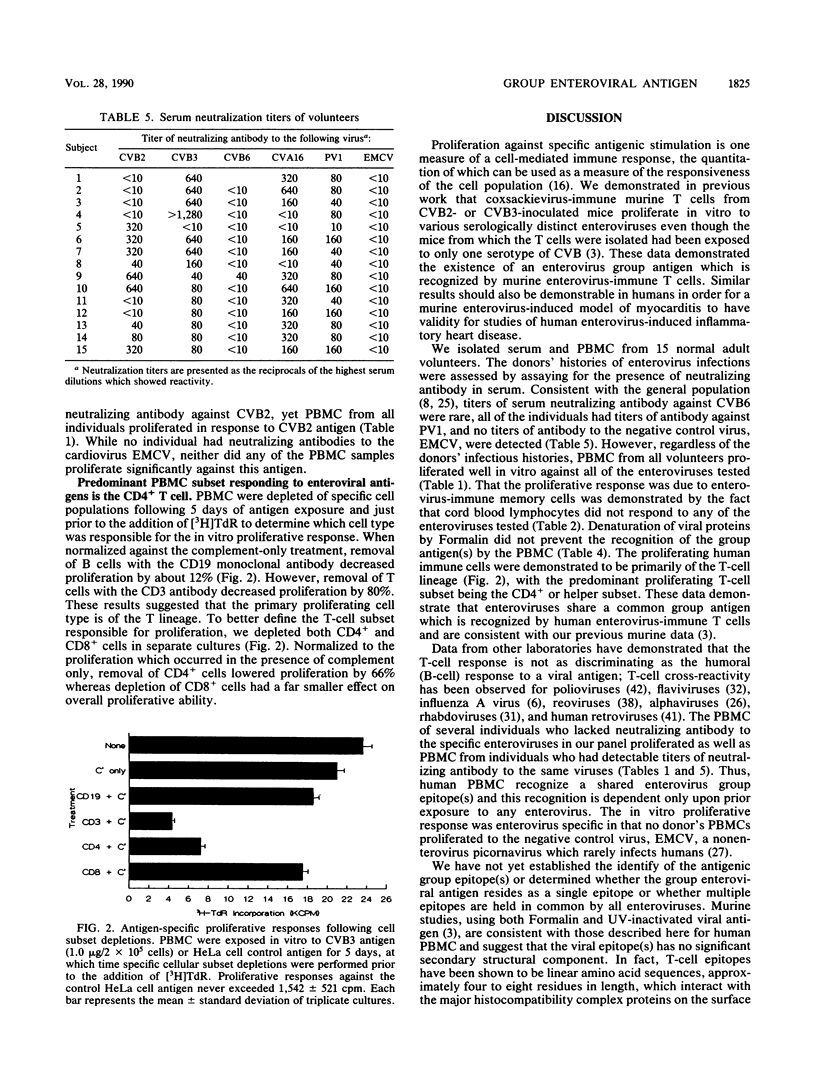
Human peripheral blood mononuclear cells from 15 normal, healthy adult volunteers proliferated in vitro against a panel of enteroviral antigens, including coxsackievirus B3, coxsackievirus B2, coxsackievirus B6, coxsackievirus A16, and poliovirus 1. No proliferation against the cardiovirus encephalomyocarditis virus occurred. Lymphocytes obtained from cord blood drawn from seven neonates were uniformly nonresponsive to enteroviral antigens. Although serum neutralization antibody titers indicated different exposure histories of the volunteers, only one had a titer against coxsackievirus B6, a rare isolate in the United States. The peripheral blood mononuclear cells from each volunteer responded in vitro to each enterovirus tested even though not all individuals had serum neutralizing antibody against each virus. The predominant cell type responding in vitro was the CD4+ T cell. Denaturation of viral antigen by Formalin did not prevent the recognition of the common group antigen by the T cells, indicating that noninfectious virus can also serve as antigen. These data demonstrate that human T cells recognize a common enterovirus group antigen(s).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck M. A., Chapman N. M., McManus B. M., Mullican J. C., Tracy S. Secondary enterovirus infection in the murine model of myocarditis. Pathologic and immunologic aspects. Am J Pathol. 1990 Mar;136(3):669–681. [PMC free article] [PubMed] [Google Scholar]
- Beck M. A., Tracy S. M. Murine cell-mediated immune response recognizes an enterovirus group-specific antigen(s). J Virol. 1989 Oct;63(10):4148–4156. doi: 10.1128/jvi.63.10.4148-4156.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. J., McCartney R. A. A study of Coxsackie B virus infections, 1972-1983. J Hyg (Lond) 1984 Oct;93(2):197–203. doi: 10.1017/s0022172400064718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. I. Generation of a virus-strain specific and a cross-reactive subpopulation of cytotoxic T cells in the response to type A influenza viruses of different subtypes. Cell Immunol. 1977 Oct;33(2):423–436. doi: 10.1016/0008-8749(77)90170-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deguchi H., Kitaura Y., Morita H., Kotaka M., Kawamura K. Cell-mediated immunity in Coxsackie B3 virus myocarditis in mice--in situ characterization by monoclonal antibody of mononuclear cell infiltrates. Heart Vessels Suppl. 1985;1:221–227. doi: 10.1007/BF02072397. [DOI] [PubMed] [Google Scholar]
- Easton A. J., Eglin R. P. The detection of coxsackievirus RNA in cardiac tissue by in situ hybridization. J Gen Virol. 1988 Feb;69(Pt 2):285–291. doi: 10.1099/0022-1317-69-2-285. [DOI] [PubMed] [Google Scholar]
- Finberg R., Weiner H. L., Fields B. N., Benacerraf B., Burakoff S. J. Generation of cytolytic T lymphocytes after reovirus infection: role of S1 gene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):442–446. doi: 10.1073/pnas.76.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Gomez P. T., Duffey P. S., Grant J. A., Trent D. W., Witherspoon S. M., Paque R. E. Characterization and myocarditic capabilities of coxsackievirus B3 variants in selected mouse strains. J Virol. 1984 Nov;52(2):598–605. doi: 10.1128/jvi.52.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauntt C. J., Trousdale M. D., LaBadie D. R., Paque R. E., Nealon T. Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol. 1979;3(3):207–220. doi: 10.1002/jmv.1890030307. [DOI] [PubMed] [Google Scholar]
- Grist N. R., Bell E. J., Assaad F. Enteroviruses in human disease. Prog Med Virol. 1978;24:114–157. [PubMed] [Google Scholar]
- Grun J. B., Schultz M., Finkelstein S. D., Crowell R. L., Landau B. J. Pathogenesis of acute myocardial necrosis in inbred mice infected with coxsackievirus B3. Microb Pathog. 1988 Jun;4(6):417–430. doi: 10.1016/0882-4010(88)90027-7. [DOI] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K., Steinman H. G., Buzzerd P. M. Studies of the blastogenic response of murine lymphocyte. 3. Specific viral transformation. Proc Soc Exp Biol Med. 1972 Oct;141(1):106–109. doi: 10.3181/00379727-141-36726. [DOI] [PubMed] [Google Scholar]
- Herskowitz A., Beisel K. W., Wolfgram L. J., Rose N. R. Coxsackievirus B3 murine myocarditis: wide pathologic spectrum in genetically defined inbred strains. Hum Pathol. 1985 Jul;16(7):671–673. doi: 10.1016/s0046-8177(85)80149-0. [DOI] [PubMed] [Google Scholar]
- Hori H., Matoba T., Shingu M., Toshima H. The role of cell mediated immunity in coxsackie B viral myocarditis. Jpn Circ J. 1981 Dec;45(12):1409–1414. doi: 10.1253/jcj.45.1409. [DOI] [PubMed] [Google Scholar]
- Huber S. A., Weller A., Herzum M., Lodge P. A., Estrin M., Simpson K., Guthrie M. Immunopathogenic mechanisms in experimental picornavirus-induced autoimmunity. Pathol Immunopathol Res. 1988;7(4):279–291. doi: 10.1159/000157123. [DOI] [PubMed] [Google Scholar]
- Lerner A., Wilson F. M., Reyes M. P. Enteroviruses and the heart (with special emphasis on the probable role of coxsackieviruses, group B, types 1-5). II. Observations in humans. Mod Concepts Cardiovasc Dis. 1975 Mar;44(3):11–15. [PubMed] [Google Scholar]
- Longson M., Cole F. M., Davies D. Isolation of a Coxsackie virus group B, type 5, from the heart of a fatal case of myocarditis in an adult. J Clin Pathol. 1969 Nov;22(6):654–658. doi: 10.1136/jcp.22.6.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller H. M., Powars D. F., Horowitz R. E., Portnoy B. Fatal myocarditis associated with ECHO virus, type 22, infection in a child with apparent immunological deficiency. J Pediatr. 1967 Aug;71(2):204–210. doi: 10.1016/s0022-3476(67)80073-8. [DOI] [PubMed] [Google Scholar]
- McManus B. M., Gauntt C. J., Cassling R. S. Immunopathologic basis of myocardial injury. Cardiovasc Clin. 1988;18(2):163–184. [PubMed] [Google Scholar]
- Moore M., Kaplan M. H., McPhee J., Bregman D. J., Klein S. W. Epidemiologic, clinical, and laboratory features of Coxsackie B1-B5 infections in the United States, 1970-79. Public Health Rep. 1984 Sep-Oct;99(5):515–522. [PMC free article] [PubMed] [Google Scholar]
- Mullbacher A., Marshall I. D., Blanden R. V. Cross-reactive cytotoxic T cells to alphavirus infection. Scand J Immunol. 1979;10(4):291–296. doi: 10.1111/j.1365-3083.1979.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Whitton J. L., Lewicki H., Tishon A. Fine dissection of a nine amino acid glycoprotein epitope, a major determinant recognized by lymphocytic choriomeningitis virus-specific class I-restricted H-2Db cytotoxic T lymphocytes. J Exp Med. 1988 Aug 1;168(2):559–570. doi: 10.1084/jem.168.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. L., Zinkernagel R. M. Cross-reactive cytotoxic T cells to serologically distinct vesicular stomatitis virus. J Immunol. 1980 May;124(5):2301–2308. [PubMed] [Google Scholar]
- Rothman A. L., Kurane I., Zhang Y. M., Lai C. J., Ennis F. A. Dengue virus-specific murine T-lymphocyte proliferation: serotype specificity and response to recombinant viral proteins. J Virol. 1989 Jun;63(6):2486–2491. doi: 10.1128/jvi.63.6.2486-2491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoub B. D., Johnson S., McAnerney J. M., Dos Santos I. L., Klaassen K. I. Epidemic Coxsackie B virus infection in Johannesburg, South Africa. J Hyg (Lond) 1985 Oct;95(2):447–455. doi: 10.1017/s0022172400062872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan J. F., Donnenberg A. D., Aurelian L., Elpern D. J. Immunity to herpes simplex virus type 2. IV. Impaired lymphokine production during recrudescence correlates with an imbalance in T lymphocyte subsets. J Immunol. 1982 Jul;129(1):326–331. [PubMed] [Google Scholar]
- Strongwater S. L., Dorovini-Zis K., Ball R. D., Schnitzer T. J. A murine model of polymyositis induced by coxsackievirus B1 (Tucson strain). Arthritis Rheum. 1984 Apr;27(4):433–442. doi: 10.1002/art.1780270411. [DOI] [PubMed] [Google Scholar]
- Tilzey A. J., Signy M., Banatvala J. E. Persistent coxsackie B virus specific IgM response in patients with recurrent pericarditis. Lancet. 1986 Jun 28;1(8496):1491–1492. doi: 10.1016/s0140-6736(86)91519-9. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Walker B. D., Flexner C., Paradis T. J., Fuller T. C., Hirsch M. S., Schooley R. T., Moss B. HIV-1 reverse transcriptase is a target for cytotoxic T lymphocytes in infected individuals. Science. 1988 Apr 1;240(4848):64–66. doi: 10.1126/science.2451288. [DOI] [PubMed] [Google Scholar]
- Wang K. G., Sun L. Z., Jubelt B., Waltenbaugh C. Cell-mediated immune responses to poliovirus. I. Conditions for induction, characterization of effector cells, and cross-reactivity between serotypes for delayed hypersensitivity and T cell proliferative responses. Cell Immunol. 1989 Apr 1;119(2):252–262. doi: 10.1016/0008-8749(89)90242-6. [DOI] [PubMed] [Google Scholar]
- Wolfgram L. J., Beisel K. W., Herskowitz A., Rose N. R. Variations in the susceptibility to Coxsackievirus B3-induced myocarditis among different strains of mice. J Immunol. 1986 Mar 1;136(5):1846–1852. [PubMed] [Google Scholar]
- el-Khatib M. R., Chason J. L., Ho K. L., Silberberg B., Lerner A. M. Coxsackievirus B4 myocarditis in mice: valvular changes in virus-infected and control animals. J Infect Dis. 1978 Apr;137(4):410–420. doi: 10.1093/infdis/137.4.410. [DOI] [PubMed] [Google Scholar]


