Abstract
2-Chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine (atrazine, ATR) is a toxicologically important and widely used herbicide. Recent studies have shown that it can elicit neurological, immunological, developmental, and biochemical alterations in several model organisms, including in mice. Because disposition data in mice are lacking, we evaluated ATR's metabolism and tissue dosimetry after single oral exposures (5–250 mg/kg) in C57BL/6 mice using liquid chromatography/mass spectrometry (Ross and Filipov, 2006). ATR was metabolized and cleared rapidly; didealkyl ATR (DACT) was the major metabolite detected in urine, plasma, and tissues. Plasma ATR peaked at 1 h postdosing and rapidly declined, whereas DACT peaked at 2 h and slowly declined. Most ATR and metabolite residues were excreted within the first 24 h. However, substantial amounts of DACT were still present in 25- to 48-h and 49- to 72-h urine. ATR reached maximal brain levels (0.06–1.5 μM) at 4 h (5–125 mg/kg) and 1 h (250 mg/kg) after dosing, but levels quickly declined to <0.1 μM by 12 h in all the groups. In contrast, strikingly high concentrations of DACT (1.5–50 μM), which are comparable with liver DACT levels, were detectable in brain at 2 h. Brain DACT levels slowly declined, paralleling the kinetics of plasma DACT. Our findings suggest that in mice ATR is widely distributed and extensively metabolized and that DACT is a major metabolite detected in the brain at high levels and is ultimately excreted in urine. Our study provides a starting point for the establishment of models that link target tissue dose to biological effects caused by ATR and its in vivo metabolites.
Atrazine (ATR) is a triazine herbicide that effectively inhibits photosynthesis in broadleaf weeds and grasses (Environmental Protection Agency, 2003). In the United States, ATR is applied on crops such as corn, sugarcane, pineapples, macadamia nuts, and sorghum, as well as on highway and railroad rights-of-way and on evergreen trees (Environmental Protection Agency, 2003). For some time, ATR was the most widely used agricultural pesticide in the United States and only recently has the use of glyphosate surpassed it (Kolpin et al., 2006). Nevertheless, large amounts of ATR are still being used annually (approximately 65–80 million pounds/year; Environmental Protection Agency, 2003).
Several epidemiological studies have investigated the link between ATR exposure and adverse human health outcomes (Hoppin et al., 2002; MacLennan et al., 2002; De Roos et al., 2003; Hessel et al., 2004). However, the human data at present are equivocal. In some cases ATR exposure is associated with negative health outcomes, whereas in others it is not. One major drawback of virtually all the existing epidemiological studies is the fact that ATR exposure has not been precisely quantified or estimated. In this regard, exposure-only studies, i.e., studies where health outcomes have not been assessed, indicate that pesticide applicators and farmers during spraying are exposed to appreciable levels of ATR because detectable amounts of ATR and its metabolites are found in their saliva and urine (Hines et al., 2006). Moreover, a recent report indicated that not only ATR applicators but also their families are at risk of high level of ATR exposure (Curwin et al., 2007).
Although data on health effects of ATR in humans are not complete, an increasing number of animal studies report that ATR exposure causes reproductive, developmental, and immunological alterations in laboratory rodents (Cooper et al., 1996, 2000; Narotsky et al., 2001; Pruett et al., 2003; Filipov et al., 2005; Giusi et al., 2006; Rowe et al., 2006). The brain is another potential target organ for ATR as several in vitro and in vivo studies have suggested that excessive exposure to ATR may be neurotoxic (Das et al., 2000, 2001; Rodriguez et al., 2005; Giusi et al., 2006; Coban and Filipov, 2007). Most of the above-mentioned studies have been performed in rats (Cooper et al., 1996, 2000; Narotsky et al., 2001; Rodriguez et al., 2005). However, mice are increasingly used in toxicology research, and the number of studies using mice for the assessment of potential adverse consequences of ATR exposure is increasing (National Toxicology Program, 1994; Pruett et al., 2003; Filipov et al., 2005; Giusi et al., 2006; Rowe et al., 2006; Coban and Filipov, 2007).
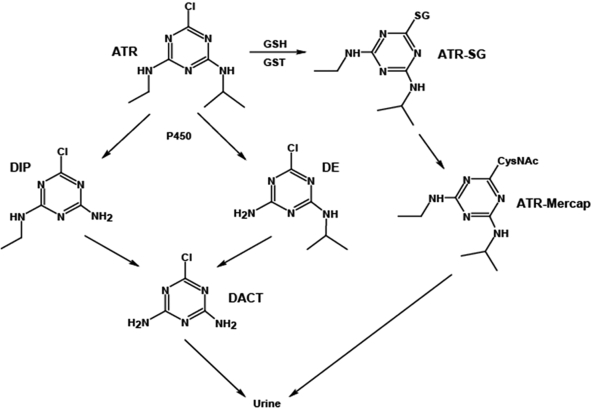
In vitro studies indicate that ATR is metabolized primarily by cytochromes P450 (P450s) and, to a much lesser extent, by glutathione transferases (GSTs; Fig. 1) (Adams et al., 1990; Hanioka et al., 1999b). The major P450-derived metabolites of ATR detected in these in vitro studies are the N-dealkylated products desethyl atrazine (DE) and desisopropyl atrazine (DIP). However, didealkyl atrazine (DACT) is the major in vivo metabolite of ATR detected in rat plasma (Brzezicki et al., 2003; McMullin et al., 2003) and in mouse plasma and urine (Ross and Filipov, 2006). In humans, major ATR metabolites detected in urine of occupationally exposed subjects are DE, DIP, and DACT (Catenacci et al., 1993). Earlier studies reported that DE and DIP were the two primary ATR metabolites found in urine of occupationally exposed humans (Ikonen et al., 1988; Catenacci et al., 1990). However, very recent evidence, using modern analytical technology, indicates that DACT is the most frequently detected human urinary metabolite of ATR (Barr et al., 2007).
Fig. 1.
Biotransformation of ATR in mammals. See Table 1 for detailed definition of abbreviations.
Except for an earlier limited study in rats where ATR and some of its metabolites were determined in pooled liver, kidney, and brain samples from animals orally exposed to ATR (6 or 12 mg/kg) for 7 days (Gojmerac and Kniewald, 1989), peer-reviewed data on tissue dosimetry of ATR and its metabolites in any mammalian species are lacking. The main objective of this study was to provide a detailed description of ATR's metabolism in mice. Our additional objectives were to 1) determine the levels of ATR and/or its metabolites in potential target tissues such as the brain and 2) to determine how these tissue levels correlate with levels of ATR and its metabolites in plasma and urine, which is an accessible biological fluid. By obtaining plasma, urine, and tissue concentration data for ATR and its major mammalian metabolites, one might be able to extrapolate urinary levels to possible maximal levels in target tissues.
Materials and Methods
Chemicals and Reagents. ATR (98% purity), DE (99.5%), DIP (98%), DACT (96%), and simazine (99%) were purchased from ChemService, Inc. (West Chester, PA). For oral exposure, ATR was dissolved in corn oil. For liquid chromatography/mass spectrometry (LC/MS) analysis, stock solutions (10 mM) of ATR, DE, DIP, and simazine [used as internal standard (IS)] were prepared in ethanol, and DACT (50 mM) was dissolved in dimethyl sulfoxide. Atrazine-mercapturate (ATR-mercap) and atrazine-glutathione conjugate (ATR-SG) were synthesized by a modification of the method of Lucas et al. (1993), as we described in detail elsewhere (Ross and Filipov, 2006). N-Acetyl cysteine and glutathione (GSH), used for the synthesis of ATR-mercap and ATR-SG, were purchased from Sigma-Aldrich (St. Louis, MO).
In Vivo Studies. Animals. Male C57BL/6 mice (3–4 months old) were used in this study. Animals were housed (5 mice/cage) during acclimatization and throughout the experiment in a room maintained under constant temperature (22°C) on a 12-h light/dark cycle, with water and food available ad libitum. Animals were treated in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication 86-23). All of the experimental procedures were approved in advance by the Institutional Animal Care and Use Committee of Mississippi State University. A minimum of 5 mice/dose/sample collection time point were used. Mice used for urine collection were placed in individual metabolic cages 1 day before experimentation and maintained there until final tissue collection, as described in detail below.
ATR administration. After an overnight fast, mice (n = 5–8/dose/time point) were exposed to single doses of ATR (5, 25, 125, and 250 mg/kg b.wt.) by oral gavage. This dose regimen was selected to closely resemble the doses used in the National Toxicology Program study (National Toxicology Program, 1994) and by Pruett et al. (2003) in mice, as well as by Brzezicki et al. (2003) in their study in rats. An additional dose range consideration was the fact that documented exposures of more than 200,000 people to ATR levels above the acute reference dose (RfD) have been reported (Environmental Protection Agency, 2003); the acute RfD was derived from a no observed adverse effect level of 10 mg/kg/day and a lowest observed adverse effect level of 70 mg/kg/day (Environmental Protection Agency, 2003). In comparison with the National Toxicology Program study, we added a lower (5 mg/kg) dose and excluded the highest (500 mg/kg) dose used in the National Toxicology Program study (National Toxicology Program, 1994) because of the significant decrement in body weight observed at that dose.
Average body weight of the mice immediately before ATR administration was 25.3 ± 0.5 g (mean ± S.E.M.). ATR was dissolved in corn oil, and control animals were exposed to the corn oil vehicle (100 μl/25 g mouse, or 4 ml/kg b.wt.). At each time point, mice were euthanized with CO2, and blood samples were obtained by cardiac puncture. Liver, kidney, brain, spleen, and thymus were also harvested and frozen at –80°C for future analysis of ATR and its metabolites. For urine and fecal material collection, mice were placed in individual metabolic cages (Nalgene Nunc International Corp., Rochester, NY) and acclimated overnight before ATR administration. Twenty-four-hour collections of urine and feces from each mouse were performed for up to 96 h after ATR administration. After each daily collection, cages and collection cups were thoroughly cleaned, and a new collection was initiated. Thus, the samples are not cumulative, i.e., each 24-h sample represents the urinary/fecal excretion that occurred from 0 to 24, 24 to 48, 48 to 72, and 72 to 96 h, respectively.
Data collection. At 0.5, 1, 2, 4, 6, 12, 24, 48, and 96 h after ATR administration, groups of mice (5–8/dose) were euthanized, and plasma and tissues were harvested for subsequent analyses. Urine samples from the mice placed in metabolic cages were collected at 24, 48, 72, and 96 h after the single ATR administration. After collection, samples were processed for LC/MS analysis of ATR and its metabolites as described in detail below.
Sample Preparation and Analytical Procedure. Urine. The procedure for analyzing ATR and its metabolites in mouse urine is adapted from our preliminary study (Ross and Filipov, 2006). In brief, urine samples (0.5 ml) were adjusted to pH 9 with NH4OH, followed by 1 volume of acetonitrile containing IS (simazine) to deproteinize urine. The samples were placed on ice for 15 min and then centrifuged (16,000g, 5 min, 4°C). The supernatant was evaporated to dryness under nitrogen, and the residue was redissolved in water (1 ml). The aqueous samples were applied to a C-18 Sep Pak column (3-ml bed volume; Waters, Milford, MA) that had been preconditioned with 100% methanol (2 ml) and equilibrated with 100% water (2 ml). After sample loading, the column was washed with 5 ml of water, and the analytes subsequently were eluted in 100% methanol (3 ml). The methanol eluate was evaporated to dryness under nitrogen, and the residue was redissolved in 200 μl of water. Aliquots (10 μl) were subsequently injected onto the LC/MS.
Plasma. Analysis of mouse plasma was performed by procedures developed in our previous study (Ross and Filipov, 2006). In brief, mouse plasma sample (250-μl aliquots) was added to a microcentrifuge tube and mixed with 1 ml of acetonitrile containing the IS. The samples were placed on ice for 15 min and then centrifuged at 16,000g for 5 min at 4°C. The supernatants were evaporated to dryness under nitrogen with slight heating (40°C). The residue was reconstituted in 400 μl of 0.05% (v/v) acetic acid, passed through a syringe filter (0.22 μm), and injected (10 μl) onto the LC/MS.
Tissue extracts. Mouse tissues (liver, kidney, brain, spleen, and thymus) were accurately weighed and homogenized in aqueous acetic acid (0.01% v/v) as a 15% (w/v) mixture using a Potter-Elvehjem tissue grinder or a small-tip sonicator (Branson Sonifier 250, Branson Ultrasonics, Danbury, CT). Each tissue homogenate was vigorously vortexed for 3 min, and an aliquot of the homogenate (typically 400 μl) was mixed with the IS (10 nmol). Four volumes of ice-cold acetonitrile was added to the spiked homogenate, mixed, and the samples placed on ice for 15 min. After centrifugation (16,000g, 5 min, 4°C), the supernatants were transferred to clean test tubes and evaporated to dryness under nitrogen. The residues were redissolved in 1:1 (v/v) 0.05% acetic acid/ethanol (400 μl), passed through a syringe filter (0.22 μm), and an aliquot (10–25 μl) was analyzed by LC/MS. Analyte recoveries from tissue extracts and method sensitivity were assessed using an “add-back” approach that was previously described for plasma and urine (Ross and Filipov, 2006). Recovery of analytes from tissues after this extraction procedure was typically ≥70%. DACT provided the poorest recovery (∼70%) of all the tissue analytes examined, which was consistent with our previous study with urine and plasma samples (Ross and Filipov, 2006). The concentrations of analytes in each tissue and/or biofluid are expressed in micromolar units.
LC/MS analytical procedure. LC/electrospray ionization/MS analysis of tissue, plasma, and urine extracts was performed on a Surveyor MSQ single quadrupole system (Thermo Electron Corporation, Waltham, MA). The mobile phase solvents were a blend of solvent A (0.1% v/v acetic acid in water) and solvent B (0.1% v/v acetic acid in methanol). After injection of the sample (10–25 μl) onto a C-8 column (4.6 × 100 mm; Thermo Electron Corporation), the analytes were eluted with the following gradient program: 0 min (98% A, 2% B), 15 min (25% A, 75% B), 20 min (25% A, 75% B), 25 min (98% A, 2% B). The flow rate was 1 ml/min, and the column eluate was split postcolumn (1:1), half the eluate being directed to a UV detector, the other half to the MS. The UV wavelength was set to 230 nm. Ions were introduced into the MS by electrospray ionization in the positive ion mode. The MS single quadrupole was operated in single-ion mode with seven analytes (ATR and its major metabolites: DACT, DIP, DE, ATR-mercap, and ATR-SG, plus simazine, the IS; see Table 1 for m/z values) monitored throughout the chromatographic run. Calibration standards for each analyte were prepared in tissue, plasma, and urine matrices from naive untreated mice. The calibration standards in each matrix were worked up for LC/MS analysis in the exact manner as described for the unknown samples. An equivalent amount of IS was added to both unknown sample and calibrant before sample workup and LC/MS analysis. Quality control samples were prepared with each sequence of samples to be run on the LC/MS. Blank samples (solvent blanks) were interspersed in the sequence to ensure no intersample carryover was occurring.
TABLE 1.
Description of analytes and the molecular ions monitored during chromatographic runs
| Group | Purity | Lot Number | Analyte Name | Generic Name | Acronym | m/za |
|---|---|---|---|---|---|---|
| % | ||||||
| 1 | 97.5b | —c | N-Acetyl-S-[4-(ethylamino)-6-(isopropylamino)-1,3,5-triazin-2-yl]-L-cytseine | Atrazine-mercapturate | ATR-mercap | 343 |
| 2 | 96 | 285-100A | 2-Chloro-4,6-diamino-s- triazine | Didealkyl atrazine | DACT | 146 148 |
| 3 | 98 | 295-74A | 2-Chloro-4-(ethylamino)-6-amino-s-triazine | Desisopropyl atrazine | DIP | 174 176 |
| 4 | 99.5 | 284-85B | 2-Chloro-4-amino-6-(isopropylamino)-s-triazine | Desethyl atrazine | DE | 188 190 |
| 5 | 98 | 301-49A | 2-Chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine | Atrazine | ATR | 216 218 |
| 6 | 99b | —c | S-[4-(ethylamino)-6-(isopropylamino)-1,3,5-triazin-2-yl]-L-glutathione | Atrazine-glutathione | ATR-SG | 244.1d 487.2 |
| 7 | 99 | 332-44A | 2-Chloro-4,6-bis(ethylamino)-s-triazine (internal standard) | Simazine | SIM | 202 |
Ions monitored by single-ion monitoring during LC/MS runs.
Purity determined by high-performance liquid chromatography/UV analysis of the synthesized product. All of the other purities were provided by ChemService, Inc. (Lot numbers are indicated for each compound purchased from ChemService, Inc.).
—, Synthesis described in Ross and Filipov (2006).
Doubly protonated ion.
Detection and quantitation limits of analytical method. The detection and quantitation limits for ATR and its metabolites in mouse plasma and urine were previously reported in Ross and Filipov (2006). To estimate the limits of detection (LOD) and quantitation (LOQ) of each analyte in tissue extracts, the lowest calibration standard containing all the target compounds was injected onto the LC/MS seven consecutive times to estimate the average base peak intensity for each analyte, and to determine the S.D. of the baseline noise over 100 consecutive scan events immediately adjacent to the analyte peak. The signal-to-noise (S/N) ratios in each analytical run were estimated using the following calculation: S/N = (base peak intensity of analyte – baseline noise intensity)/(S.D. of baseline noise intensity), and the average values were calculated. For each analyte, LOD and LOQ values were determined assuming S/N = 3 and S/N = 10, respectively. See Table 2 for the LOD and LOQ values for the primary analytes in tissue extracts.
TABLE 2.
Detection and quantitation limits of target analytes in tissuesa
| Analyte | Limit of Detectionb | Limit of Quantitationc |
|---|---|---|
| μM | μM | |
| DACT | 0.45 | 1.5 |
| DIP | 0.09 | 0.31 |
| DE | 0.07 | 0.25 |
| ATR-mercap | 0.05 | 0.18 |
| ATR-SG | 0.05 | 0.18 |
| ATR | 0.01 | 0.05 |
Values are based on tissue wet weight (g), assuming 1 g ≈ 1 ml.
Limit of detection; S/N = 3.
Limit of quantitation; S/N = 10.
Results
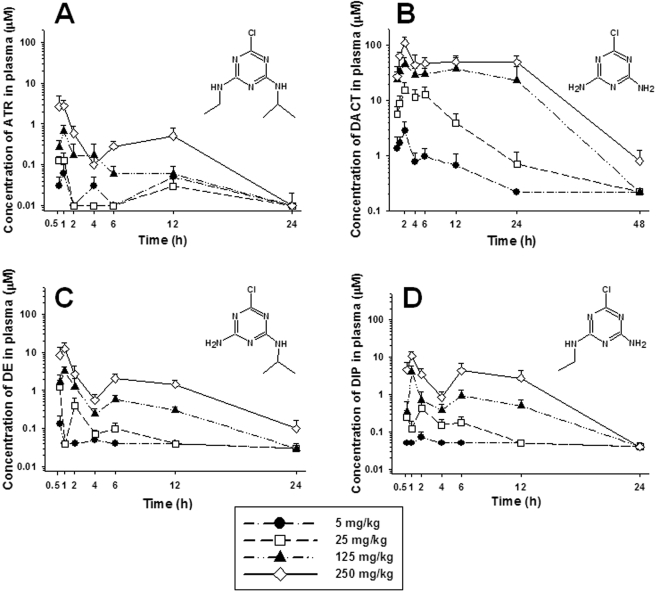
Plasma Concentrations. The maximum amount of ATR in mouse plasma was detected 1 h postdosing at all the dose levels, followed by a rapid decline in concentration (Fig. 2A). An apparent biphasic concentration curve was observed, with a second peak in plasma ATR levels noted at 12 h. This may reflect a discontinuity in the absorption rate of ATR from the gastrointestinal tract after an oral dose. By 24 h, the plasma ATR levels were lower than the detection limit of the LC/MS assay. Whereas ATR was rapidly cleared from mouse plasma, the levels of the didealkylated metabolite DACT concomitantly increased and reached peak levels at 2 h postdosing at each dose level. DACT was clearly the most abundant metabolite found in the plasma. The peak concentration of DACT reached ∼100 μM following the highest dose of ATR, and the maximum DACT levels observed were found to be dose-dependent. Meanwhile, the clearance of DACT from mouse plasma was slow during the initial 24-h period, particularly for the high-dose animals (Fig. 2B). The estimated t1/2 values for DACT during the initial 24-h period were 7.5 and 42 h following the lowest and highest ATR doses, respectively (Table 3). Plasma levels of the two monodealkylated metabolites, DIP and DE, were found to be significantly lower than DACT during this timeframe (Fig. 2, C and D). Both metabolites were approximately 10-fold lower in concentration than DACT at each time point examined, indicating that each was rapidly converted to DACT in vivo. ATR-mercap, which has been advocated as a human biomarker of ATR exposure (Lucas et al., 1993), was also detected in the mouse plasma by our analytical method (data not shown). After exposure to the highest (250 mg/kg) dose of ATR, the peak level of ATR-mercap was observed at 2 h, although the concentration (∼0.1 μM) was low compared with the other metabolites. All the other time points exhibited levels of ATR-mercap that were below the LOD.
Fig. 2.
Concentration of ATR and its metabolites in mouse plasma determined by LC/MS at the indicated time points after single ATR treatments at four different dose levels. A, concentration of ATR. B, concentration of DACT. C, concentration of DE. D, concentration of DIP. Each time point represents the mean ± S.E.M.; n = 5, 8, 8, 8, 6, 6, and 8 mice/dose for plasma samples collected at 0.5, 1, 2, 4, 6, 12, and 24 h, respectively.
TABLE 3.
AUC for each major metabolite
|
Analyte
|
AUCa
|
|||
|---|---|---|---|---|
| 5 mg/kg | 25 mg/kg | 125 mg/kg | 250 mg/kg | |
| μM-h | μM-h | μM-h | μM-h | |
| ATR | 0.68 | 0.54 | 2.0 | 9.6 |
| DE | 0.92 | 2.0 | 10 | 38 |
| DIP | 1.1 | 2.5 | 13 | 58 |
| DACTb | 18 (7.5) | 140 (4.8) | 750 (35) | 1250 (42) |
Obtained from the 0- to 24-h plasma concentration data using the trapezoid function in SigmaPlot (Systat Software, Inc., San Jose, CA).
Estimated plasma half-lives (h) for DACT are indicated by values in parentheses: data were estimated from the 0- to 24-h time period (Fig. 2B).
Of all the metabolites targeted in plasma, only DACT was detectable by 48 h and only in plasma samples obtained from mice dosed with 250 mg/kg ATR (Fig. 2B). Seventy-two-hour plasma samples from all the dose groups were also analyzed, but all the metabolites (including DACT) were below the detection limits of the assay by this time point. Thus, in contrast to the 0- to 24-h time period, it seems that DACT was rapidly cleared from the plasma of high-dose animals during the 24- to 48-h time period.
Plasma area under the curves (AUCs) for ATR and DACT are reported in Table 3, as are plasma half-lives for DACT. When plasma AUC values for DACT were plotted against ATR dose, a significant linear correlation was observed (r2 = 0.9872; p = 0.0064). The increase in plasma DACT half-life during the 0- to 24-h time period as a function of ATR dose is likely owing to DACT being a terminal metabolite of ATR and the abundant amounts produced in mice that can only be cleared via urinary excretion. Because of the bimodal distribution of ATR in plasma, which probably results from uneven gastrointestinal absorption rates and rapid metabolism, it is difficult to estimate ATR's half-life in plasma after oral gavage.
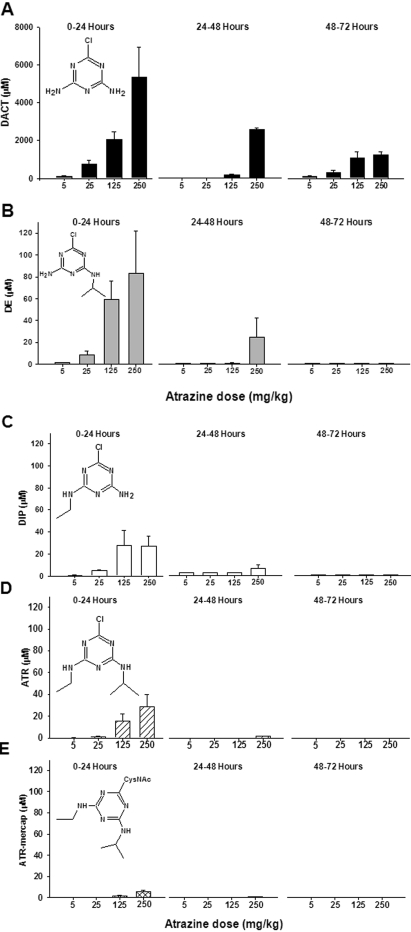
Urine Concentrations. The concentrations of ATR and its metabolites in the 0- to 24-, 24- to 48-, and 48- to 72-h cumulative urine samples were determined (Fig. 3, A–E). The analytes are presented in Fig. 3 in order of decreasing abundance. The levels of DACT measured in the 0- to 24-h urine were 2 orders of magnitude higher than ATR or its monodealkylated metabolites (Fig. 3A). The concentration of DACT reached as high as 5400 μM in 0- to 24-h urine following the highest dose of ATR. This is substantially higher than we reported previously (Ross and Filipov, 2006), but in that preliminary study we only collected a spot urine sample from mice at 24 h after ATR treatment. In the current study, we have collected cumulative daily urine samples in metabolic cages. Subsequent analysis of urine samples collected between 24 to 48 and 48 to 72 h after ATR treatment also detected significant quantities of DACT (Fig. 3A). In the 72- to 96-h urine samples, DACT levels were below the detection limit of the LC/MS assay (data not shown), indicating that the bulk of the DACT had been excreted from the mice within 72 h after ATR administration. The next most abundant metabolite detected in urine was DE (Fig. 3B). Concentrations of DE reached as high as ∼80 μM in 0- to 24-h urine, with significantly lower quantities found in the 24- to 48-h and 48- to 72-h urine samples. Levels of DIP in urine (Fig. 3C) were lower than DE but were comparable with the amounts of urinary ATR (Fig. 3D). ATR was detected in significant amounts in the 0- to 24-h urine after exposure to the two highest doses of ATR (Fig. 3D); the estimated concentrations were ∼15 and 28 μM after the 125- and 250-mg/kg doses, respectively. It is interesting to note that the levels of ATR-mercap detected in mouse urine were much lower than all the other metabolites analyzed, reaching only ∼6 μM after administration of the highest ATR dose (Fig. 3E). Except for DACT, the other ATR metabolites were found at very low levels, if at all, 2 to 3 days after the administration of ATR. Urine seems to be the major excretion pathway of ATR metabolites because limited analysis of fecal extracts did not reveal significant amounts of the major metabolites, although ATR was detected in fecal extracts and is probably a result of unabsorbed compound that had traversed the gastrointestinal tract (data not shown).
Fig. 3.
Concentration of ATR and its metabolites in mouse urine after single ATR treatments at four different dose levels. Cumulative 0- to 24-, 24- to 48-, and 48- to 72-h urine samples were collected and analyzed for DACT (A), DE (B), DIP (C), ATR (D), and ATR-mercap (E). Note the difference in y-axis scales for DACT versus other analytes. Each time point represents the mean ± S.E.M.; n = 8 mice/dose for 0- to 24-h urine samples and 5 mice/dose for 24- to 48- and 48- to 72-h urine samples.
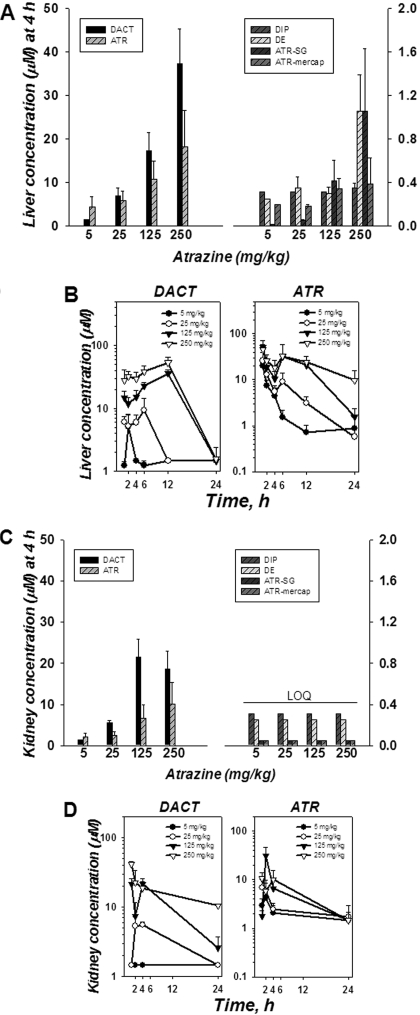
Liver and Kidney Concentrations. By 4 h postdosing, most ATR had been absorbed from the gastrointestinal tract, and ATR and all its metabolites were detectable in liver at this particular time point. The concentrations of ATR and DACT in liver at 4 h were far greater than the other metabolites (Fig. 4A). Because ATR and DACT were the most abundant analytes detected in liver, the time course for these two compounds is shown in Fig. 4B. The level of DACT in liver increased in a dose-dependent manner, reaching a maximum of ∼50 μM by 12 h after the highest ATR dose; its levels rapidly declined by 24 h (Fig. 4B). ATR reached maximum levels in liver (∼50 μM) at 2 h, which then gradually declined over 24 h (Fig. 4B). For comparison, ATR concentrations were never greater than 5 μM in the plasma over the entire time course. The concentration ratio of DACT/ATR in liver at 4 h increased in a linear manner with ATR dose, which suggested that the P450 metabolic pathway had not become saturated even at the highest administered dose of ATR. Furthermore, DIP and DE were found in only modest concentrations in liver at all the time points compared with DACT and ATR. Meanwhile, the glutathione conjugate of ATR (ATR-SG) was detectable in liver (the only tissue in which it was detected) but only at low concentrations (≤1 μM) after the two highest doses of ATR (Fig. 4A).
Fig. 4.
Concentration of ATR and its metabolites in mouse liver (A and B) and kidney (C and D) after single ATR treatments at four different dose levels. Bar graphs represent the distribution of ATR and metabolites in liver (A) and kidney (C) at 4 h, respectively. Note scale differences of y-axes. LOQ, all the analytes were less than the limit of quantitation. Time course of DACT and ATR concentrations in liver (B) and kidney (D), respectively. Each time point represents the mean ± S.E.M.; n = 7, 8, 8, 5, 3, and 2 mice/dose for liver and n = 6, 5, 8, 5, 3, and 2 mice/dose for kidney tissue samples collected at 1, 2, 4, 6, 12, and 24 h, respectively.
When the levels of DACT and ATR in the kidney were determined at 4 h, generally lower concentrations of each compound were found compared with liver (Fig. 4C). However, the ratios of DACT to ATR in the kidney for each dose group were similar to that seen in the liver (Fig. 4A). A time course of DACT and ATR levels in kidney is shown in Fig. 4D. The levels of DE and DIP were exceedingly low in the kidney (below limit of quantitation), and ATR-SG and ATR-mercap were not detectable at all in this organ.
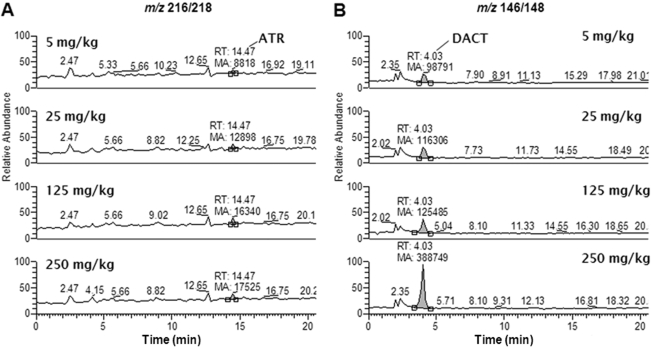
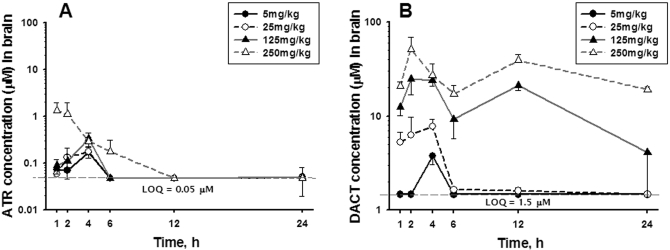
Brain Concentrations. Figure 5 shows representative LC/MS chromatograms of brain extracts obtained from mice 4 h after being dosed with ATR at 5, 25, 125, and 250 mg/kg. This particular time point exhibited significant quantities of DACT and detectable amounts of ATR in all the dose groups. Modest amounts of ATR were detected in the brains (Fig. 6A). For example, maximum levels of ATR in the brain (∼1–1.5 μM) were found 1 to 2 h after the highest dose. However, by 12 h, the levels of ATR had declined to <0.1 μM in all the dose groups. Yet, low levels of ATR were still detectable in the brains of all the dose groups at 24 h. In contrast to ATR, DACT reached significant levels in the brains of all the dose groups, being detected as high as ∼50 μM at 2 h after the highest dose (Fig. 6B). The levels of DACT in the brain were dose-dependently increased and like ATR were also found to be persistent. For example, DACT was still detectable at significant levels in the brain at the 24-h time point in all the dose groups. None of the monodealkylated metabolites, on the other hand, could be detected in brain extracts in our study.
Fig. 5.
Representative LC/MS ion chromatograms of brain extracts prepared from mice that had been treated with ATR at 5, 25, 125, and 250 mg/kg. Brains were collected 4 h after the indicated dose of ATR and processed as described under Materials and Methods. Single-ion monitoring chromatograms for ATR (m/z 216/218; A) and DACT (m/z 146/148; B) in brain extracts from each dose group. RT, retention time for target analyte peak; MA, mass area for target analyte peak.
Fig. 6.
Concentration of ATR (A) and DACT (B) in mouse brain at the indicated time points after single ATR treatments at four different dose levels. Each time point represents the mean ± S.E.M.; n = 8, 8, 8, 6, 6, and 8 mice/dose for brain tissue samples collected at 1, 2, 4, 6, 12, and 24 h, respectively.
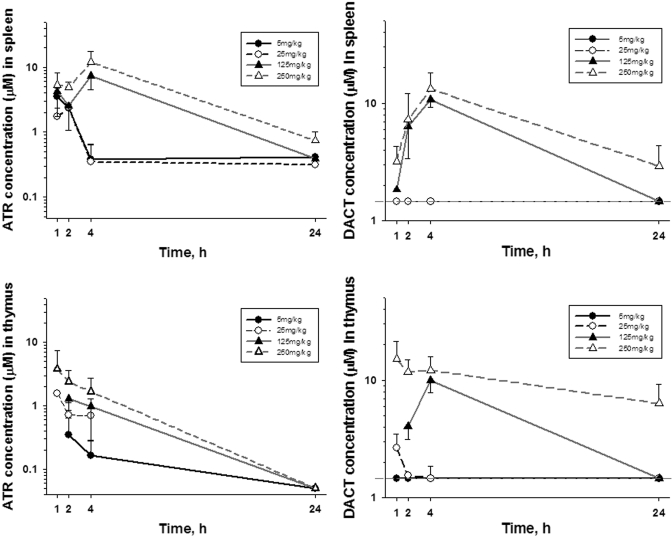
Spleen and Thymus Concentrations. At the two low doses of ATR (5 and 25 mg/kg), the maximum levels of ATR (∼2–3 μM) detected in the spleen were found at 1 h (Fig. 7A). After the two high doses (125 and 250 mg/kg), the maximum levels of atrazine (∼7–11 μM) were observed at 4 h. By 24 h, splenic levels of ATR were significantly reduced yet were still above the limit of quantitation. The major metabolite detected in spleen was DACT. Peak levels of DACT (∼10–15 μM) were detected at 4 h after the two highest doses of ATR (Fig. 7B), with little DACT detectable by 24 h. In contrast, little or no DACT could be detected in spleen after the two lowest doses at any time point. Finally, lower concentrations of ATR were detected in thymus (Fig. 7C) compared with that found in the spleen, whereas levels of DACT in thymus were generally comparable with those found in spleen (Fig. 7D). Except for the high-dose groups (DACT only), the levels of ATR and DACT in the thymus were below the limit of quantitation at 24 h.
Fig. 7.
Concentration of ATR and DACT in mouse spleen (A and B) and thymus (C and D), respectively, at the indicated time points after single ATR treatments at four different dose levels. Each time point represents the mean ± S.E.M. of n = 5 mice, except for the 24-h samples (n = 2).
Discussion
Accurate quantification of pesticides and their metabolites in biofluids and tissues of experimental model organisms is an important goal of toxicology research as it will link target tissue dose to the biological effects caused by such chemicals. Although definitive human data on adverse effects associated with ATR exposure are not available, ATR causes several biological effects in multiple tissues of experimental rodents. Thus, ATR exposure: 1) causes several reproductive abnormalities, primarily associated with perturbation of luteinizing hormone homeostasis, in female (Cooper et al., 1996, 2000; Narotsky et al., 2001) and male (Stoker et al., 2002) rats; 2) decreases thymic and splenic cellularity and causes other immune system perturbations in female (Pruett et al., 2003) and male (Filipov et al., 2005) mice; and 3) affects the nervous system of male rodents (Rodriguez et al., 2005; Coban and Filipov, 2007), and, apparently, the developing nervous system is particularly sensitive (Giusi et al., 2006). Moreover, because of the continued widespread use and the physiochemical properties of ATR, estimates indicate that more than 200,000 people in the United States are exposed above the acute RfD each year (Environmental Protection Agency, 2003). Indeed, human biomonitoring studies have detected and quantified levels of several ATR metabolites in human urine from high-, low-, and environmentally exposed individuals (Barr et al., 2007). Still, occupational exposure to ATR is the greatest concern (Hines et al., 2006; Curwin et al., 2007).
This study is the first to report levels of ATR and its metabolites in critical tissues such as brain, thymus, and spleen after a single oral exposure to a dose range of ATR. Animal studies have shown that these tissues are adversely affected by ATR (National Toxicology Program, 1994; Filipov et al., 2005; Karrow et al., 2005; Rodriguez et al., 2005; Pruett et al., 2006; Coban and Filipov, 2007). Our data suggest that ATR is widely distributed and extensively metabolized in mice. DACT is the most abundantly produced ATR metabolite by far and is detected in multiple tissues, plasma, and readily excreted in urine. Consistent with previous metabolism studies in rats (Brzezicki et al., 2003; McMullin et al., 2003), in mice, ATR undergoes successive rounds of oxidative N-dealkylation reactions, presumably catalyzed by P450s (Hanioka et al., 1999a), eventually leading to DACT (Fig. 1). It is noteworthy that DACT is not formed in significant quantities during mouse hepatic microsomal incubations (Ross and Filipov, 2006) or by freshly isolated rat hepatocytes (McMullin et al., 2007). The higher levels of DE compared with DIP in mouse urine that we noted are consistent with the metabolite profile observed in human urine of ATR-exposed subjects (Barr et al., 2007). Furthermore, compared with DACT and ATR, DIP and DE were found in only modest concentrations in liver, suggesting that they were rapidly converted to DACT in the liver and/or that DACT is formed in the gut epithelium and subsequently transported to the liver. It is interesting to note that the maximum concentration of ATR determined in liver at 4 h was 18 μM. This is well below the published Km value for ATR in mouse liver microsomes (Hanioka et al., 1999b), indicating a highly efficient liver metabolism and a lack of saturation of the P450 biotransformation pathway. In support, plasma DACT AUC values (Table 3) correlated with ATR dose (r2 = 0.9872). In contrast to P450-catalyzed metabolism of ATR, GSH conjugation of ATR seems to be a minor pathway of ATR metabolism in mice. This is based on the fact that, relative to DACT, we detected very low quantities of ATR-SG and ATR-mercap. As a recent report indicated that GSH conjugation of ATR in humans is a minor biotransformation pathway (Barr et al., 2007), it seems that mice and humans appear to use this pathway to metabolize ATR only sparingly.
Besides providing a profile for ATR and its metabolites in human urine, Barr et al. (2007) also concluded, based on the slow rate of GSH conjugation of ATR, that using ATR-mercap as an exposure biomarker can underestimate exposure. Thus, they recommended that future biomonitoring studies include the suite of ATR metabolites that are detected in urine. DACT would seem to be the best candidate metabolite for such studies because of its abundance. However, the disadvantages of using DACT as a biomarker are its lack of specificity, chromatographic properties on LC columns, and ionization behavior. DACT is formed from the structurally related herbicide simazine (Adams et al., 1990), and because of its polarity it elutes off reversedphase high-performance liquid chromatography columns in the presence of a large number of polar endogenous molecules that are present in biofluids and tissue extracts. This can result in ion suppression effects and a corresponding loss in sensitivity for DACT in mass spectrometers.
Few reported studies have quantified ATR and its metabolites in rodent tissues after in vivo ATR exposure. One study using [14C]ATR reported that total [14C]ATR-derived radiocarbon (i.e., parent compound and all the metabolites) could be detected in all the tissues tested (Bakke et al., 1972), with levels in each tissue gradually decreasing from 2 to 8 days postexposure. It is interesting to note that the rate of decline in the brain was slower than the decline observed in liver and kidney tissues (Bakke et al., 1972). The high concentrations of DACT in the brain and the ability to detect ATR in the brain are notable findings of our study. The apparent persistence of low amounts of ATR in the brain may be because of its lipophilicity and its consequently slow rate of clearance from this lipid-rich tissue, which has sparse amounts of P450 enzyme. On the other hand, because none of the monodealkylated metabolites could be detected in brain extracts in our study, DACT probably entered the brain as a result of partitioning and/or transport of this metabolite through the blood-brain barrier. Indeed, the high levels of DACT observed may warrant neurotoxicological studies of this metabolite, particularly because DACT levels slowly declined in the brain. Using isolated synaptic vesicles preparations, we observed that ATR dose-dependently inhibited vesicular uptake of the neurotransmitter dopamine. The lowest effective concentration was 1 μM (Hossain and Filipov, 2008), which is in the range of the brain ATR levels that we observed in the present study. DACT, at concentrations as high as 100 μM, which is again in the range of the brain levels observed in the current study, was ineffective. These data suggested that ATR is perhaps the primary compound that acutely affects the brain and that metabolism of ATR, at least in terms of its acute effects on the brain, is a detoxication mechanism. However, recent reports indicated that both ATR and DACT can covalently react with proteins forming stable protein adducts (Dooley et al., 2006, 2008). Several proteins were identified in these studies that were adducted by DACT, but the functional consequence of this adduction is currently unknown. Nevertheless, these findings coupled with our current brain DACT data suggest the possibility that the neurotoxicological effects associated with chronic ATR exposure may be related to the protein-damaging properties of DACT. This hypothesis will need further experimentation to verify.
Besides effects on the nervous system, ATR exposure can adversely affect the immune system, with thymus being affected more than the spleen shortly after exposure (National Toxicology Program, 1994; Pruett et al., 2003; Filipov et al., 2005; Karrow et al., 2005; Rowe et al., 2006). However, thymus cellularity seems to recover from the effects of ATR, whereas splenic cellularity is reduced up to 7 weeks after exposure termination (Filipov et al., 2005). Our current data indicate that low amounts of ATR are present in the thymus and DACT is not detectable following low doses. Thus, some of the effects of ATR exposure on the thymus may be indirect, i.e., mediated by activation of the stress axis, which is observed after acute ATR exposure (Pruett et al., 2003). On the other hand, because ATR and DACT were detectable in the spleen, their ability to form protein adducts (Dooley et al., 2006, 2008) may play a role in the longer-lasting effects of ATR exposure on the spleen (Filipov et al., 2005).
In conclusion, ATR is widely distributed and extensively metabolized in mice; DACT is far and away the major metabolite formed in vivo and is ultimately excreted in urine. Both ATR and DACT were found in high concentrations in liver and kidneys, and markedly high levels of DACT were detected in the brain that seemed to persist over 24 h. Furthermore, ATR and DACT levels were determined in two other potential target organs of this herbicide, the thymus and spleen. In contrast to DACT, the monodealkylated metabolites DE and DIP were found in low quantities in all the tissues examined. The large ratios of DACT/monodealkylated metabolite that are found in tissues are in concordance with the ratios of DACT/monodealkylated metabolite found in urine. Furthermore, ATR-mercap was barely detectable in plasma and tissues, which corresponds well with the low amounts detectable in urine. These data will help to establish urine as an appropriate surrogate matrix for reverse dosimetry analysis of pesticides, such as ATR.
Acknowledgments
We thank S. C. Sistrunk, A. B. Norwood, and Dr. A. Borazjani for providing technical support for the completion of this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
This work was supported by the National Institutes of Health National Center for Research Resources [Grant P20-RR017661].
Parts of this work were previously as follows: Filipov NM, Ross MK, Pinchuk LM, Borazjani A, and Coban A (2007) Metabolism and health effects of atrazine exposure in the mouse. 46th Annual Meeting of the Society of Toxicology; 2007 Mar 25–29; Charlotte, NC; (2008) 47th Annual Meeting of the Society of Toxicology; 2008 Mar 16–20; Seattle, WA. Society of Toxicology, Reston, VA.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
doi:10.1124/dmd.108.024927.
ABBREVIATIONS: ATR, atrazine, 2-chloro-4-(ethylamino)-6-(isopropylamino)-s-triazine; P450, cytochrome P450; GST, glutathione transferase; DE, desethyl atrazine; DIP, desisopropyl atrazine; DACT, didealkyl atrazine; LC/MS, liquid chromatography/mass spectrometry; IS, internal standard; ATR-mercap, atrazine-mercapturate; ATR-SG, atrazine-glutathione conjugate; GSH, glutathione; RfD, reference dose; LOD, limit of detection; LOQ, quantification limit; S/N, signal/noise ratio; AUC, area under the curve.
References
- Adams NH, Hodgson E, and Levi PE (1990) In vitro studies of the metabolism of atrazine, simazine, and terbutryn in several vertebrate species. J Agric Food Chem 38 1411–1417. [Google Scholar]
- Bakke JE, Larson JD, and Price CE (1972) Metabolism of atrazine and 2-hydroxyatrazine by the rat. J Agric Food Chem 20 602–607. [DOI] [PubMed] [Google Scholar]
- Barr DB, Panuwet P, Nguyen JV, Udunka S, and Needham LL (2007) Assessing exposure to atrazine and its metabolites using biomonitoring. Environ Health Perspect 115 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezicki JM, Andersen ME, Cranmer BK, and Tessari JD (2003) Quantitative identification of atrazine and its chlorinated metabolites in plasma. J Anal Toxicol 27 569–573. [DOI] [PubMed] [Google Scholar]
- Catenacci G, Barbieri F, Bersani M, Ferioli A, Cottica D, and Maroni M (1993) Biological monitoring of human exposure to atrazine. Toxicol Lett 69 217–222. [DOI] [PubMed] [Google Scholar]
- Catenacci G, Maroni M, Cottica D, and Pozzoli L (1990) Assessment of human exposure to atrazine through the determination of free atrazine in urine. Bull Environ Contam Toxicol 44 1–7. [DOI] [PubMed] [Google Scholar]
- Coban A and Filipov NM (2007) Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem 100 1177–1187. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Goldman JM, Parrish MB, and Tyrey L (1996) Effect of atrazine on ovarian function in the rat. Reprod Toxicol 10 257–264. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, Goldman JM, and McElroy WK (2000) Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci 53 297–307. [DOI] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, and Alavanja MC (2007) Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann Occup Hyg 51 53–65. [DOI] [PubMed] [Google Scholar]
- Das PC, McElroy WK, and Cooper RL (2001) Alteration of catecholamines in pheochromocytoma (PC12) cells in vitro by the metabolites of chlorotriazine herbicide. Toxicol Sci 59 127–137. [DOI] [PubMed] [Google Scholar]
- Das PC, McElroy WK, and Cooper RL (2000) Differential modulation of catecholamines by chlorotriazine herbicides in pheochromocytoma (PC12) cells in vitro. Toxicol Sci 56 324–331. [DOI] [PubMed] [Google Scholar]
- De Roos AJ, Zahm SH, Cantor KP, Weisenburger DD, Holmes FF, Burmeister LF, and Blair A (2003) Integrative assessment of multiple pesticides as risk factors for non-Hodgkin's lymphoma among men. Occup Environ Med 60 E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley GP, Prenni JE, Prentiss PL, Cranmer BK, Andersen ME, and Tessari JD (2006) Identification of a novel hemoglobin adduct in Sprague Dawley rats exposed to atrazine. Chem Res Toxicol 19 692–700. [DOI] [PubMed] [Google Scholar]
- Dooley GP, Reardon KF, Prenni JE, Tjalkens RB, Legare ME, Foradori CD, Tessari JE, and Hanneman WH (2008) Proteomic analysis of diaminochlorotriazine adducts in Wistar rat pituitary glands and LbetaT2 rat pituitary cells. Chem Res Toxicol 21 844–851. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (2003) Interim Reregistration Eligibility Decision for Atrazine, pp 2005 (EPA-HQ-OPP-2003-0072-0002), U.S. Environmental Protection Agency, Washington, DC.
- Filipov NM, Pinchuk LM, Boyd BL, and Crittenden PL (2005) Immunotoxic effects of short-term atrazine exposure in young male C57BL/6 mice. Toxicol Sci 86 324–332. [DOI] [PubMed] [Google Scholar]
- Giusi G, Facciolo RM, Canonaco M, Alleva E, Belloni V, Dessi'-Fulgheri F, and Santucci D (2006) The endocrine disruptor atrazine accounts for a dimorphic somatostatinergic neuronal expression pattern in mice. Toxicol Sci 89 257–264. [DOI] [PubMed] [Google Scholar]
- Gojmerac T and Kniewald J (1989) Atrazine biodegradation in rats–a model for mammalian metabolism. Bull Environ Contam Toxicol 43 199–206. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, and Ando M (1999a) In vitro metabolism of chlorotriazines: characterization of simazine, atrazine, and propazine metabolism using liver microsomes from rats treated with various cytochrome P450 inducers. Toxicol Appl Pharmacol 156 195–205. [DOI] [PubMed] [Google Scholar]
- Hanioka N, Jinno H, Tanaka-Kagawa T, Nishimura T, and Ando M (1999b) In vitro metabolism of simazine, atrazine and propazine by hepatic cytochrome P450 enzymes of rat, mouse and guinea pig, and oestrogenic activity of chlorotriazines and their main metabolites. Xenobiotica 29 1213–1226. [DOI] [PubMed] [Google Scholar]
- Hessel PA, Kalmes R, Smith TJ, Lau E, Mink PJ, and Mandel J (2004) A nested case-control study of prostate cancer and atrazine exposure. J Occup Environ Med 46 379–385. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Deddens JA, Lu C, Fenske R, and Striley CA (2006) Mixed-effect models for evaluating multiple measures of atrazine exposure among custom applicators. J Occup Environ Hyg 3 274–283. [DOI] [PubMed] [Google Scholar]
- Hoppin JA, Umbach DM, London SJ, Alavanja MC, and Sandler DP (2002) Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med 165 683–689. [DOI] [PubMed] [Google Scholar]
- Hossain MM and Filipov NM (2008) Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology 248 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen R, Kangas J, and Savolainen H (1988) Urinary atrazine metabolites as indicators for rat and human exposure to atrazine. Toxicol Lett 44 109–112. [DOI] [PubMed] [Google Scholar]
- Karrow NA, McCay JA, Brown RD, Musgrove DL, Guo TL, Germolec DR, and White KL Jr (2005) Oral exposure to atrazine modulates cell-mediated immune function and decreases host resistance to the B16F10 tumor model in female B6C3F1 mice. Toxicology 209 15–28. [DOI] [PubMed] [Google Scholar]
- Kolpin DW, Thurman EM, Lee EA, Meyer MT, Furlong ET, and Glassmeyer ST (2006) Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci Total Environ 354 191–197. [DOI] [PubMed] [Google Scholar]
- Lucas AD, Jones AD, Goodrow MH, Saiz SG, Blewett C, Seiber JN, and Hammock BD (1993) Determination of atrazine metabolites in human urine: development of a biomarker of exposure. Chem Res Toxicol 6 107–116. [DOI] [PubMed] [Google Scholar]
- MacLennan PA, Delzell E, Sathiakumar N, Myers SL, Cheng H, Grizzle W, Chen VW, and Wu XC (2002) Cancer incidence among triazine herbicide manufacturing workers. J Occup Environ Med 44 1048–1058. [DOI] [PubMed] [Google Scholar]
- McMullin TS, Andersen ME, Tessari JD, Cranmer B, and Hanneman WH (2007) Estimating constants for metabolism of atrazine in freshly isolated rat hepatocytes by kinetic modeling. Toxicol In Vitro 21 492–501. [DOI] [PubMed] [Google Scholar]
- McMullin TS, Brzezicki JM, Cranmer BK, Tessari JD, and Andersen ME (2003) Pharmacokinetic modeling of disposition and time-course studies with [14C]atrazine. J Toxicol Environ Health A 66 941–964. [DOI] [PubMed] [Google Scholar]
- Narotsky MG, Best DS, Guidici DL, and Cooper RL (2001) Strain comparisons of atrazine-induced pregnancy loss in the rat. Reprod Toxicol 15 61–69. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (1994) NTP Report on the Immunotoxicity of Atrazine (CAS no. 1912-24-9) in Female B6C3F1 Mice (IMM94002), National Institute of Environmental Health Science, Research Triangle Park, NC.
- Pruett SB, Fan R, and Oppenheimer S (2006) Greater than additive suppression of TLR3-induced IL-6 responses by administration of dieldrin and atrazine. J Immunotoxicol 3 353–362. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, and Hebert P (2003) Modeling and predicting immunological effects of chemical stressors: characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol Sci 75 343–354. [DOI] [PubMed] [Google Scholar]
- Rodriguez VM, Thiruchelvam M, and Cory-Slechta DA (2005) Sustained exposure to the widely used herbicide atrazine: altered function and loss of neurons in brain monoamine systems. Environ Health Perspect 113 708–715. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ross MK and Filipov NM (2006) Determination of atrazine and its metabolites in mouse urine and plasma by LC-MS analysis. Anal Biochem 351 161–173. [DOI] [PubMed] [Google Scholar]
- Rowe AM, Brundage KM, Schafer R, and Barnett JB (2006) Immunomodulatory effects of maternal atrazine exposure on male Balb/c mice. Toxicol Appl Pharmacol 214 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker TE, Guidici DL, Laws SC, and Cooper RL (2002) The effects of atrazine metabolites on puberty and thyroid function in the male Wistar rat. Toxicol Sci 67 198–206. [DOI] [PubMed] [Google Scholar]