Abstract
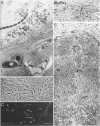
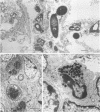
A murine model of focal hepatic candidiasis which we suggest simulates certain conditions of this clinical variant of systemic candidiasis in leukemic patients is described. We have shown that outbred mice inoculated with Candida albicans by the oral-intragastric route as infants (6 days old) and then immunocompromised by cyclophosphamide and cortisone acetate treatment 2 weeks later demonstrate systemic spread of the opportunistic pathogen to the liver, lungs, spleen, and kidneys. Treatment with the immunosuppressive drugs cyclophosphamide and cortisone acetate resulted in alteration of the normal integrity of the mucosal epithelium of the gut as well as in granulocytopenia. Approximately 55% of the animals with C. albicans infections in the liver demonstrated hepatic abscesses. After these same infected, immunocompromised animals were treated with suboptimal dosages of antifungal agents (cilofungin or amphotericin B), either by intraperitoneal or subcutaneous (s.c.) routes, persistent hepatic abscesses were fewer in number and delimited by a distinct outer layer of host tissue but still contained large numbers of the viable pathogen. Blood cell counts indicated that these antifungal drug-treated animals had reestablished approximately the same number of leukocytes per microliter of blood as estimated prior to the immunocompromising drug treatment. Similar conditions in leukemic patients who were in remission and who were undergoing antifungal drug therapy for systemic candidiasis have been reported. Clearance of hepatic infections in mice was accomplished by using appropriate concentrations of amphotericin B administered by daily intraperitoneal or s.c. injection for 5 to 7 days or cilofungin by continuous s.c. infusion for 7 days. However, systemic antifungal therapy did not significantly reduce numbers of C. albicans cells in the stomach and esophagus. Persistent foci of gastrointestinal colonization by C. albicans, especially in the region of the cardial-atrium fold of the stomach of these mice, are reservoirs of the opportunistic pathogen from which reinfection may occur, leading to relapse of systemic candidiasis.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannatyne R. M., Cheung R., Devlin H. R. Microassay foramphotericin B. Antimicrob Agents Chemother. 1977 Jan;11(1):44–46. doi: 10.1128/aac.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Vecchiarelli A., Cenci E., Sbaraglia G., Perito S., Cassone A. A comparison of experimental pathogenicity of Candida species in cyclophosphamide-immunodepressed mice. Sabouraudia. 1984;22(5):409–418. doi: 10.1080/00362178485380661. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Anaissie E. J. Chronic systemic candidiasis. Eur J Clin Microbiol Infect Dis. 1989 Oct;8(10):855–857. doi: 10.1007/BF01963770. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988 Feb;11 (Suppl A):411–426. doi: 10.1016/0195-6701(88)90220-4. [DOI] [PubMed] [Google Scholar]
- Christiansen K. J., Bernard E. M., Gold J. W., Armstrong D. Distribution and activity of amphotericin B in humans. J Infect Dis. 1985 Nov;152(5):1037–1043. doi: 10.1093/infdis/152.5.1037. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R. An animal model for oropharyngeal, esophageal and gastric candidosis. Mycoses. 1990 Jan;33(1):7–19. doi: 10.1111/myc.1990.33.1.7. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Lynn K. T., Seshan K. R., Pope L. M. Gastrointestinal and systemic candidosis in immunocompromised mice. J Med Vet Mycol. 1989;27(6):363–380. doi: 10.1080/02681218980000491. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Seshan K. R., Pope L. M., Yancey R. J. Morphological aspects of gastrointestinal tract invasion by Candida albicans in the infant mouse. J Med Vet Mycol. 1988 Jun;26(3):173–185. [PubMed] [Google Scholar]
- Estey E. H., Keating M. J., McCredie K. B., Bodey G. P., Freireich E. J. Causes of initial remission induction failure in acute myelogenous leukemia. Blood. 1982 Aug;60(2):309–315. [PubMed] [Google Scholar]
- Gold J. W. Opportunistic fungal infections in patients with neoplastic disease. Am J Med. 1984 Mar;76(3):458–463. doi: 10.1016/0002-9343(84)90665-x. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Cole G. T., Pope L. M. Animal models for candidiasis. Curr Top Med Mycol. 1985;1:57–116. doi: 10.1007/978-1-4613-9547-8_3. [DOI] [PubMed] [Google Scholar]
- Guentzel M. N., Herrera C. Effects of compromising agents on candidosis in mice with persistent infections initiated in infancy. Infect Immun. 1982 Jan;35(1):222–228. doi: 10.1128/iai.35.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. S., Myles C., Pratt K. J., Washington J. A. Cilofungin (LY121019), an antifungal agent with specific activity against Candida albicans and Candida tropicalis. Antimicrob Agents Chemother. 1988 Sep;32(9):1331–1335. doi: 10.1128/aac.32.9.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haron E., Feld R., Tuffnell P., Patterson B., Hasselback R., Matlow A. Hepatic candidiasis: an increasing problem in immunocompromised patients. Am J Med. 1987 Jul;83(1):17–26. doi: 10.1016/0002-9343(87)90492-x. [DOI] [PubMed] [Google Scholar]
- Herrera C., Guentzel M. N. Mice with persistent gastrointestinal Candida albicans as a model for antifungal therapy. Antimicrob Agents Chemother. 1982 Jan;21(1):51–53. doi: 10.1128/aac.21.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. L., Barnett J. L., Appelman H. D., Nostrant T. Candida hepatitis. Histopathologic diagnosis. Am J Surg Pathol. 1988 Sep;12(9):716–720. doi: 10.1097/00000478-198809000-00008. [DOI] [PubMed] [Google Scholar]
- Lewis J. H., Patel H. R., Zimmerman H. J. The spectrum of hepatic candidiasis. Hepatology. 1982 Jul-Aug;2(4):479–487. doi: 10.1002/hep.1840020415. [DOI] [PubMed] [Google Scholar]
- Livstone E. M., Sheahan D. G., Behar J. Studies of esophageal epithelial cell proliferation in patients with reflux esophagitis. Gastroenterology. 1977 Dec;73(6):1315–1319. [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Mehta R., Lopez-Berestein G., Hopfer R., Mills K., Juliano R. L. Liposomal amphotericin B is toxic to fungal cells but not to mammalian cells. Biochim Biophys Acta. 1984 Mar 14;770(2):230–234. doi: 10.1016/0005-2736(84)90135-4. [DOI] [PubMed] [Google Scholar]
- Meunier F., Gérard M., Richard V., Debusscher L., Bleiberg H., Malengrau A. Hepatic candidosis in a patient with acute leukemia. Mycoses. 1989 Aug;32(8):421–426. [PubMed] [Google Scholar]
- Meunier F., Lambert C., Van der Auwera P. In-vitro activity of cilofungin (LY121019) in comparison with amphotericin B. J Antimicrob Chemother. 1989 Sep;24(3):325–331. doi: 10.1093/jac/24.3.325. [DOI] [PubMed] [Google Scholar]
- Musial C. E., Cockerill F. R., 3rd, Roberts G. D. Fungal infections of the immunocompromised host: clinical and laboratory aspects. Clin Microbiol Rev. 1988 Oct;1(4):349–364. doi: 10.1128/cmr.1.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Pazin G. J., Allen C. M. Disseminated candidiasis. Changes in incidence, underlying diseases, and pathology. Am J Clin Pathol. 1977 Jul;68(1):29–38. doi: 10.1093/ajcp/68.1.29. [DOI] [PubMed] [Google Scholar]
- Perfect J. R., Hobbs M. M., Wright K. A., Durack D. T. Treatment of experimental disseminated candidiasis with cilofungin. Antimicrob Agents Chemother. 1989 Oct;33(10):1811–1812. doi: 10.1128/aac.33.10.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L. M., Cole G. T. Comparative studies of gastrointestinal colonization and systemic spread by Candida albicans and nonlethal yeast in the infant mouse. Scan Electron Microsc. 1982;(Pt 4):1667–1676. [PubMed] [Google Scholar]
- Pope L. M., Cole G. T., Guentzel M. N., Berry L. J. Systemic and gastrointestinal candidiasis of infant mice after intragastric challenge. Infect Immun. 1979 Aug;25(2):702–707. doi: 10.1128/iai.25.2.702-707.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H., Fischedick A. R., Peters P. E., von Lengerke H. J. Candida-Abszesse in Leber und Milz. Sonographische und computertomographische Morphologie. Dtsch Med Wochenschr. 1986 May 23;111(21):816–820. doi: 10.1055/s-2008-1068537. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stone H. H., Kolb L. D., Currie C. A., Geheber C. E., Cuzzell J. Z. Candida sepsis: pathogenesis and principles of treatments. Ann Surg. 1974 May;179(5):697–711. doi: 10.1097/00000658-197405000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Taguchi H., Nishimura K., Miyaji M., Nakamura A., Nakajima H. Studies on detection of Candida antigen in the sera of mice inoculated orally with Candida albicans. Mycopathologia. 1988 Oct;104(1):7–17. doi: 10.1007/BF00437918. [DOI] [PubMed] [Google Scholar]
- Tashjian L. S., Abramson J. S., Peacock J. E., Jr Focal hepatic candidiasis: a distinct clinical variant of candidiasis in immunocompromised patients. Rev Infect Dis. 1984 Sep-Oct;6(5):689–703. doi: 10.1093/clinids/6.5.689. [DOI] [PubMed] [Google Scholar]
- Thaler M., Pastakia B., Shawker T. H., O'Leary T., Pizzo P. A. Hepatic candidiasis in cancer patients: the evolving picture of the syndrome. Ann Intern Med. 1988 Jan;108(1):88–100. doi: 10.7326/0003-4819-108-1-88. [DOI] [PubMed] [Google Scholar]
- Verdeguer A., Fernández J. M., Esquembre C., Ferris J., Ruiz J. G., Castel V. Hepatosplenic candidiasis in children with acute leukemia. Cancer. 1990 Feb 15;65(4):874–877. doi: 10.1002/1097-0142(19900215)65:4<874::aid-cncr2820650408>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]