Abstract
Introduction
In the industrialized countries 67 million people suffer from glaucoma, which represents the third most common cause of blindness and thus has a high economic impact. Early diagnosis of glaucoma, which does not necessarily involve raised intraocular pressure, is essential because by the time the patient notices functional impairment the damage is irreversible. Early treatment can decrease the rate of blindness 20 years later by about 50%.
Methods
Selective literature review and clinical investigation of early glaucoma detection and of screening methods.
Results
Currently, no evidence-based recommendations for glaucoma screening can be found in the literature. No single method or combination of screening procedures can be recommended unambiguously on economic grounds.
Discussion
From the clinical perspective sensitive, specific, and cost-effective glaucoma screening seems feasible. The high-risk group would need to be defined on the basis of age and family history. A two-stage screening process would then have to be established with initial computer-supported telemedical sorting followed by telemedical ophthalmological diagnosis of cases selected for clarification.
Keywords: glaucoma, prevention, early detection of disease, ophthalmology, telemedicine
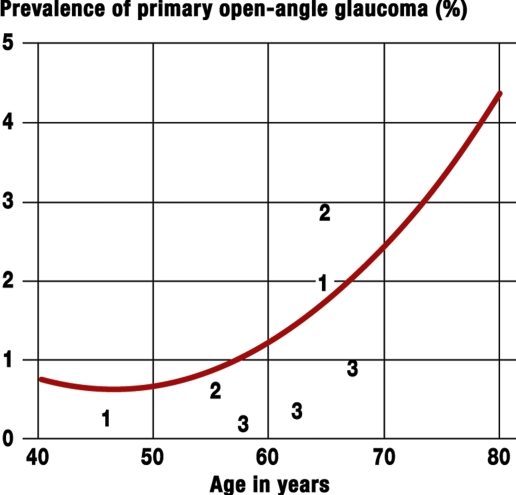
Glaucoma disease is progressive and poses an important challenge to healthcare systems. In the industrialized countries, some 67 million people have glaucoma; the prevalence is 2 to 3% in persons older than 40 (1). From the 60th year of life, the prevalence doubles with every decade of life (2) (figure 1). The rate rises from 0.1% in the fourth decade to 9.7% in the eighth decade (3). In Germany, the proportion of people older than 65 was 19.3% in 2005; it will increase to 28.7% in 2030 and 34.1% in 2050. These calculations are based on data from the Federal Statistical Office (4, 5). Assuming mean prevalence rates of glaucoma of 1.0%, 2.9%, and 5.3% for the age ranges 50 to 64, 65 to 79, and older than 80 years, respectively, and taking into account the demographic changes in Germany up to 2050, the number of glaucoma patients is set to rise from 710 000 to more than 970 000 in 2030 and to 1.01 million patients afterwards. The calculated population prevalence will almost double from 0.86% in 2005 to 1.26% in 2030 to 1.60% in 2050 (table 1a and 1b). Estimates for Germany even now assume some 950 000 patients with primary open-angle glaucoma (6). Ocular hypertension with raised intraocular pressure is regarded as merely one of several risk factors that can occur in isolation without morphological or functional signs of glaucoma. Some 25 to 50% of patients with glaucoma related atrophy of the optic nerve have normal pressure glaucoma – their intraocular pressure is not raised (7).
Figure 1.
Prevalence of glaucoma as a function of age. Calculations of the incidence and prevalence of open-angle glaucoma are based on the glaucoma model of Quigley and Vitale (2). Data from 14 different population based studies went into this model. The model calculations relate to white (Caucasian) people. Mean prevalence rates from different publications are also shown. The values were taken from publications as follows: 1 from (3), 2 from (e5), and 3 from (e6).
Table 1a. Calculated glaucoma prevalence in Germany.
| Age | 2005 | 2030 | 2050 | ||||
|---|---|---|---|---|---|---|---|
| Prevalence (%) | Population (× 1000) | Glaucoma patients (× 1000) | Population (× 1000) | Glaucoma patients (× 1000) | Population (× 1000) | Glaucoma patients (× 1000) | |
| 50–64 | 1.0 | 15 143 | 154 | 16 203 | 165 | 14 870 | 151 |
| 65–79 | 2.9 | 12 189 | 349 | 15 931 | 457 | 13 335 | 382 |
| > 80 | 5.3 | 3681 | 196 | 6305 | 335 | 10 152 | 540 |
| > 50 | 31 013 | 709 | 38 439 | 974 | 38 357 | 1010 | |
Prevalence calculated after (2) for age range medians of 57.5 years and 72.5 years and for 84 years of age.
Population data from Federal Statistical Office (4), extrapolation of the population for 2030 and 2050 according to Federal Statistical Office (6);
we used tables A6 and A7 with "mean" population, upper limit.
Table 1b.
| Population | 2005 | 2030 | 2050 |
|---|---|---|---|
| Prevalence in population >50 years | 2.29 | 2.53 | 2.87 |
| Prevalence in total population | 0.86 | 1.26 | 1.60 |
To calculate population based prevalence values, we assumed the prevalence in people younger than 50 to be zero because reliable epidemiological values are lacking and the formula from (2) is not suitable.
Some 50% of patients are unaware that they have glaucoma (6, 8). Glaucoma is the third most common cause of blindness in industrialized nations (9). The annual incidence of glaucoma related blindness in Germany is increasing and will affect some 1900 people in 2020 (10). Some 4.5 million people worldwide will develop bilateral blindness due to primary open-angle glaucoma, and this number is set to increase to 5.9 million by 2020 (11). Since patients notice glaucoma only once functional impairments – and thus irreversible damage – have developed, early detection and early treatment are of crucial importance to avoid blindness. In glaucoma, simultaneous specific damage affects the neural fibers and astrocytes of the optic nerve and the retinal ganglion cells. Only a loss in neural fibers of at least 50% enables the proof of visual field impairment.
In Germany, glaucoma screening is recommended according to the guideline every 3 years in all persons aged 40 to 64 years and in persons older than 65 every 1 to 2 years (12). Glaucoma screening can be accessed as an "individual healthcare service" (which patients pay for) and entails a medical history, general medical eye examination with optic nerve checks and tonometry. The costs will be covered by the statutory health insurance companies only in patients in whom glaucoma is suspected or who have specific risk factors.
In this article, we will review the current situation with regard to early diagnosis of glaucoma and the available investigative methods, and will assess the medical quality of screening examinations for the detection of glaucoma.
Methods
We conducted a selective literature search of relevant German language and English language publications, focusing particularly on review articles with much cited randomized studies or meta-analyses of preventive glaucoma diagnostics and on our own scientific studies. We searched the databases of the Cochrane Library, the National Library of Medicine/National Institutes of Health, and the German Institute for Medical Documentation, using the search terms "glaucoma", "telemedicine", "screening", "evidence based", and "economic". We identified possible other relevant studies from the bibliographies of the retrieved articles. We gave preference to studies that investigated glaucoma screening in the Western industrialized nations. We assessed the relevance of the studies on the basis of our own clinical experience.
Results
Presenting and assessing the study methods
Glaucoma induced visual functional impairments have to be distinguished from glaucoma induced changes to the ocular morphology. Table 2 shows the sensitivities and specificities of the individual screening tests.
Table 2. Overview of sensitivities and specificities of investigative methods.
| Investigative method | Sensitivity (%) | Specificity (%) | Literature |
|---|---|---|---|
| Ophthalmoscopic examination | > 90 | > 90 | (e7) |
| FDT | 78.9 – 84.2 | 55.0 – 65.7 | (16) |
| FDT + fundus camera | 58.6 | 64.3 | (e8) |
| FDT | 92 | 93 | (14) |
| HRT | 15 – 70 | 83 – 98 | (15) |
| HRT | 26.5 (pre-perimetric) 97 (perimetric) | 65 – 95 | (22) |
| HRT | 25 – 100 | 87 – 97 | (23) |
| HRT | 89.5 – 94.7 | 80.6 – 90.5 | (16) |
| OCT | 39 – 79 | 80 – 83 | (15) |
| GDx | 66 – 86 | 80 – 83 | (15) |
| GDx | 86 | 90 | (24) |
| GDx | 62 – 100 | 73 – 100 | (e9) |
| Non-contact tonometry | 14 | 98 | (e8. 25) |
| Visual acuity measurement, family history, FDT, and HRT | 96.8 | 89.7 | (16) |
| RTA | 75 | >= 90 | (e10) |
FDT, frequency duplication test; HRT, Heidelberg retina tomography; OCT, optical coherence tomography; GDx, scanning laser polarimetry; RTA, retinal thickness analyzer; pre-perimetric, without perimetric confirmation of visual field defect; perimetric, with perimetric confirmation of visual field loss
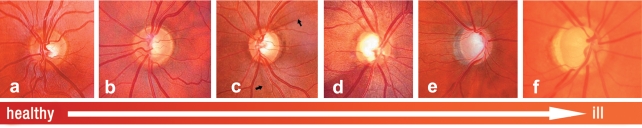
Functional perimetric investigative methods – such as the frequency doubling technique (FDT, spatial and temporal contrast sensitivity) and blue-yellow perimetry – enable detection of glaucomatous visual field defects earlier than standard perimetry because they investigate special retinal functions. Standard perimetry is more suitable for documenting progression. Papillary changes – such as a generalized or focal loss of the rim margin, focal nerve fiber defects, and parapapillary hemorrhages are pathognomonic for glaucoma (figure 2). For this reason, screening should entail assessment of the optic nerve. Screening also enables confirmation of pre-perimetric glaucoma with morphological changes that precede measurable visual field defects. The most important papillary and nerve fiber examinations include:
Figure 2.
Color papillary images showing different phases of glaucoma disease; a) healthy with regular margin, b) narrow, clearly visible nerve fiber defects in 2 h and 7 h, c) discrete focal nerve fiber defect in 12 h, d) wide nerve fiber defect in 6 h to 8 h, nerve fiber loss from 6 to 12 h, f) complete nerve fiber loss with deep papillary excavation. With permission from
Ophthalmoscopic examination.
Optic nerve head or nerve fiber photography using a non-mydriatic fundus camera to determine the relation between the excavation and papillary margins in a vertical direction (C/D ratio). Together with papillary size measurements this is an important criterion in assessing optic nerve head images with suspected glaucoma. Interobserver reliability is high, at 0.84, as is consistency with the C/D ratio determined by Heidelberg retina tomography (13).
Confocal scanning laser tomography for three-dimensional measurements of the optic nerve head with quantitative determination of papillary size and rim margin.
Scanning laser polarimetry (GDx) and optical coherence tomography (OCT) measure the layer thickness and topological distribution of the neural fibres in the papillary region.
The retinal thickness analyzer (RTA) enables measuring of the entire retinal thickness.
Tonometry is used to measure the most important treatable risk factor-intraocular pressure. Goldmann applanation tonometry of the anesthetized cornea is the current gold standard in patients whose corneal thickness is normal. In non-contact tonometry, a short gust of air is blown on to the cornea. The corneal curvature provides information on intraocular pressure.
In sum, no investigative approach provides sufficient valid data by itself to perform glaucoma screening.
In the quantitatively best screening study, the sensitivity of the frequency doubling technique (FDT) was 92% and the specificity 93% (14). The sensitivity of laser supported imaging methods for the fundus – such as Heidelberg retina tomography, optical coherence tomography, and scanning laser polarimetry – depends on the stage of the glaucoma disease and the optic nerve head size. The more advanced the glaucoma, the easier it may be diagosed. The bigger the papilla the more likely it may be mistaken for macropapilla with physiological macroexcavation without glaucoma. The smaller the papilla the more likely it is for a small excavation to be assessed as normal, although the excavation is already due to glaucomatous loss of neural fibers. Heidelberg retina tomography, optical coherence tomography, and scanning laser polarimetry achieve an increasing sensitivity – where the specificity is fixed at 83% (true negative diagnosis) – depending on the glaucoma stage and papillary size, and thus a true positive diagnosis (table 3) (15).
Table 3. Sensitivities (%) of HRT/OCT/GDx, with specificity fixed at 83% (from [15]).
| Parameter | Papillary size (mm2) | Glaucoma stage, AGIS score | |||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | ||
| HRT-II-Moorfields classification | 1.0 | 47 | 62 | 74 | 84 |
| 2.0 | 58 | 71 | 82 | 89 | |
| 3.0 | 68 | 79 | 87 | 93 | |
| Stratus OCT- nerve fiber thickness | 1.0 | 88 | 93 | 96 | 98 |
| 2.0 | 73 | 83 | 90 | 94 | |
| 3.0 | 50 | 63 | 76 | 85 | |
| GDx VCC NFI | 1.0 | 86 | 92 | 95 | 97 |
| 2.0 | 81 | 88 | 93 | 96 | |
| 3.0 | 74 | 84 | 90 | 94 | |
HRT, Heidelberg retina tomography; OCT, optical coherence tomography; GDx VCC, scanning laser polarimetry; AGIS score: 0=glaucoma without visual field defects, 9=very advanced glaucoma with pronounced visual field defects.
Sequential diagnostics under screening conditions are advantageous (16). Using simple investigative methods including a family history, visual acuity measurement, and frequency doubling technique reduced the number of participants by 59.3% in the first step. If the findings were conspicuous, Heidelberg retina tomography was initiated subsequently. The combination of these approaches, which was optimized in respect of the threshold values, yielded a sensitivity of 96.8% and a specificity of 89.7%.
However, even though hi-tech investigative methods may be applied the final responsibility for the diagnosis or suspected diagnosis lies with the ophthalmologist.
Limitations
No study has thus far investigated Heidelberg retina tomography under screening conditions. The results of the studies that investigated several approaches showed unambiguously that sensitivity, specificity, and receiver operating characteristic (ROC) curves – a method used to optimize threshold values – were improved by combining investigative methods and using appropriate algorithms.
Cost effectiveness
A British modeling study from 1997 (17) calculated the cost of different screening pathways per correct diagnosis. The authors evaluated combinations of ophthalmoscopy, tonometry, and perimetry in primary care with a prevalence of undetected glaucoma of 0.6% in the population older than 40. This screening pathway for all persons – or persons with a high glaucoma risk (numbers in parentheses) – showed, with a sensitivity of 87% (80%), a cost effectiveness ratio of (converted) Euro 1500 (Euro 1300) for all direct medical costs per true positive diagnosis, with a specificity of at least 97%. The screening was most economical when performed in the context of eye examinations for other reasons, minimizing the ophthalmoscopy expense and indirect costs and involving non-medical personnel – for example, in non-contact tonometry and modern perimetry.
A Canadian study from 1995 compared the costs of different screening pathways – mainly the initial fundus examination with tonometry and, with perimetry used only in the second examination – and treatments with the years of blindness avoided (18). Relative to the population of Quebec (more than 7 million), 354 years of blindness (39.4 cases per year) in 40 to 79 year olds could be avoided, if participation and compliance are 75%, the treatment is 50% effective, and a 3 year screening interval is adhered to. The calculated cost effectiveness ratio is Euro 70 000 per year of blindness avoided. The benefits of the avoided visual impairment were, however, not investigated. Owing to the uncertain data situation and the high costs, the authors were opposed to glaucoma screening.
The joint federal committee (G-BA) has not approved glaucoma screening (19), for the following reasons:
Intraocular pressure, when measured in isolation, is merely one risk factor and is subject to substantial diurnal variation.
It is difficult to define a pathological stage that precedes the symptoms of disease.
It is currently not known which testing method – alone or in combination – is sufficiently precise.
It is not known from which age, and at what intervals, screening makes sense.
By contrast, the Global AIGS Committee on Screening for Open-angle Glaucoma (20) and the United States Preventive Services Task Force (UPSTF) (21, e1) found good evidence that early treatment of glaucoma patients with raised intraocular pressure identified through screening reduces visual field defects and slows glaucoma progression. Quality of vision or life was not studied, however, and no recommendations were made with regard to regular glaucoma screening. Its cost effectiveness can therefore be discussed only on the basis of general considerations.
Discussion
In glaucoma screening, as a minimum, the optic nerve head, visual function, medical history, and preliminary findings – if present-should be assessed. As a second step, in case of suspicious findings, the ophthalmologist should examine anterior and posterior ocular sections, intraocular pressure, corneal thickness, optic nerve head, thickness of neural fiber layer in the papillary region, and the static visual field, to specify the diagnosis and determine the prognosis. Glaucoma screening is therefore a link in a process chain in glaucoma treatment – consisting of providing information to the public about glaucoma disease, sequential screening, and ophthalmological examination in patients with suspected glaucomatous atrophy of the optic nerve.
One of the few population based screening studies of satisfactory quality, which also showed good results for sensitivity and specificity (16), used simple methods (family history, visual acuity testing, frequency doubling technique, and Heidelberg retina tomography) that are easily integrated into screening programs, as well as an ophthalmological examination and tonometry. The sequential process was the main and crucial feature in this study.
The screening pathway recommended in the guidelines (12) in the British modeling study showed lower sensitivity and negligibly higher costs per true positive diagnosis if additional perimetry was not performed in patients at risk. However, the medical evidence regarding screening investigations is scanty. Cost as an intermediary parameter per true positive glaucoma diagnosis is practical and should take into account not only blindness but also glaucoma related visual impairments, while assessing the treatment with regard to morbidity related result variables for the comparison of screening investigations. We do not know of any modeling studies for more recent investigative methods. The question of whether glaucoma screening is cost effective can thus not be answered on the basis of the published literature or transfer of study results to Germany. A new evaluation of glaucoma screening should take into account improvements in the early detection of glaucoma on the one hand, and the demographic trends in Germany on the other hand. Owing to the higher life expectancy, the prevalence may be expected to double by 2050, and many patients will therefore have to spend more years of their lives with significant visual impairments including blindness.
From an economic perspective, no unanimous recommendation for an individual method or a combination of screening methods for glaucoma is possible, because no evidence based recommendations exist for particular individual investigative methods. A sensible combination might include a functional, morphological, and non-invasive tonometry component. Establishing methods to investigate morphology – such as Heidelberg retinal tomography, optical coherence tomography, and scanning laser polarimetry into screening is a more complex undertaking because some early glaucoma signs may already be present even though nothing is found on standard examinations. Longitudinal studies are needed, which can show the delayed development of visual field defects according to preceding pathological-morphological changes and thus prove the value of the screening method. Future studies should also investigate the most effective combination and sequence of investigative methods with a high sensitivity and specificity. In glaucoma patients identified by screening, randomized controlled intervention studies should evaluate successful treatments with regard to end point parameters and patient management – for example, visual impairment.
For patients with positive screening results, therapeutic options exist for lowering intraocular pressure. The risk of glaucoma related blindness after 20 years can thus be reduced from 27% to 14% for one eye and from 9% to 4% for both eyes (e2). The following treatments are available:
Topical drug treatment with prostaglandin analogous, beta blockers, sympathomimetic drugs, alpha receptor agonists, carboanhydrase inhibitors (the latter may also be used systemically).
Laser treatment with laser trabeculoplasty or cyclophotocoagulation.
Surgical treatments using trabeculotomy or drainage devices (9).
Lowering intraocular pressure is the most important therapeutic approach, although it is not effective in all patients because of the multifactorial pathogenesis of glaucoma disease. Complementary neuroprotective treatments have therefore been developed (e3). Studies in macaque models have shown efficacy of memantine against neural fiber loss of the optic nerve (e4). Efficacy in humans has not been shown thus far. Memantine is an NMDA (N-methyl-D-aspartate) receptor antagonist, which is licensed for the treatment of Alzheimer’s disease.
An analysis of 26 studies (e11) concluded that early treatment of raised intraocular pressure can reduce the frequency of visual field defects. The largest of these studies is the multicenter, single blinded ocular hypertension study (OHTS), which includes more than 1600 patients (e12). By using preventive drug treatment with eye drops to lower intraocular pressure by an average of 22.5%, the stability of the visual field was maintained, independent of the substance class of the eye drops. The Cochrane review by Vass et al. (e11) assessed the evidence level of the OHT study as A, meaning "adequate." An intermediate analysis of the randomized Collaborative Initial Glaucoma Treatment Study (CIGTS) (e13, e14) compared medical treatment with eye drops and trabeculectomy – a glaucoma filtration procedure – to lower intraocular pressure in more than 600 patients with primary open-angle glaucoma. No difference was found for the two therapeutic options with regard to visual field loss after 5 years. The surgically treated group had a higher incidence of cataracts than the group receiving medication. The Cochrane review by Burr et al (e15) assessed the evidence level of the CIGT study as A.
Outlook
Telemedical computer assisted sequential diagnostics
To diagnose suspected glaucoma with sufficiently high specificity requires the simultaneous assessment of several independent glaucoma specific signs. A collaborative research project SFB 539-A4 based at Erlangen-Nürnberg University is using an interdisciplinary approach including the chair for pattern recognition and the institute for epidemiology and biometrics to develop computer assisted diagnostics systems for glaucoma screening.
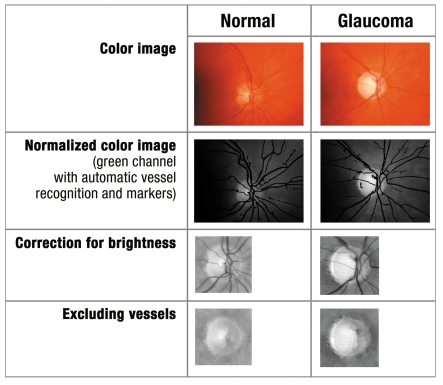
The networked combination of screening devices enables the sequential combination of automatic preclassification of all fundus images into "normal" or "requires further investigation" so as to reduce the time taken to evaluate images (figure 3). The papillary images – prepared using computer assisted techniques – that require further investigation are then telemedically evaluated by an ophthalmologist. The combination of devices initially documents the spatial and temporal contrast sensitivity, intraocular pressure, nerve fibers, and the optic nerve head. The images and data are saved on a server, processed on a computer, and automatically evaluated using several classification methods. Images whose initial findings are classified as abnormal are telemedically assessed by a specialized ophthalmologist, in a different location and at a later time. The patient can then view his or her report of findings including images electronically after verifying their legitimacy with a special username and password. The report serves as the starting point for deciding whether further ophthalmological diagnosis and treatment are needed.
Figure 3.
Image processing steps of healthy and glaucomatous papillary: - color image, normalized color image with automatic vascular recognition (only green channel), green channel image focusing on papilla, with vascular segmentation and green channel image focused on papilla excluding vessels
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Quigley A. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- 3.Wensor MD, McCarty CA, Stanislavsky YL, Livingston PM, Taylor HR. The prevalence of glaucoma in the Melbourne visual impairment project. Ophthalmology. 1998;105:733–739. doi: 10.1016/S0161-6420(98)94031-3. [DOI] [PubMed] [Google Scholar]
- 4.Statistisches Bundesamt. Bevölkerungsfortschreibung Fachserie 1 Reihe 1.3 - 2005. Wiesbaden; 2006. www-ec.destatis.de/csp/shop/sfg/bpm.html.cms.cBroker.cls?cmspath=struktur,vollanzeige.csp&ID=1019551. [Google Scholar]
- 5.Statistisches Bundesamt. Bevölkerung Deutschlands bis 2050-11. Koordinierte Bevölkerungsvorausberechnung. Wiesbaden; 2006. www-ec.destatis.de/csp/shop/sfg/bpm.html.cms.cBroker.cls?cmspath=struktur,vollanzeige.csp&ID=1020576. [Google Scholar]
- 6.BVA (Berufsverband der Augenärzte Deutschlands e.V.), DOG (Deutsche Ophthalmologische Gesellschaft e.V) Augenärzte informieren: Glaukom (grüner Star) www.augeninfo.de/patinfo/patinfo.php. [Google Scholar]
- 7.Quigley HA. Open-angle glaucoma. N Engl J Med. 1993;328:1097–1106. doi: 10.1056/NEJM199304153281507. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia. The Blue Mountains eye study. Ophthalmology. 1996;103:1661–9. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 9.Antony K, Genser D, Fröschl B. Erkennungsgüte und Kosteneffektivität von Screeningverfahren zur Erfassung von primären Offenwinkelglaukomen. HTA-Bericht. http://gripsdb.dimdi.de/de/hta/hta_berichte/hta144_bericht_de.pdf. [Google Scholar]
- 10.Knauer C, Pfeiffer N. Erblindung in Deutschland - heute und 2030 [Blindness in Germany - today and in 2030] Ophthalmologe. 2006;103:735–741. doi: 10.1007/s00347-006-1411-y. [DOI] [PubMed] [Google Scholar]
- 11.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BVA (Berufsverband der Augenärzte Deutschlands e.V.), DOG (Deutsche Ophthalmologische Gesellschaft e.V) Detektion des primären Offenwinkelglaukoms (POWG): Glaukom-Screening von Risikogruppen, Glaukomverdacht, Glaukomdiagnose, Leitlinie Nr. 15c. www.augeninfo.de/leit/leit15c.htm. [Google Scholar]
- 13.Lamoureux EL, Lo K, Ferraro JG, et al. The agreement between the Heidelberg retina tomograph and a digital nonmydriatic retinal camera in assessing area cup-to-disc ratio. Invest Ophthalmol Vis Sci. 2006;47:93–98. doi: 10.1167/iovs.05-0936. [DOI] [PubMed] [Google Scholar]
- 14.Yamada N, Chen PP, Mills RP, et al. Screening for glaucoma with frequency-doubling technology and Damato campimetry. Arch Ophthalmol. 1999;117:1479–1484. doi: 10.1001/archopht.117.11.1479. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros FA, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Influence of disease severity and optic disc size on the diagnostic performance of imaging instruments in glaucoma. Invest Ophthalmol Vis Sci. 2006;47:1008–1015. doi: 10.1167/iovs.05-1133. [DOI] [PubMed] [Google Scholar]
- 16.Robin TA, Muller A, Rait J, Keeffe JE, Taylor HR, Mukesh BN. Performance of community-based glaucoma screening using frequency doubling technology and Heidelberg retinal tomography. Ophthalmic Epidemiol. 2005;12:167–178. doi: 10.1080/09286580590969716. [DOI] [PubMed] [Google Scholar]
- 17.Tuck MW, Crick RP. The cost-effectiveness of various modes of screening for primary open angle glaucoma. Ophthalmic Epidemiol. 1997;4:3–17. doi: 10.3109/09286589709058056. [DOI] [PubMed] [Google Scholar]
- 18.Conseil d’évaluation des technologies de la santé du Québec (CETS) The screening of primary open-angle glaucoma. Montreal: CETS; 1995. xvi, 93. http://www.aetmis.gouv.qc.ca/site/download.php?f=d7375b87adf105a0600de66893aa64b7. [Google Scholar]
- 19.Gemeinsamer Bundesausschuss. Ein Glaukom-Screening ist nicht sinnvoll. www.g-ba.de/informationen/aktuell/pressemitteilungen/62/ [Google Scholar]
- 20.The AGIS Investigators. „The advanced glaucoma interventions study 7. The relationship between control of intraocular pressure and visual field deterioration“. Am J Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Preventive Services Task Force. Screening for glaucoma: recommendation statement. AHRQ publication No. 04-0548-A. Agency for Healthcare Research and Quality. www.ahrq.gov/clinic/uspstf05/glaucoma/glaucrs.htm. [Google Scholar]
- 22.Mardin CY, Horn FK, Jonas JB, Budde WM. Preperimetric glaucoma diagnosis by confocal scanning laser tomography of the optic disc. Br J Ophthalmol. 1999;83:299–304. doi: 10.1136/bjo.83.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harasymowycz PJ, Papamatheakis DG, Fansi AK, Gresset J, Lesk MR. Validity of screening for glaucomatous optic nerve damage using confocal scanning laser ophthalmoscopy (Heidelberg retina tomograph II) in high-risk populations: a pilot study. Ophthalmology. 2005;112:2164–2171. doi: 10.1016/j.ophtha.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Yamada N, Chen PP, Mills RP, et al. Glaucoma screening using the scanning laser polarimeter. J Glaucoma. 2000;9:254–261. doi: 10.1097/00061198-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Ivers RQ, Optom B, Macaskill P, Cumming RG, Mitchell P. Sensitivity and specificity of tests to detect eye disease in an older population. Ophthalmology. 2001;108:968–975. doi: 10.1016/s0161-6420(00)00649-7. [DOI] [PubMed] [Google Scholar]
- e1.U.S. Preventive Services Task Force. Screening for Glaucoma: Recommendation Statement. Ann Fam Med. 2005;3:171–172. doi: 10.1370/afm.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, Butterfield LC, Gray DT. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- e3.Weinreb RN. Glaucoma neuroprotection: What is it? Why is it needed? Can J Ophthalmol. 2007;42:396–398. [PubMed] [Google Scholar]
- e4.Hare WA, WoldeMussie E, Weinreb RN, Ton H, Ruiz G, Wijono M, Feldmann B, Zangwill L, Wheeler L. Efficacy and safety of memantine treatment for reduction of changes associated with experimental glaucoma in monkey, II: Structural measures. Invest Ophthalmol Vis Sci. 2004;45:2640–2651. doi: 10.1167/iovs.03-0567. [DOI] [PubMed] [Google Scholar]
- e5.Jonasson F, Damji KF, Arnarsson A, Sverrisson T, Wang L, Sasaki H, Sasaki K. Prevalence of open-angle glaucoma in Iceland: Reykjavik Eye Study. Eye. 2003;17:747–753. doi: 10.1038/sj.eye.6700374. [DOI] [PubMed] [Google Scholar]
- e6.Wolfs RC, Grobbee DE, Hofman A, de Jong PT. Risk of acute angle-closure glaucoma after diagnostic mydriasis in nonselected subjects: the Rotterdam Study. Invest Ophthalmol Vis Sci. 1997;38:2683–2687. [PubMed] [Google Scholar]
- e7.Goldbloom R, Battistra RN, Anderson G, Beaulieu MD, Elford RW, Feightner JW, Feldman W, Logan AG, Morrison B, Offord D, Patterson C, Spitzer WO, Wange E, Mickelson P, Dingle J, MacMillan H, Micleod R, Moutquin JM. Periodic health examination, 1995 update: 3. Screening for visual problems among elderly patients. CMAJ. 1995;152:1211–1222. [PMC free article] [PubMed] [Google Scholar]
- e8.Detry-Morel M, Zeyen T, Kestelyn P, Collignon J, Goethals M Belgian Glaucoma Society. Screening for glaucoma in a general population with the non-mydriatic fundus camera and the frequency doubling perimeter. Eur J Ophthalmol. 2004;14:387–393. doi: 10.1177/112067210401400505. [DOI] [PubMed] [Google Scholar]
- e9.De Mul M, De Bont AA, Reus NJ, Lemij HG, Berg M. Improving the quality of eye care with tele-ophthalmology: shared-care glaucoma screening. J Telemed Telecare. 2004;10:331–336. doi: 10.1258/1357633042602107. [DOI] [PubMed] [Google Scholar]
- e10.Tanito M, Itai N, Ohira A, Chihara E. Reduction of posterior pole retinal thickness in glaucoma detected using the retinal thickness analyzer. Ophthalmology. 2004;111:265–275. doi: 10.1016/j.ophtha.2003.05.023. [DOI] [PubMed] [Google Scholar]
- e11.Vass C, Hirn C, Sycha T, Findl O, Bauer P, Schmetterer L. Medical interventions for primary open angle glaucoma and ocular hypertension. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD003167.pub3. CD003167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK, 2nd, Wilson MR, Gordon MO. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829-30. [DOI] [PubMed] [Google Scholar]
- e13.Lichter PR, Musch DC, Gillespie BW, Guire KE, Janz NK, Wren PA, Mills RP CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–1953. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- e14.Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE, Mills RP CIGTS Study Group. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology. 2001;108:1954–1965. doi: 10.1016/s0161-6420(01)00874-0. [DOI] [PubMed] [Google Scholar]
- e15.Burr J, Azuara-Blanco A, Avenell A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2005;(2) doi: 10.1002/14651858.CD004399.pub2. CD004399. [DOI] [PubMed] [Google Scholar]