Abstract
Introduction
Gamma-hydroxybutyric acid (GHB, "liquid ecstasy") and its legal pro-drugs gamma-butyrolactone and 1,4-butanediol are gaining in importance as recreational drugs in Germany. The effects of these substances are comparable with those of alcohol or benzodiazepines. Because of the wide availability of GHB physicians are increasingly being confronted with cases of intoxication.
Methods
This review is based on a selective literature search as well as on the authors’ own experience and on information provided by the GIZ-Nord Poisons Centre, Göttingen, Germany.
Results
Consumption of a high dose of GHB or its prodrugs leads to severe intoxication with respiratory depression and coma. Only supportive therapy can be offered; no antidote is available.
Discussion
In any patient with impaired consciousness of unknown cause, the possibility of intoxication with GHB must be considered. Chemical detection of GHB in blood or urine is possible only using specific analytical methods and only within a short time frame (<12 h). Because of the short half-life of GHB, intoxications treated in intensive care units rarely show any complications. However, a number of fatalities have occurred. The potential abuse of GHB as a date rape drug must be borne in mind.
Keywords: liquid ecstasy, gamma-hydroxybutyric acid, drug abuse, designer drugs, diagnosis
Gamma-hydroxybutyric acid (GHB) is gaining in importance as an illegal drug in Germany (1). In spite of its popular name, "liquid ecstasy," the substance is neither chemically nor pharmacologically related to ecstasy substances such as methylenedioxyamphetamine or methylenedioxymethamphetamine (MDA or MDMA). Rather, its effects are comparable to those of alcohol or benzodiazepines. Because of its wide availability, clinicians are increasingly confronted with cases of GHB intoxication (1, 2). This article provides information about the substance and legal regulations.
History of the drug
As early as in the 1980s, GHB was offered in the United States in fitness clubs and health centers. Especially bodybuilders used GHB because of its renowned muscle building effects by increased release of growth hormones and its aphrodisiac effect (3, 4). After numerous cases of intoxication occurred over the following years, the US Food and Drug Administration (FDA) reclassified the drug as prescription-only in 1990 (5, 6). However, this did not put a stop to misuse of the drug. It gained in popularity; its users consumed "liquid ecstasy" or "soap" on the club scene as a party drug or at private events, as an alternative to, or in combination with, alcohol.
From the US the new drug spread to the United Kingdom, France, and Switzerland. Since the beginning of the 21st century, GHB has also been produced in Germany. According to a study by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), consumption of GHB or gamma-butyrolactone (GBL) is not as widespread as consumption of other illegal drugs. Data have shown that the prevalence of GHB use within the preceding month in young people in their leisure time rarely exceeds 3%. However, there are also signs of increased consumption in certain population subgroups, certain social environments, or geographical areas, and consumption in the private arena seems to be becoming more common (1). Depending on the study and target group surveyed, the lifetime prevalence was 3% to 19%.
In spite of the comparatively low popularity of GHB, cases of intoxication – some with a lethal result – have been increasing (6, 7, 8). Exact numbers of deaths in Germany or other countries are currently not available.
Biological effects
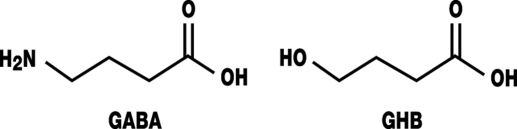
Gamma-hydroxybutyric acid is a derivative of the endogenous neurotransmitter gamma-aminobutyric acid (GABA) (figure). It can pass the blood-brain barrier. GHB was first synthesized in 1960 and licensed in Europe as an intravenous narcotic (9). Although it is still commercially available in Germany, it is rarely used, mainly because of:
Figure.
Structural formula of gamma-aminobutyric acid (GABA) and gamma-hydroxybutyric acid (GHB)
Insufficient analgesic effects that necessitate combination with an analgesic;
Narrow therapeutic spectrum and therefore greater occurrence of adverse effects, such as seizures or vomiting;
Controlling the dosage and effects is difficult;
Insufficiently calculable duration of effect (3).
Since studies have shown that GHB has a positive effect in patients with narcolepsy and cataplexy, GHB was licensed for this in the US in 2002 and in Europe in 2005. These therapies have many side effects (10, 11).
Dosage
GHB is administered intravenously for the purpose of sedation and orally for most other indications (table 1). Misuse is mostly done by oral administration in liquid form – neat or diluted in drinks. Occasionally, capsules are ingested, which often contain GHB as a sodium salt.
Table 1. Therapeutic use of GHB (adapted from [9, 11, 13]).
| Dosage | Effect |
|---|---|
| 1.7–3.5 g/day (p.o.) | Alleviates withdrawal symptoms in heroin addiction |
| 3.5 g/day (p.o.) | Alleviates withdrawal symptoms in alcohol addiction |
| 4.0–7.0 g/70 kg KG (IV) | Anesthesia |
| 4.5–9.0 g/day (p.o.) | Treatment of narcolepsy with cataplexy |
GHB, gamma-hydroxybutyric acid
GHB has a narrow therapeutic spectrum. The effects are dose dependent and range from a slight state of intoxication, similar to alcohol intoxication, to coma, cardiovascular arrest, and respiratory arrest (table 2) (4, 12, 13).
Table 2. GHB misuse (adapted from [13]).
| Individual dose | Effect |
|---|---|
| 1.0–2.0 g (p.o.) | Relaxation, anxiolysis, euphoria, sedation |
| 2.5–3.0 g (p.o.) | Nausea, vomiting, myoclonic seizures, bradycardia, amnesia |
| 3.0–4.0 g (p.o.) | Loss of consciousness |
| > 4.0 g | Respiratory depression, coma |
GHB, gamma-hydroxybutyric acid
Owing to the extremely fast metabolism of GHB, the desired or undesirable side effects last only one to four hours, depending on the dose. A typical patient is admitted with severely impaired consciousness and suddenly wakes up, usually after a very short time. As a rule GHB does not have longer term side effects, so the patients feel fit and want to leave the hospital – also thanks to the mnemic effect – to "return to the party." This phenomenon is known as the "fast-in, fast-out" effect (14).
Pharmacodynamics
In spite of numerous studies, the mechanism of action of GHB is still not known. According to the literature, several factors seem to influence the neurotransmitter balance. It was found shortly after introduction of the drug to the market that GHB is present as an endogenous substance in the brain. Here it is present as a breakdown product but also most probably as a precursor of GABA (15).
A possible mechanism of action that is under discussion is that in the context of the GABA metabolism, GHB functions as a neuromodulator in the brain. Further, studies have shown that GHB leads to a rise or fall in dopamine concentrations in the brain – depending on the dose – and influences the cholinergic system and the serotonergic system like neurosteroids and opioids.
It has been shown that in the millimolar concentration range – above endogenous concentrations – GHB activates the G-protein coupled GABAB receptors and, like GABA, inhibits the Ca2+ inflow and opens the potassium channels.
Further the possible presence of a G-protein coupled GHB receptor in the brain is under discussion. This is said to be stimulated by endogenous concentrations in the micromolar range. It is assumed that after exogenous administration, GHB acts as an agonist on the GABAB and the GHB receptors (14).
Pharmacokinetics
Resorption and distribution
After oral administration, GHB is absorbed very rapidly; maximum plasma concentrations are reached after 25 to 45 minutes. The distribution throughout the body is rapid and follows a two-compartment model (7). The distribution volume is smaller than that of ethanol, at 0.4 to 0.6 L/kg.
Biosynthesis, metabolism, and elimination
GHB is formed from GABA by means of succinsemialdehyde reductase via the intermediate product succinsemialdehyde.
Via the enzyme GHB dehydrogenase, GHB can be oxidated back into succinsemialdehyde and then reaches the citrate cycle, after a further oxidation step, mainly as succinate. Only a small proportion of GHB is excreted in an unchanged form through the kidneys (<2%).
The half-life of GHB is about 20 to 40 minutes, but there are indications that the elimination kinetics of GHB is not linear after administration of therapeutic doses (16).
Legal regulations
GHB was put under the narcotics law in the US since 2000 and is subject to the strictest categorization (schedule I).
Since 1 March 2002, GHB has been under the narcotics law in Germany too: Gamma-hydroxybutyric acid and its salts are listed in appendix III of the German Narcotics Act (BtMG). According to the law, possession of GHB or dealing GHB is a crime. As a ready-made preparation, GHB can be prescribed and traded.
Legal alternatives to GHB
Since GHB is subject to the Narcotics Act, legal substitutes are currently being consumed in Germany. These include gamma-butyrolactone (GBL), for example, or 1,4 butandiol (1,4-BD). These substances are metabolized into GHB after oral administration, or they are transformed by means of a simple chemical reaction into GHB by the dealers or users themselves. Instructions on how to synthesize these substances and even whole "building sets" are available from the internet in a "customer friendly" format.
Since the actual effective substance is GHB, and from a pharmacological perspective GBL and 1,4-BD are therefore pro-drugs, their consumption may result in the same effects, side effects, and symptoms of intoxication (17, 18). For this reason, the FDA warned the public as early as in 1999 not to consume GBL containing products and called for a voluntary stop to the trade of the products. The GHB substitutes have thus far not been included in the German Narcotics Act, owing to their widespread use as solvents and the associated consequences for the chemical industry. They can therefore be traded and consumed without penalty. However, a voluntary monitoring system of the German Federal Criminal Office has been in force since 2002. Since not all traders of the chemicals are participating, however, it is still easy for potential consumers to buy GBL or 1,4-BD.
Intoxication
Symptoms
The symptoms of gamma-hydrobutyric acid poisoning are partly similar to those of alcohol poisoning. Headaches, nausea, vomiting, vertigo, and speech impairment may be present. Higher concentrations may lead to bradycardia, seizures, respiratory depression to the point of respiratory arrest and impaired consciousness to the point of coma (table 2). In contrast to opioid intoxication, patients usually do not have miosis. A further diagnostic pointer may be non-responsiveness to ex juvantibus administration of naloxone or flumenazil.
Because of the rapid absorption and the rapid onset of effect, no precise data about the development of symptoms over time can be given. A typical characteristic is a rapid-onset and sudden clinical picture that then develops in a dose dependent fashion. Patients usually recover within six to eight hours after receiving symptom oriented treatment (19). In severe intoxication, myoclonus or seizures followed by loss of consciousness have often been described. It is important to bear in mind possible GBH intoxication in certain risk groups.
Number of cases of GHB poisoning
The number of cases of GHB intoxication is rising not only in Europe but also in Germany (1). For a long time, very few cases of GHB intoxication were seen in Hamburg, but since the end of 2006, severe cases of intoxication requiring intensive medical care have been seen regularly. This does not affect a particular clientele since GHB and its analogues can be acquired and consumed not only as prepared medications but also as chemicals. Most of the consumers are aged between 20 and 40 and have prior experience with other drugs (1).
According to current information from the GIZ-Nord Poisons Centre, Göttingen, the number of cases of intoxication notified to the center rose notably in 2007 (table 3).
Table 3. Number of cases of intoxication notified to GIZ-Nord Poisons Centre, Göttingen.
| Substance | 2006 | 2007 | 01/2008–03/2008 |
|---|---|---|---|
| Gamma-hydroxybutyric acid | 16 | 28 | 7 |
| Gamma-butyrolactone | 5 | 10 | 0 |
| 1,4-butandiol | 1 | 1 | 0 |
From the end of 2006 to April 2008, 18 clinically relevant cases of intoxication occurred in the investigated samples that incurred toxicological questions at the institute for forensic medicine at the University Hospital in Hamburg-Eppendorf, in which analysis confirmed ingestion of GHB or GBL. Further, within the area of the institute, three young women and one man died and GHB or GBL intoxication was confirmed. Since samples are sent to the forensic laboratory not in all cases of intoxication in the Hamburg region, a much larger number can be assumed.
Treatment
The treatment of GHB intoxication relies mainly on monitoring in the intensive care setting and stabilizing vital functions. Depending on the clinical picture, protective reflexes, frequency of breathing, and blood gas controls, the indication for endotracheal intubation and temporary ventilator treatment need to be assessed (19). Particular attention is required for the tendency to vomit even in patients with severely impaired consciousness and therefore a clearly increased risk of aspiration. Emergency physicians should be prepared to intubate. Symptomatic cardiac arrhythmias should initially be treated with atropine. If the patient does not respond, the indication for temporary pacemaker therapy should be assessed. In patients with seizures, treatment with benzodiazepines is recommended, while at the same time, attention has to be paid to the patient’s impaired consciousness (2). Administering active charcoal is mostly not beneficial because of the rapid resorption and metabolization, but this may be discussed in the context of a mixed indication.
Although its mechanism of action resembles that of benzodiazepines, the benzodiazepine antagonist flumazenil is equally ineffective as an antidote as the opioid antagonist naloxone (4). However, according to specialist information about GHB as an anesthetic, the symptoms of intoxication can be antagonized by giving physostigmine. However, studies have not shown conclusive results (20). Especially in patients with bradycardic arrhythmias, the risk is so overwhelming that administration of physostigmine is regarded as unadvisable (2).
Dependency
Initial studies after clinical administration of GHB in patients with narcolepsy have not shown any cases of misuse or of tolerance developing. More recent studies, however, indicate the potential for the drug being psychologically and physically addictive (21, 21). Withdrawal symptoms resemble those of alcohol withdrawal and occur about one to six hours after the most recent administration. Treatment is difficult. Benzodiazepines, barbiturates, or neuroleptics/antipsychotics have been among the drugs used (2, 22).
Abuse of GHB as a date rape drug
GHB and its pro-drugs are colorless liquids, transparent and almost entirely flavorless. They can thus be mixed with drinks and administered to unsuspecting victims without their noticing. The victim’s defenselessness is exploited to commit sexual attacks or robberies.
Especially in combination with alcohol, GHB can result in impaired consciousness including coma with anterograde amnesia. Studies have shown that GHB is one of the substances most often used for this purpose in the US (6, 23). Similar cases have also been reported from Germany and other European countries (24).
Laboratory tests/analysis
GHB can be confirmed in human blood or urine specimens or in remnants of drinks via tests such as gas chromatography combined with mass spectrometry (25).
Thus far, no immunological rapid test for GHB has been developed, which constitutes a problem. Rapid diagnosis in hospital or less well equipped laboratories is therefore impossible. If a patient is admitted with suspected GHB intoxication, the specimen can be sent to a specialist laboratory. The substance can then usually be confirmed within two to three hours. Information about laboratories with the required spectrum of analytic tests, which also provide emergency services, can be obtained from the Poisons Centres.
Because of the extremely short half-life of GHB in the body, the currently assumed maximum time span during which GHB can be confirmed in blood samples is five to eight hours and in urine samples, about 12 hours (23). It is therefore important that the specimens – serum or urine – are obtained as early as possible. This should be borne in mind especially in cases of possibly concealed administration ("Someone put something into my beer"), because after a longer time period has passed, GHB – by contrast to other substances – cannot be confirmed any longer.
In a forensic scenario – for example, a suspected involuntary ingestion of centrally effective substances – the specimen should be frozen as early on as possible because an increase in GHB concentrations – owing to higher temperatures and subsequent bacterial activity – cannot be excluded. If an acute intoxication is suspected, it is sufficient to keep specimens in the fridge, at temperatures of 2 to 8 degrees Celsius.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Thematic Papers - GHB and its precursor GBL: an emerging trend case study. 2008 ( http://www.emcdda.europa.eu/html.cfm/index7079EN.html) [Google Scholar]
- 2.Reeves J, Duda R. GHB/GBL intoxication and withdrawal: a review and case presentation. Addictive Disorders & Their Treatment. 2003;2:25–28. [Google Scholar]
- 3.Couper FJ, Logan BK. Determination of gamma-hydroxybutyrate (GHB) in biological specimens by gas chromatography-mass spectrometry. J Anal Toxicol. 2000;24:1–7. doi: 10.1093/jat/24.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Templeton A, Vonesch H-J. Intoxikation mit GHB („liquid ecstasy“) Schweiz Med Forum. 2005;5:115–116. [Google Scholar]
- 5.Nightingale S. From the Food and Drug Administration (warning about GHB) J Am Med Assoc. 1991;265:1802. [PubMed] [Google Scholar]
- 6.ElSohly MA, Salamone SJ. Prevalence of drug used in cases of alleged sexual assault. J Anal Toxicol. 1999;23:141–146. doi: 10.1093/jat/23.3.141. [DOI] [PubMed] [Google Scholar]
- 7.Brenneisen R, ElSohly MA, Murphy TP, Passarelli J, Russmann S, Sala-mone SJ, Watson DE. Pharmacokinetics and excretion of gamma-hydroxybutyrate (GHB) in healthy subjects. J Anal Toxicol. 2004;28:625–630. doi: 10.1093/jat/28.8.625. [DOI] [PubMed] [Google Scholar]
- 8.Mazarr-Proo S, Kerrigan S. Distribution of GHB in tissues and fluids following a fatal overdose. J Anal Toxicol. 2005;29:398–400. doi: 10.1093/jat/29.5.398. [DOI] [PubMed] [Google Scholar]
- 9.Fachinformation. Somsanit(R) Injektionslösung. Köhler Chemie GmbH. 2007 Mai [Google Scholar]
- 10.Fuller D, Hornfeldt C. From club drug to orphan drug: sodium oxybate (Xyrem) for the treatment of cataplexy. Pharmacotherapy. 2003;23:1205–1209. doi: 10.1592/phco.23.10.1205.32756. [DOI] [PubMed] [Google Scholar]
- 11.Fachinformation. Xyrem(R) 500mg/ml Lösung zum Einnehmen. UCB Pharma Ltd. 2005 Dez [Google Scholar]
- 12.Bernasconi R, Mathivet P, Bischoff S, Marescaux C. Gamma-hydroxybutyric acid: an endogenous neuromodulator with abuse potential? TiPS. 1999;20:135–141. doi: 10.1016/s0165-6147(99)01341-3. [DOI] [PubMed] [Google Scholar]
- 13.Stein M. Stellungnahme zur nicht geringen Menge von Gamma-Hydroxybuttersäure. Toxichem und Krimtech. 2003;70:87–92. [Google Scholar]
- 14.Drasbek K, Jensen JC. K. Gamma-hydroxybutyrate - a drug of abuse. Acta Neurol Scand. 2006;114:145–156. doi: 10.1111/j.1600-0404.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 15.Maitre M. The gamma-hydroxybutyrate signalling system In Brain: Organization and functional implications. Progr Neurobiol. 1997;51:337–361. doi: 10.1016/s0301-0082(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 16.Palatini P, Tedeschi L, Frison G. Dose-dependent absorption and elimination of gamma-hydroxybutyric acid in healthy volunteers. European J Clin Pharmacol. 1993;45:353–356. doi: 10.1007/BF00265954. [DOI] [PubMed] [Google Scholar]
- 17.Marinetti LJ, Isenschmid DS, Hepler BR, Kanluen S. Analysis of GHB and 4-methyl-GHB in postmortem matrices after long-term storage. J Anal Toxicol. 2005;29:41–47. doi: 10.1093/jat/29.1.41. [DOI] [PubMed] [Google Scholar]
- 18.Duer WC, Byers KL, Martin JV. Application of a convenient extraction procedure to analyze gamma-hydroxybutyric acid in fatalities involving gamma-hydroxybutyric acid, gamma-butyrolactone, and 1,4-butanediol. J Anal Toxicol. 2001;25:576–582. doi: 10.1093/jat/25.7.576. [DOI] [PubMed] [Google Scholar]
- 19.Mühlendahl vKE, Oberdisse U, Bunjes R, Brockstedt M. Vergiftungen im Kindesalter. 4 Auflage. Stuttgart: Thieme Verlag; 2003. [Google Scholar]
- 20.Traub S, Nelson L, Hoffman R. Physostigmine as a treatment for gamma-hydroxybutyrate toxicity: a review. J Clin Toxicol. 2002;40:781–787. doi: 10.1081/clt-120015839. [DOI] [PubMed] [Google Scholar]
- 21.Galloway G, Frederick S, Staggers F, Gonzoles M, Stalcup S, Smith D. Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction. 1997;92:89–96. [PubMed] [Google Scholar]
- 22.Dyer JE, Roth B, Hyma B. Gamma-hydroxybutyrate withdrawal syndrome. Ann Emerg Med. 2001;37:147–153. doi: 10.1067/mem.2001.112985. [DOI] [PubMed] [Google Scholar]
- 23.LeBeau MA, Andollo W, Hearn W, et al. Recommendation for toxicological investigations of drug-facilitated sexual assaults. J Forensic Sci. 1999;44:227–230. [PubMed] [Google Scholar]
- 24.European Monitoring Centre for Drugs and Drug Addiction. EMCDDA Technicla Datsheets - Sexual assaults facilitated by drugs or alcohol. 2008 http://www.emcdda.europa.eu/html.cfm/index50537EN.html. [Google Scholar]
- 25.Crookes CE, Faulds MC, Forrest ARW, Galloway JH. A reference range for endogenous gamma-hydroxybutyrate in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2004;28:644–649. doi: 10.1093/jat/28.8.644. [DOI] [PubMed] [Google Scholar]