Abstract
Introduction
Peripartum hemorrhage is one of the leading causes of maternal death worldwide (25%).
Methods
Selective literature review, including international guidelines, for assessment of the causes and optimal management of this condition.
Results
The major causes of hemorrhage are uterine atony, placenta previa, and abruptio placentae. The diagnosis of hemorrhage is suspected from its clinical manifestations and confirmed by ultrasonography. In placenta previa, the placenta is implanted in the lower uterine segment and may cover the internal cervical os. Placenta previa is more common in older and multiparous mothers, as well as in mothers who have previously undergone a cesarean section. Placental abruption is defined as separation of the placenta from the uterine wall before delivery of the infant. The risk factors for this condition include preeclampsia, advanced maternal age, and trauma. When it presents with manifestations of acute blood loss, premature abruption placentae must be diagnosed rapidly and treated without delay to save the life of the mother and child. A rare, but highly lethal, cause of bleeding is amniotic fluid embolism, which manifests itself with sudden and unexplained peripartum respiratory distress and cardiovascular collapse. Amniotic fluid embolism is associated with high fetal and maternal mortality (20% and 60% to 80%, respectively) even when it is optimally treated.
Discussion
Peripartum hemorrhage is an important source of maternal and fetal morbidity and mortality. The prognosis for both mother and child can be markedly improved if the risk factors for hemorrhage are recognized and the problem is treated rapidly and appropriately when it arises.
Keywords: third-trimester hemorrhage, placenta previa, maternal mortality, preterm abruption of placenta, amniotic fluid embolism, uterine atony
After thromboembolism, hemorrhage in late pregnancy is the second commonest cause of maternal death worldwide (1, 2).
For patients who have previously undergone a cesarean section and who have had placenta increta in a subsequent pregnancy, hemorrhage after another cesarean delivery is the most dangerous complication.
About 140 000 women die annually worldwide from a complication of postpartum hemorrhage (5). In Europe, one to two hemorrhage-related maternal mortalities are to be expected per 100 000 live births.
The learning goals of this article are to
become aware of the importance of quickly identifying the risk group and providing effective treatment for peripartum hemorrhage, because the prognosis of hemorrhagic complications can be improved if the patients are treated rapidly at a center qualified to manage this disorder;
internalize the main therapeutic steps for the acute case, since severe hemorrhage (>1500 mL) can also occur in a normal population.
The therapeutic options available for hemorrhage of late pregnancy were analyzed on the basis of a selective literature review. With the aid of "PubMed," the authors searched literature from the period 1980 to 2008 using the search terms "postpartum hemorrhage," "postpartum maternal mortality," "amniotic fluid embolism," and "placenta previa."
Mortality.
About 140 000 women die annually worldwide from postpartum bleeding resulting from a complication.
The majority of the studies quoted in this article comprise retrospective analyses, guidelines, case reports, and review articles (4, 5, 6). Randomized studies or extensive meta-analyses on this topic are not currently available.
Etiology and pathogenesis of peripartum hemorrhage
Principally four mechanisms are capable of inducing peripartum hemorrhage (e1, e2):
The main cause is uterine atony (at up to 75%)
Placental disorders such as placenta previa and problems of placental detachment
Traumatic birth injuries such as cervical lacerations, vaginal lacerations, or uterine rupture
Coagulation disorders resulting from amniotic fluid embolism and consumption-dilution coagulopathy.
Blood loss of >1500 mL may be expected to lead rapidly to hemorrhagic shock. The deficient perfusion of all organs may cause the shock process to progress swiftly into multiorgan failure with an unfavorable prognosis. The organs affected are the kidneys, central nervous system, heart, lungs, and liver. The inadequate perfusion of the placenta associated with severe postpartum hemorrhage additionally represents a risk for the fetus. Especially premature placental abruption represents a high risk of intrauterine fetal demise (about 10%).
Main causes of peripartum hemorrhage.
Uterine atony
Placental disorders
Birth trauma injuries
Coagulation disorders
Within a short time, an irreversibly lethal situation can develop which may be the result of inadequate diagnosis and/or a delay in starting therapy. This may have the following causes:
Transfusions of red blood cell concentrates given too late
Failure to adequately replace coagulation factors
Inadequate surgical management (e3).
Diagnosis
The documentation of risk factors such as family history of hemorrhage, increased personal bleeding tendency, and medication history is diagnostically important. Questions relating to the duration and severity of bleeding are decisive for the primary assessment of blood loss that has already occurred. The number of sanitary napkins used by the patient and the passage of coagulum may be important indicators.
An immediate vaginal speculum examination and determination of the blood loss by measuring in milliliters are necessary to assess the current intensity of bleeding. An ultrasound screen to evaluate the following aspects may also contribute to the diagnosis:
Fetal wellbeing
Placenta localization
Hematoma formation
Free fluid in the abdomen.
Basic diagnosis.
Primary assessment of previous blood loss
Determination of current blood loss by speculum examination
Ultrasound examination as further measure
Intraoperatively, blood loss can usually be accurately assessed from the contents of the aspiration device. The use of measuring cups and weighing of swabs and compresses and blood clots are indispensable for the measurement of blood loss after vaginal delivery. The coagulum weight is multiplied by a factor of three to obtain the approximate blood loss in milliliters. Immediately postpartum, the evaluation of uterine contractility by manual palpation is the first and decisive procedure.
Assessment of the cardiovascular parameters is important not only for diagnosis but also for treatment supervision. This includes monitoring of blood pressure and heart rate, if appropriate the measurement of arterial blood pressure and assessment of peripheral oxygen saturation, as well as the measurement of central venous pressure through a central venous catheter.
Clinical signs of impending shock such as pallor, cold sweats, cold extremities, restlessness, heart rate >100 bpm, and blood pressure <100/70 mmHg are important parameters especially for evaluating the severity of bleeding that is not externally visible. Urine production is monitored through an indwelling catheter. The minimum output must be more than 60 mL/h.
Laboratory diagnostic tests should be performed promptly. The start of treatment must not, however, be delayed by waiting for the laboratory results. Rather, treatment should be modified in the light of blood results as and when they become available.
Routine laboratory diagnostic parameters include hemoglobin and platelet levels, fibrinogen, partial thromboplastin time (PTT), and INR (International Normalized Ratio) values. Additional rapid point-of-care testing for coagulation diagnosis is possible by means of thromboelastography (7).
A simple coagulation test suitable for use at any time is the clot observation test. This involves carefully filling about 5 mL of whole blood into an uncoated glass tube and inverting it every 30 seconds. A clot should form after about 8 to 10 min. At low fibrinogen concentrations clot formation is delayed or fails to occur. Clot dissolution within 30 to 60 min indicates the presence of hyperfibrinolysis (e4) (table 1).
Table 1. Differential diagnoses of bleeding in late pregnancy*1.
| Placenta previa | Premature placental detachment | Antepartum hemorrhage (normal slight bleeding at start of delivery) | |
| Bleeding | profuse, bright to outside | slight, dark to outside, profuse towards inside | slight |
| Pain | none | continuous pain | contraction dependent |
| Labor pains | soft, no labor pains | woody hard uterus with permanent uterine tone | regular uterine activity |
| Cervix | unripe | unripe | sufficiently ripe |
| Circulation | stable | unstable, shock | stable |
| Coagulation | normal | disturbed | normal |
| Ultrasound | placenta in front of cervical os | retroplacental clot | normal ultrasound findings |
| *1 modified from (25) |
Non-pregnancy associated causes of hemorrhage include bleeding from the external cervical os (ectopic bleeding, frequent after coitus) or bleeding associated with cervical cancer. In most cases these require no acute action.
General therapeutic interventions
Invisible bleeding.
Clinical signs of shock such as pallor, cold sweats, cold extremities, restlessness, HR >100 bpm, BP <100/70 mmHg are important parameters for assessing severity of blood loss, especially for not outwardly visible bleeding.
The primary goal is the early treatment of the cause of bleeding. Placement of wide-bore venous access and, if necessary, central venous catheters creates the essential conditions for effective treatment.
Adequate volume therapy, for example with hydroxyethyl starch or Ringer’s solution, can prevent cardiovascular decompensation (e5).
Young, healthy women may compensate volume deficiency for long periods, which may lead to an incorrect estimation of the actual blood loss. Besides maintaining vital signs, timely supply of blood products for effective coagulation therapy is indispensable.
There is no agreed target haemoglobin level. With severe bleeding, however, it should be attempted to achieve a target Hb of >8 g/dL because of rheological factors, as at normal Hb values the platelets are pushed aside towards the vessel surface by the red cells (e6) and are therefore already at the site of action if lesions develop. In anemia, the platelets migrate into the middle of the vessel and are not present at the vascular wall. In emergencies, patients are given uncrossed O-negative packed cells. It should be remembered that the blood group is usually known since it is entered in the patient’s maternity record card. If there is no time pressure, compatible crossed RBC transfusions are given.
Therapeutic measures.
Adequate volume therapy is required to prevent cardiovascular decompensation.
The target fibrinogen value for heavy bleeding should be >150 mg/dL (7, e7). The fibrinogen value can be increased most rapidly by administering 4 to 6 g fibrinogen concentrate. This is equivalent to giving 8 to 12 volumes of fresh frozen plasma (FFP). It should be remembered that FFP still has to be thawed. If the bleeding persists, the additional administration of cryoprecipitates is recommended (prothrombin complex concentrates [PPSB]; 25 IU/kg body weight). Because of their special method of manufacture, precipitates are particularly rich in fibrinogen, fibronectin, and blood coagulation factors. Platelet replacement is indicated at a platelet count <50 000/µL. If hyperfibrinolysis is proven by thromboelastogram (TEG) analysis or the clot observation test, antifibrinolytic agents should be administered, and in doubtful cases treatment should be initiated on suspicion. Based on existing literature data, however, the value of antifibrinolytic therapy cannot yet be conclusively assessed. The agent of choice is tranexamic acid in a dosage of 1 mg/kg body weight/h (e8).
If all therapeutic options have been exhausted, the use of recombinant factor VIIa (rFVIIa) in a dosage of 90 µg/kg body weight as a loading dose is indicated. However, this will only be effective if certain basic preconditions are present (normal temperature, normal acidity, normal fibrinogen and platelet levels) (8, e9).
Stabilization of blood circulation.
The target fibrinogen value in severe bleeding should be >150 mg/dL. The fibrinogen value can be rapidly increased by administering 4 to 6 g fibrinogen concentrate.
The surgical techniques are described for the specific clinical entities.
Specific clinical entitiesUterine atony
The main cause of postpartum bleeding is uterine atony (75% of cases). This occurs in 2% to 8% of all deliveries (12). Lacking compression of the placental bed can lead to severe, persistent bleeding (e14). About 80% of women with postpartum hemorrhage have risk factors. Overdistension of the uterine muscles by multiple pregnancies, fetal macrosomia and polyhydramnion as well as a protracted delivery play a predominant role.
Symptoms and clinical course
Typical symptoms are continuous or intermittent, gushing hemorrhages starting immediately postpartum. The extent of postpartum blood loss is usually underestimated by 30% to 50% (13). Maternal cardiovascular depression (hypotension, nausea, impaired consciousness) may be the first indicator of major postpartum blood loss.
Diagnosis
The blood present in the uterine cavity is expressed through the abdominal wall by manual compression of the uterus. This allows an assessment both of the blood loss that has already occurred and of the uterine tone. Typical of atony is a soft, boggy consistency of the uterus with a high fundus. Physiologically, uterine tone is hard immediately after the birth of the placenta and the fundus is just above and slightly beside the navel. Severe bleeding resulting from birth trauma can be ruled out by a vaginal speculum examination. Additional sonographic screening makes it possible to rapidly exclude large placenta residues and demonstrate hematoma formations in other cavities such as the abdomen and retroperitoneum as well as paravaginal hematomas.
Treatment
Uterine atony.
The main cause of postpartum bleeding is uterine atony, which accounts for 75% of all postpartum hemorrhages.
Active management of the third stage of labor can reduce blood loss. This involves the intravenous administration of 3 to 6 IU oxytocin during the first few minutes after passage of the infant’s shoulders and rapid tying and slight traction of the umbilical cord (box 1). In most cases the need for hysterectomy can be avoided by surgical treatment with uterine compression sutures. Reducing the size of the placental adhesion surface and the uterine contraction usually produce adequate hemostasis. The "B-Lynch suture“ method first described in 1997 is now used successfully in various modifications (18–22). If the uterine arteries are also ligated, the uterine blood circulation can be reduced.
If these measures are insufficient, supracervical hysterectomy is necessary. Ligation of the internal iliac artery should only be performed by experienced surgeons (e19).
In embolization of the uterine artery, arterial catheter embolization of the uterine arteries can be successfully used in patients with stable cardiovascular and coagulation conditions and provided that the appropriate logistics are in place. This method is also to be used as the last resort after hysterectomy with persistent bleeding (11, e20).
Placenta previa
This is a common placental insertion disorder (1:200 pregnancies) in which the internal cervical os is partially or completely covered with placental tissue. The risk of perioperative bleeding is additionally influenced to a great extent by placenta implantation disorders. The differential diagnosis of placental implantation disorders is decisive for further therapy. The following conditions are distinguished:
Placenta accreta: the placental villi extend into the decidua basalis
Placenta increta: the placental villi extend into the myometrium
Placenta percreta: the placental tissue invades the adjacent organs.
The main risk factors for placental implantation disorders are curettages and previous cesarian sections.
Symptoms and clinical course
The main symptom is severe, painless, bright red bleeding which occurs completely unexpectedly, usually in a uterus without birth contractions (e10). The first hemorrhage usually ceases without intervention. If the hemorrhage recurs, the intensity of bleeding increases. The final diagnosis is made sonographically (abdominal and transvaginal) (table 2).
Table 2. Sonography in placenta previa.
| Transabdominal sonography, diagnosis possible in 90% | Transvaginal sonography |
| Fetal condition? | Invasion of cervix? Length of cervix |
| Estimated fetal weight based on head and abdomen measurement | Invasion of myometrium? Bladder? |
| Localization of placenta | |
| Invasion of cervix? Myometrium? Bladder? |
Diagnosis
Conservative management of uterine atony.
Uterine tamponade and manual uterine compression are the mechanical treatment options for uterine atony.
Misoprostol, sulprostone, methylergotamine and oxytocin are available for pharmacological therapy.
Specific clinical entities.
Placental implantation disorders may frequently cause hemorrhage during pregnancy. The main risk factors for implantation disorders are curettages and previous cesarian sections.
Whether an invasion of the placenta into the cervix is present can be assessed in 90% of cases by transabdominal sonography. In suboptimal examination conditions such as a posterior wall placenta, which is difficult to inspect because it is covered by the head of the fetus, the diagnostic procedure should be continued with vaginal sonography. For known placenta previa with suspected presence of placenta accreta/increta/percreta, care at a center equipped with the necessary logistic resources is required. This center should have rapid access to stored blood, a round-the-clock standby team for cesearian section and an intensive care unit for adults and newborns.
Sonographic signs of a placental implantation disorder are:
Absence of the retroplacental hypoechoic zone
Lacunar appearance of the placenta close to the myometrium with transition to the muscles ("Swiss cheese holes")
Color coded sonography shows intensive and turbulent blood flow from the placenta into the myometrium.
Suspected placenta percreta with bladder invasion can be confirmed by cystoscopy. Speculum insertion allows a more accurate assessment of the intensity of bleeding but is without relevance for the diagnosis of placenta previa.
Treatment: active approach
For life-threatening hemorrhage with hemodynamic decompensation of the pregnant woman (volume deficiency shock), prompt cesarian delivery is indicated because of the risks for the mother. If there are also signs of fetal impairment (abnormal cardiotocogram [CTG]), immediate cesarian section is indicated regardless of the severity of bleeding. If hemorrhage occurs after 34/0 weeks of gestation, prolongation of pregnancy is no longer indicated even if the bleeding is slight.
Treatment: temporizing approach
Treatment for placental implantation disorder.
Continuation of pregnancy under inpatient monitoring is justifiable if bleeding is minor and fetal well-being is demonstrable.
If bleeding is slight and fetal wellbeing is established, continuation of the pregnancy under hospital conditions is justified. If placenta accreta or placenta increta is suspected, the patient should be transferred to a maximum medical care center. Between 24 + 0 and 34 + 0 weeks of gestation, fetal lung maturation is additionally induced by administering 2 x 12 mg intramuscular betamethasone at a 24 hour interval. Until induction of lung maturation is complete, inhibition of uterine contractility with calcium channel blockers, beta-sympathomimetics or oxytocin antagonists is additionally necessary. Rh negative pregnant women receive 300 µg anti-D immunoglobulin as prophylaxis. Maternal cardiovascular functions are maintained by infusion therapy and blood replacement from Hb <10 g/dL (hematocrit <30%), the transfusion rate depending on the intensity of the bleeding. Discharge to ongoing outpatient care may be justified depending on the local circumstances. This is only permissible, however, if the obstetric clinic can be reached within a reasonable period of 15 minutes. Patients with complete central placenta previa or placental villi invasion of the myometrium are to be exempted from this rule. The patient should be informed of the risk of bleeding associated with vaginal intercourse.
Surgical approach
The surgical approach adopted depends to a very great extent on the sonographic findings. The uterine incision line is determined sonographically. As far as possible, the fetus should not be extracted transplacentally and the opening of uteroplacental vessels should be avoided. No relevant randomized studies have been performed, but it is clinically helpful if severe bleeding does not occur before or during fetal extraction. After extraction of the fetus and planned removal of the placenta, active placental detachment is attempted with intravenous administration of the contraction-inducing agent oxytocin. If there is already a sonographic suspicion of a placental abruption, such as placenta accreta or placenta increta, then the various alternatives are to be discussed with the patient preoperatively. If no further children are wished, hysterectomy without a prior attempt to detach the placenta is to be preferred since this procedure involves fewer risks for cardiovascular stability.
If there is a wish to have more children, it can be attempted to leave the placenta in situ. The patient should then be informed about the increased risk of bleeding and infection during the puerperium. Secondary abruption of the placenta usually takes place many weeks after childbirth, when a suitable check on the inflammatory parameters is indicated. If the placenta is incompletely removed and there is no heavy bleeding it can be attempted to leave the placental remnants in situ as a primary measure and then remove them in a secondary step (surgically or spontaneously) (9, e11). If the attempt at detachment fails and bleeding persists, timely surgical revision is indicated.
Uterine devascularization
A 95% success rate has been described for stepwise uterine devascularization (10). The internal uterine artery is ligated about 2.5 cm beyond the bifurcation. The vessel is double ligated and not severed. This is regarded as the method of last resort because of the risk of venous injury (e12).
Imaging procedures.
Abdominal and transvaginal sonography are helpful for peripartum bleeding and can detect specific pathological conditions such as placental insertion disorders.
With stable cardiovascular conditions and the possibility of emergency intervention radiology, bilateral embolization of the uterine arteries is a possible option (11).
The anesthetic technique is to be selected in consultation with the anesthetist. In most cases the intervention can be performed under spinal anesthesia. If increased intraoperative bleeding is probable due to placenta accreta with hysterectomy, intubation anesthesia is to be preferred (e13).
Premature placenta detachment
About 1% of all pregnant women may be expected to suffer premature placental detachment, in which the normally positioned placenta detaches partially or completely from the surface of the uterus before delivery. With considerable premature detachment, half of the fetuses show signs of deficient oxygen supply evidenced by an abnormal cardiotocogram. 15% of the fetuses with signs of deficient oxygen supply have already died intrauterinely. This represents a fetal mortality of 0.75 per thousand with reference to all pregnancies. The causes of premature placental detachment are multifactorial (box 2).
Symptoms and clinical course
The symptoms correspond to the degree of severity of detachment. A typical sign of pronounced premature placental detachment is a painful uterus with permanent uterine contraction (woody hard uterus). Vaginal bleeding need not necessarily be present. Massive hemorrhage with retroplacental hematoma formation may also be present without vaginal bleeding. The blood loss is usually underestimated since the inward bleeding is not usually clinically detectable.
The release of large amounts of tissue thromboplastin introduces a high risk of developing a disseminated intravascular coagulation process. The probability of intrauterine fetal demise depends on the degree of placental detachment.
Diagnosis
In addition to the clinical symptoms, sonography is of great diagnostic importance. Premature detachment of the placenta leading to fetal impairment can in most cases also be reliably diagnosed sonographically. The expected hypoechoic space-occupying lesion is usually absent in the ultrasound scan, with the hematoma presenting as a partly echodense, partly hypoechoic structure which may be confused with the placenta on superficial inspection. The imaging procedure should therefore be performed by an experienced examiner.
Hysterectomy versus wish for children.
If there is a wish to have more children despite a placental attachment disorder, it may be attempted to leave the placenta in situ.
In addition to assessing the fetal condition, the maternal vital signs (respiration, blood pressure, heart rate) are to be recorded. Rapid laboratory diagnosis (hemoglobin assay) allows an objective estimation of the blood loss that has already occurred. A decrease in platelets and fibrin with an increase in fibrin cleavage products and a prolonged partial thromboplastin time (PTT) indicate consumption or loss of coagulation factors due to blood loss and hematoma formation.
Treatment: active approach
With an extensive form of placental detachment with abnormal CTG, cesarian section must be performed immediately. With intrauterine fetal demise, spontaneous delivery is attempted subject to sufficient cervical maturity. If examination reveals cervical immaturity and massive hematoma, cesearian section is also performed for a dead fetus.
Treatment: temporizing approach
If the fetal findings are normal (biometry, CTG findings) and the pregnant woman’s cardiovascular situation is stable without severe abdominal pain, a conservative approach is justified for extreme prematurity. Up to 34 + 0 weeks of gestation, fetal lung maturity should be induced with betamethasone (2 x 12 mg at 24 h interval).
Anaphylactoid syndrome of pregnancy
Premature placental detachment.
Premature placental detachment is to be expected in about one percent of all pregnant women. The symptom is permanent uterine contraction (woody hard uterus).
With peripartum hemorrhage, the possibility of anaphylactoid syndrome of pregnancy (previously known as amniotic fluid embolism) should also be considered. The bleeding is caused by a coagulation disorder which is induced by coagulation activation of amniotic fluid constituents. Amniotic fluid constituents enter the maternal circulation through endocervical veins, the placenta insertion site, or uterine or vaginal traumas. Anaphylactoid syndrome of pregnancy is rare (1: 80 000 deliveries). A high maternal mortality of 60% to 80% is to be expected even when treatment is optimal (e23). Only 15% of survivors have no neurological complications (25).
Symptoms and clinical course
Anaphylactoid syndrome of pregnancy (Amniotic fluid embolism).
The incidence is 1 : 80 000 deliveries
The clinical symptoms resemble those of anaphylactic or septic shock (e24, e25). Acute cardiovascular decompensation (and possibly cardiac arrest) usually occurs rapidly, with dyspnea, cyanosis (possibly with respiratory arrest), and acute hypoxia. Tonic-clonic seizures may also develop. Additionally, this is followed by a disseminated intravascular coagulation disorder with massive hemorrhage.
Diagnosis
The clinical entity is always determined by diagnosis through exclusion. Clinical conditions exhibiting a similarly dramatic course must be ruled out, such as pulmonary embolism, aspiration pneumonia, myocardial infarction, eclampsia, intracerebral hemorrhage, medication induced anaphylaxis and post-hemorrhagic coagulopathy.
Treatment
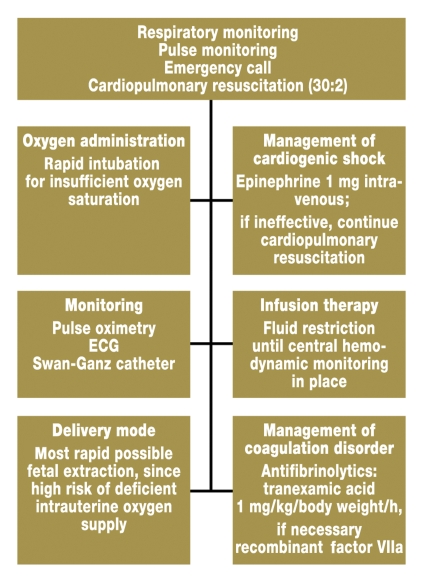
The primary intervention is stabilization of the patient’s cardiorespiratory situation (figure 3). For coagulation management, antifibrinolytic therapy (tranexamic acid 1 mg/kg body weight/h) should be initiated at an early stage. Insufficient data are available regarding treatment with recombinant factor VIIa (rFVIIa) for anaphylactoid syndrome of pregnancy. Successful treatment has been documented in individual case reports (26).
Figure 3.
Procedure for acute amniotic fluid embolism
Discussion
Anaphylactoid syndrome of pregnancy (Amniotic fluid embolism).
High maternal mortality (60% to 80%) is to be expected even with optimal treatment.
The treatment of patients with severe peripartum hemorrhage is only substantiated by data with a low evidence level. Close interdisciplinary cooperation between obstetricians, midwife, anesthetists, intensive care physicians, and coagulation specialists is therefore the essential requirement for effective treatment. Local medical facilities should thus be regularly reviewed to ensure availability of the required therapeutic resources in each specific case.
Key messages.
Peripartum hemorrhage is the second commonest cause of maternal death associated with pregnancy and childbirth.
The main risk factors for peripartum hemorrhage are disorders of placental attachment after cesarian section. The increase in the rate of cesarian sections may be expected to result in an increased incidence of peripartum hemorrhage.
The most accurate possible measurement of blood loss is necessary to assess the severity of bleeding.
In the management of peripartum hemorrhage, besides administration of red cell concentrates, adequate fibrin replacement is the primary therapeutic goal.
In the presence of risk factors for peripartum hemorrhage, the patient should be managed at a center with the required logistic resources (intensive care unit for adults, intensive care unit for newborns, blood bank).
Box 1. Conservative management of uterine atony.
-
Manual uterine compression
Holding of the uterus for at least 15 minutes
-
Emptying the bladder
Placement of an indwelling catheter
-
Oxytocin
-
Methylergotamine
Slow intravenous administration of up to 0.1 mg Caution: possibility of cardiac side effects!
-
Sulprostone
Prostaglandin of choice (1 ampoule equivalent to 500 µg in 500 mL infusion solution). Dosage 1.7 mL/min. Maximum daily dose: 1500 µg (14)
-
Misoprostol
-
Uterine tamponade
Intrauterine introduction of gauze packing or balloon catheters; helpful for diffuse bleeding from placental detachment surface; the evidence on uterine atony is in dispute (16)
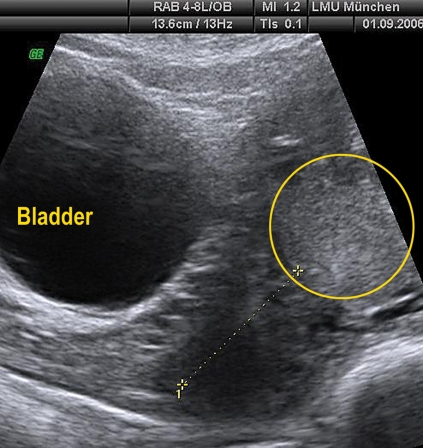
Abdominal sonography in placenta previa totalis. No evidence of placenta accreta or increta with good demarcation of the uterine wall. The dotted line shows the position of the cervix, the placenta is circled.
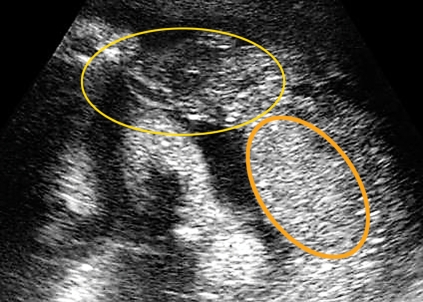
Transabdominal sonographic scan of premature marginal placental abruption with partly hypoechoic, partly echodense blood coagulum. Circled in yellow: hematoma associated with premature abruption; orange line shows the site of the normal placenta position.
Box 2. Causes and risk factors of premature placental detachment.
Hypertensive diseases of pregnancy
Factor V Leiden mutation
Hyperhomocysteinemia MTHFR mutation
Chronic hypertension
Advanced maternal age
Diabetic vasculopathy
Nephropathy
Fibroids
Alcohol and cocaine abuse
Multiple pregnancy, polyhydramnios
Abdominal trauma (traffic accident)
Further Information. This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education.
Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Chambers of Physicians of the German federal states (Länder). CME points of the Chambers of Physicians can be acquired only through the Internet by the use of the German version of the CME questionnaire within 6 weeks of publication of the article. See the following website: www.aerzteblatt.de/cme
Participants in the CME program can manage their CME points with their 15-digit "uniform CME number" (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the www.aerzteblatt.de website under "meine Daten" ("my data"), or upon registration. The EFN appears on each participant’s CME certificate.
The solutions to the following questions will be published in volume 45/2008. The CME unit "Primary Osteoporosis – Guideline Based Diagnosis and Therapy" (volume 33/2008) can be accessed until 26 September 2008.
For volume 41/2008 we plan to offer the topic "Hereditary Cancers."
Solutions to the CME questionnaire in volume 28–29/2008:
Zernikow B: Pain Therapy in Childhood and Adolescence: 1d, 2d, 3a, 4d, 5c, 6c, 7b, 8c, 9a, 10a
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1:
How many women die annually worldwide of a complication resulting from postpartum bleeding?
About 40 000 women
About 90 000 women
About 140 000 women
About 190 000 women
About 240 000 women
Question 2:
Which of the following examinations is used to confirm the diagnosis in late pregnancy hemorrhage?
Computed tomography
Cardiotocography
Placental scintigraphy
Ultrasonography
Radiography
Question 3:
Which pathophysiological mechanisms are responsible for inducing anaphylactoid syndrome of pregnancy (amniotic fluid embolism)?
Obstruction of the pulmonary arteries by amniotic fluid
Obstruction of the pulmonary arteries by amniotic fluid constituents (lanugo hairs)
Thrombus formation by amniotic fluid constituents
Anaphylactic reaction to amniotic fluid constituents
Passage of more than 350 mL amniotic fluid into the maternal vascular system
Question 4:
What is the first therapeutic measure for hypovolemic shock?
Immediate intubation
Placement of an indwelling catheter
Volume replacement
Administration of recombinant factor VIIa
Embolization of the bleeding vessels
Question 5:
What should be done in the acute treatment of anaphylactoid syndrome of pregnancy (amniotic fluid embolism)?
Intravenous administration of heparin
Cardiorespiratory stabilization
Continuous hemodialysis
Short-term oxygen administration
Immediate cesarian section
Question 6:
Which laboratory diagnostic parameter is primarily informative for diagnosing known peripartum bleeding?
Liver function tests
Clotting screen
Urea and electrolyte assay
Full blood count
Platelet count
Question 7:
How high is maternal mortality from anaphylactoid syndrome of pregnancy (amniotic fluid embolism) (%)?
0% to 20%
20% to 40%
40% to 60%
60% to 80%
80% to 100%
Question 8:
What is the treatment of choice for an extensive form of placental detachment with abnormal CTG?
Inhibition of labor to stop further detachment
Administration of contraction inducing agents to increase uterine tone
Delivery by cesarian section
Embolization of the uterine artery
Continuation of pregnancy
Question 9:
What treatment should be used as the last resort for consumption coagulopathy?
Administration of red blood cell concentrates
Administration of aprotinin
Administration of recombinant factor VIIa
Surgical treatment of the bleeding vessels
Hysterectomy
Question 10:
Which examination is most useful for diagnosing placenta previa?
Palpation of the cervical os
Visualization of the lower edge of the placenta with a speculum
Abdominal CT
MRI for imaging the cervical os
Transvaginal and transabdominal sonography
Acknowledgments
Translated from the original German by mt-g.
Footnotes
Conflict of Interest Statement
The authors declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Hellgren M. Hemostasis during normal pregnancy and puerperium. Semin Thromb Hemost. 2003;29:125–130. doi: 10.1055/s-2003-38897. [DOI] [PubMed] [Google Scholar]
- 2.Kainer F. Blutungen im 3. Trimenon. In: Schneider H, Husslein P, Schneider KTM, et al., editors. Geburtshilfe. Berlin, Heidelberg, New York: Springer; 2000. pp. 539–553. [Google Scholar]
- 3.Welsch H. Müttersterblichkeit während Geburt und Wochenbett bei vaginaler Entbindung und Sectio caesarea. Gynäkologe. 1997;30:742–756. [Google Scholar]
- 4.AWMF-Leitlinie der Deutschen Gesellschaft für Gynäkologie und Geburtshilfe (DGGG) Plazentationsstörungen bei Status nach Sectio - Risk-Management zur Vermeidung von Müttersterbefällen 015/046 2005 [Google Scholar]
- 5.Royal College of Obstetricians and Gynaecologists (RCOG) Guideline No 27; Placenta previa and placenta accreta: Diagnosis and management. London: RCOG Press; 2005. [Google Scholar]
- 6.American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists: postpartum hemorrhage. Obstet Gynecol. 2006;108:1039–1047. doi: 10.1097/00006250-200610000-00046. [DOI] [PubMed] [Google Scholar]
- 7.Heindl B, Delorenzo C, Spannagl M. High dose fibrinogen administration for acute therapy of coagulopathy during massive perioperative transfusion. Anaesthesist. 2005;54:787–790. doi: 10.1007/s00101-005-0865-7. [DOI] [PubMed] [Google Scholar]
- 8.Rath W, Schierbrock S, Heilmann L. Rekombinanter Faktor VIIa - eine neue vielversprechende Option zur Behandlung schwerer peripartaler Blutungskomplikationen Geburtsh Frauenheilk. 2006;66:833–840. [Google Scholar]
- 9.Henrich W, Fuchs I, Ehrenstein T, Kjos S, Schmider A, Dudenhausen JW. Antenatal diagnosis of placenta percreta with planned in situ retention and methotrexate therapy in a women infected with HIV. Ultrasound Obstet Gynecol. 2002;20:90–93. doi: 10.1046/j.1469-0705.2002.00691.x. [DOI] [PubMed] [Google Scholar]
- 10.Abd Rabbo SA. Stepwise uterine devascularization: a novel technique for management of uncontrolled postpartum hemorrhage with preservation of the uterus. Am J Obstet Gynecol. 1994;171:694–700. doi: 10.1016/0002-9378(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 11.Ojala K, Perala J, Karienami J, et al. Arterial embolization and prophylactic catherization for the treatment of severe obstetric hemorrhage. Acta Obstet Gynecol Scand. 2005;84:1075–1080. doi: 10.1111/j.0001-6349.2005.00727.x. [DOI] [PubMed] [Google Scholar]
- 12.Joseph KS, Rouleau J, Kramer MS, Young DC, Liston RM, Baskett TF. Investigation of an increase in postpartum haemorrhage in Canada. BJOG. 2007;114:751–759. doi: 10.1111/j.1471-0528.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 13.Crombach G. Operative Behandlung schwergradiger postpartaler Blutungen. Gynäkologe. 2000;33:286–297. [Google Scholar]
- 14.Crochetiere C. Obstetric emergencies. Anesthesiol Clin North Am. 2003;21:111–125. doi: 10.1016/s0889-8537(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 15.Surbek DV, et al. Oral misoprostol for third stage of labor: a randomized placebo-controlled trial. Obstet Gynecol. 1999;94:255–258. doi: 10.1016/s0029-7844(99)00271-9. [DOI] [PubMed] [Google Scholar]
- 16.Seror J, Allouche C, Elhaik S. Use of Sengstaken-Blakemore tube in massive postpartum hemorrhage: a series of 17 cases. Acta Obstet Gynecol Scand. 2005;84:660–664. doi: 10.1111/j.0001-6349.2005.00713.x. [DOI] [PubMed] [Google Scholar]
- 17.B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B-Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. Br J Obstet Gynaecol. 1997;104:372–375. doi: 10.1111/j.1471-0528.1997.tb11471.x. [DOI] [PubMed] [Google Scholar]
- 18.Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstet Gynecol. 2002;99:502–506. doi: 10.1016/s0029-7844(01)01643-x. [DOI] [PubMed] [Google Scholar]
- 19.Ghezzi F, Cromi A, Ucella S, Raio L, Bolis P, Surbek D. The Hayman technique: a simple method to treat postpartum haemorrhage. BJOG. 2007;114:362–365. doi: 10.1111/j.1471-0528.2006.01204.x. [DOI] [PubMed] [Google Scholar]
- 20.Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during caesarean delivery. Obstet Gynecol. 2000;96:129–131. doi: 10.1016/s0029-7844(00)00852-8. [DOI] [PubMed] [Google Scholar]
- 21.Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstet Gynecol. 2005;106:569–572. doi: 10.1097/01.AOG.0000168434.28222.d3. [DOI] [PubMed] [Google Scholar]
- 22.Kainer F, Schiessl B, Kästner R. Geburtshilfliche Notfälle. Geburtsh u Frauenheilk. 2003;28:161–184. [Google Scholar]
- 23.Clarke J, Butt M. Maternal collapse. Curr Opin Obstet Gynecol. 2005;17:157–160. doi: 10.1097/01.gco.0000162185.53478.34. [DOI] [PubMed] [Google Scholar]
- 24.Gilmore DA, Wakim J, Secrest J, Rawson R. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120–16. [PubMed] [Google Scholar]
- 25.Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158–1167. doi: 10.1016/0002-9378(95)91474-9. [DOI] [PubMed] [Google Scholar]
- 26.Prosper SC, Goudge CS, Lupo VR. Recombinant factor VIIa to successfully manage disseminated intravascular coagulation from amniotic fluid embolism. Obstet Gynecol. 2007;109:524–525. doi: 10.1097/01.AOG.0000235711.11817.8c. [DOI] [PubMed] [Google Scholar]
- e1.Deneux-Tharaux C, Camora E, Bouvier-Colle MH, et al. Postpartum maternal mortality and cesarean delivery. Obstet Gynecol. 2006;108:541–548. doi: 10.1097/01.AOG.0000233154.62729.24. [DOI] [PubMed] [Google Scholar]
- e2.UNFPA. Maternal mortality uptdate 2002: a focus on emergency obstetric care. New York: United Nations Population Fund; 2003. [Google Scholar]
- e3.Dildy GA. Postpartum hemorrhage: new management options. Clin Obstet Gynecol. 2002;45:330–344. doi: 10.1097/00003081-200206000-00005. [DOI] [PubMed] [Google Scholar]
- e4.Poe MF. Clot observation test for clinical diagnosis of clotting defects. Anesthesiology. 1959;20:825–829. doi: 10.1097/00000542-195911000-00013. [DOI] [PubMed] [Google Scholar]
- e5.Konje JC, Walley RJ. Bleeding in late pregnancy. In: James DK, Steer PJ, Weiner CP, Gonik B, et al., editors. High risk pregnancy. London Philadelphia: W.B.Saunders; 1994. pp. 119–136. [Google Scholar]
- e6.Hardy JF, Moerloose P de, Samama CM. Massive transfusion and coagulopathy: Pathophysiology and implications for clinical management. Can J Anaest. 2006;53([Suppl 6]):40–58. doi: 10.1007/BF03022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e7.Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of hemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- e8.Gai MY, Wu LF, Su OF, et al. Clinical observation of blood loss reduced by tranexamic acid during and after caesarean setion: a multi-center, randomized trial. Eur J Obstet Gynecol Reprod Biol. 2004;112:154–157. doi: 10.1016/s0301-2115(03)00287-2. [DOI] [PubMed] [Google Scholar]
- e9.Segal S, Shemesh IY, Blumeenthal, et al. Treatment of obstetric hemorrhage with recombinant activated factor VII (rFVIIa) Arch Gynecol Obstet. 2003;268:266–267. doi: 10.1007/s00404-002-0409-1. [DOI] [PubMed] [Google Scholar]
- e10.Kay HH. Placenta previa and Abruption. In: Scott JR, Gibbs RS, Karlan BY, Haney AF, et al., editors. Danforth´s Obstetrics and Gynaecology. Pphiladelphia, Baltimmore, London Lippincott: Williams & Wilkins; 2003. pp. 365–379. [Google Scholar]
- e11.Timmermans S, Arjanneke G, van Hof C, Duvekot JJ. Conservative management of abnormally invasive placentation. Obstet Gynecol Surv. 2007;62:529–529. doi: 10.1097/01.ogx.0000271133.27011.05. [DOI] [PubMed] [Google Scholar]
- e12.Bouwmeester FW, Bolte AC, van Geijn HP. Pharmalogical and surgical therapy of primary postpartum hemorrhage. Curr Pharmaceut. 2005;11:759–773. doi: 10.2174/1381612053381882. [DOI] [PubMed] [Google Scholar]
- e13.Love CD, Wallace EM. Pregnancies complicated by placenta previa: what is appropriate management? Br J Obstet Gynaecol. 1996;103:864–867. doi: 10.1111/j.1471-0528.1996.tb09903.x. [DOI] [PubMed] [Google Scholar]
- e14.Schuurmans N, Mackinnin C, Lane C, et al. Prevention and management of postpartum haemorrhage. J Soc Obset Gynaecol Can. 2000;88:271–281. [Google Scholar]
- e15.Prendiville WJ, Elbourne D, McDonald S. Active versus expectant management in the third stage of labour. Cochrane Database Syst Rev. 2000;(3) doi: 10.1002/14651858.CD000007. CD000007. Review. [DOI] [PubMed] [Google Scholar]
- e16.Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing Caesarean section. Br J Anaesth. 2007;98:116–119. doi: 10.1093/bja/ael302. [DOI] [PubMed] [Google Scholar]
- e17.Lapaire O, Schneider MC, Stoz F, Surbek DV, et al. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean section. Int J Gynecol Obstet. 2006;95:2–7. doi: 10.1016/j.ijgo.2006.05.031. [DOI] [PubMed] [Google Scholar]
- e18.Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2007;(1) doi: 10.1002/14651858.CD003249.pub2. CD003249, DOI10.1002/4651858. [DOI] [PubMed] [Google Scholar]
- e19.Distler W. Ligatur der Arteria iliaca interna bei lebensbedrohlichen Blutungen im Beckenbereich. Gynäkologe. 2008;41:107–110. [Google Scholar]
- e20.Soncini E, Pelicelli A, Larini P, Marcato C, Monaco D, Grignaffini A. Uterine artery embolization in the treatment and prevention of postpartum hemorrhage. Int J Gynaecol Obstet. 2007;96:181–185. doi: 10.1016/j.ijgo.2006.12.010. [DOI] [PubMed] [Google Scholar]
- e21.Aurangzeb I, George L, Raoof S. Amniotic fluid embolism. Crit Care Clin. 2004;20:643–650. doi: 10.1016/j.ccc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- e22.Biron-Andreani C, Morau E, Schved JF, et al. Amniotic fluid embolism with haemostasis complications: primary fibrinogenolysis or disseminated intravascular coagulation? Pathophysiol Haemost Thromb. 2003;33:170–177. doi: 10.1159/000077826. [DOI] [PubMed] [Google Scholar]
- e23.Peitsidou A, Peitsidis P, Tsekoura V, Spathi A, Tzaneti A, Samanta E, Siampalioti G, Kioses E. Amniotic fluid embolism managed with success during labour: report of a severe clinical case and review of literature. Arch Gynecol Obstet. 2008;277:271–275. doi: 10.1007/s00404-007-0489-z. [DOI] [PubMed] [Google Scholar]
- e24.Tuffnell DJ. Amniotic fluid embolism. Curr Opin Obstet Gynecol. 2003;15:119–122. doi: 10.1097/00001703-200304000-00006. [DOI] [PubMed] [Google Scholar]
- e25.Kretzschmar M, Zahm DM, Remmler K, Pfeiffer L, Victor L, Schirmeister W. Pathophysiological and therapeutic aspects of amniotic fluid embolism (anaphylactoid syndrome of pregnancy): case report with lethal outcome and overview. Anaesthesist. 2003;52:419–426. doi: 10.1007/s00101-003-0482-2. [DOI] [PubMed] [Google Scholar]