Abstract
Background
In recent years, the market for dietary supplements has grown. International products are readily available for purchase over the Internet. We report 17 cases of poisoning with a single product, said to be of purely herbal origin, that was bought over the Internet. A complete declaration of the ingredients was not available.
Methods
We performed a retrospective study of cases of poisoning documented by the Göttingen and Freiburg poison information centers from 2005 to 2008. In 4 cases, we were able to perform toxicological analyses of leftover capsules and urine samples.
Results
The manifestations of poisoning in the 17 documented cases included malaise, tachycardia, headache, agitation, arterial hypertension, nausea, vomiting, dyspnea, insomnia, left-sided chest pressure, elevated temperature, and, in two cases, psychosis after the substance was combined with atomoxetine and methylphenidate and with citalopram, olanzapine, and chlorprothixene. The frequency of cases rose markedly in the last year of the study. The toxicological analyses of all samples studied revealed sibutramine. The dose in each capsule was nearly twice the maximum daily dose sibutramine in the medication containing this substance that is licensed for use in Germany.
Conclusions
Products available without a prescription whose contents are claimed to be purely herbal may nonetheless contain synthetic substances in concentrations far above the therapeutic range and may be a cause of poisoning. When taking the history of a patient possibly suffering from an intoxication, the physician should ask specifically about drugs, dietary supplements, and so-called lifestyle products that were obtained without a prescription. It would be desirable for the contents of all such products to be declared, as required by law, so that their suitability for the market can be checked.
Keywords: sibutramine, weight-reducing drugs, dietary supplements, pharmaceutical industry, Internet
In recent years, the supply and sales of herbal food supplements have grown (1). 18.6% of the adult US population report that they took herbal medicines in the course of 2002, in comparison with 12.1% in 1997 and 2.5% in 1990 (2– 4). Herbal medicines are marketed in pharmacies, drugstores and directly, with advertising either in the printed media or, increasingly, from international Internet suppliers, with direct delivery (1, 5).
Many products are delivered as different formulations, without any or any adequate declaration of the ingredients, or information on the recommended dosages or side effects. Translations of product information are often missing from foreign products (6– 8). Product descriptions with references to "traditional medicine," "natural components," or ingredients known to be harmless imply that there is no risk in using the products.
But herbal products can be risky. There have been numerous cases of unexpected side effects and interactions from toxic herbal components, contamination during cultivation (4, 9), inappropriate processing, or uncritical use. In 1992 in England, a group of young women suffered interstitial renal fibrosis after using herbal weight loss drugs. Moreover, liver damage has been described after taking Teucrium chamaedrys (wall gamander) (10).
Substances which must be declared and licensed in the sense of the German Medicines Act are added to some herbal formulations in relevant concentrations (4, 8, 10– 15). Investigators have found corticosteroids, indometacin, phenytoin (8), promethazine, chlomethiazole, chlorpheniramine, diclofenac, chlordiazepoxide, hydrochlorothiazide, triamterene, diphenhydramine, and sildenafil (13) in allegedly herbal products. In recent years, not the substances themselves, but their active metabolites, such as N-bidesmethylsibutramine (15) or N-nitrosofenfluramine (14– 16) have been added to some weight loss preparations.
Products are monitored by national agencies. Regulations and recommendations on marketing herbal products used to be specific for each country, but have now been harmonized by the FDA (Food and Drug Administration) and the EMEA (European Medicines Agency; Committee on Herbal Medicinal Products, CHMP) (6, 14, 17– 20, e1).
We will now report on a Chinese weight loss preparation which can be purchased over the Internet (21– 23). This contains undeclared concentrations of sibutramine at concentrations greater than those in licensed medicines (24). This first led to intoxications in 2005 (25), with a striking number of enquiries to poison information centers in recent months. Cases between 2005 and June 2008 will be considered. According to the Internet advertisements, the product contains herbal ingredients and is declared as a food supplement. A synthetic drug (active substance) has been detected analytically and identified as sibutramine.
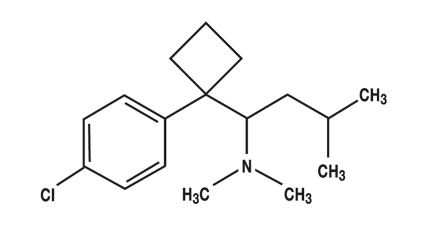
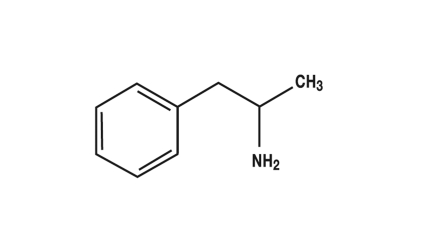
Sibutramine (figure 1) has been approved in Germany as an anti-obesity drug. It has structural similarities to amphetamine (figure 2) and is a serotonin-noradrenaline reuptake inhibitor (SNRI) (24, e2– e4). The recommended daily dose is 5 to 15 mg (e2). If a low initial dose is not sufficiently effective, it may be increased to a maximum daily dose of 15 mg (24). After oral administration, sibutramine is rapidly absorbed and is subject to marked first pass metabolism. The primary metabolites (norsibutramine and dinorsibutramine) are responsible for the activity and inhibit the reuptake of noradrenalin and serotonin from the synaptic cleft (24, e2, e3). The half-life is given as 14 h or 16 h (24, e4). Elimination is largely hepatic. No change in the half-life has been found up to a 30 mg dose, so that it can be assumed that plasma concentrations are dose dependent up to this dose (24). Metabolism mainly occurs through cytochrome P450, subtype 3A4 (CYP3A4), so that plasma concentrations may be increased if sibutramine is combined with other drugs metabolized by this enzyme (for example, ketoconazole, erythromycin, and cimetidine [e5]). In addition, activity may be enhanced in combination with other substances which increase the concentration of serotonin or noradrenaline in the synaptic cleft or which act as serotonin agonists because of structural similarity (e6). For these reasons, certain combinations are contraindicated, particularly with some weight loss drugs, such as other SSRIs, MAO inhibitors, amphetamines, triptans, or ergotamines, and some opioids (24, e2, e3).
Figure 1.
Sibutramine
Figure 2.
Amphetamine
The dose of sibutramine in a capsule of the product corresponds to almost twice the maximal daily dose for prescription drugs licensed in Germany (24).
Methods
The evaluation was performed retrospectively at the centers in Freiburg (Baden-Württemberg) and Göttingen (Hamburg, Bremen, Schleswig-Holstein, and Lower Saxony). The patients were not recorded by name and we were not provided with their medical records. The data were therefore based on consultation records. Case documentation included age, sex, toxin, dosage, mode of intake, latent period, symptoms, therapeutic measures already performed, recommended therapy and the severity of the intoxication according to the "Poisoning Severity Score" (PSS) (e7).
Results
Since 2005, 17 cases of health problems after taking Chinese slimming capsules have been reported to the poison information centers in Freiburg and Göttingen. These affected 15 women aged from 14 to 38 years (median 20 years) and two men of unknown age were affected.
The severity of the poisoning was assessed in 12 cases as "mild," in three cases as "intermediate," in one case as "severe," and in one case as not evaluable (e7).
The following symptoms were reported on visiting the doctor:
Malaise (n = 13), tachycardia (n = 7), headache (n = 4), agitation (n = 5), arterial hypertension (n = 3), nausea (n = 4), dyspnea (n = 3), vomiting (n = 3), insomnia (n = 2), left-sided chest pressure (n = 1), and elevated temperature (n = 1).
A 17-year old female patient also suffered from minor creatine kinase (CK) elevation. She had taken 1 to 3 capsules daily over a period of 5 weeks, with a body weight of 40 kg.
A 14-year old girl had taken the slimming capsules together with her current therapy with atomoxetine and methylphenidate. Under this combination therapy, the patient had to be admitted to hospital for psychiatric treatment for agitation and confusion (e7).
One man had to be admitted to hospital for acute psychosis after taking the slimming pills. As medication, he had been taking chlorprothixene, olanzapine, and cipramil (e7).
The urine of four patients was investigated toxicologically and the primary metabolite norsibutramine was detected. The administered product was provided for two patients and this was subjected to quantitative analysis. In a sample from 2005, 32.7 mg sibutramine (25) was detected and in a sample from 2008 28.3 mg sibutramine. A formulation licensed in Germany contains 15 mg sibutramine per capsule (as sibutramine hydrochloride monohydrate).
Discussion
Our experience is that responsible physicians are often unaware of their patients’ use of "herbal" tablets, purchased on the Internet and regarded as being harmless. Because of the advertising information and the package leaflet, patients and doctors do not regard these as being real drugs (4, 5, e8). As a consequence, the symptoms reported by the patients were often not regarded as side effects from an overdose or as severe interactions with previously administered psychotherapeutics. Administration of the same Chinese slimming pill was only identified in all 17 cases after a specific medical history had been taken, including food supplements. A causal connection was suspected and this could be confirmed in four cases from the toxicological analysis of the capsule content and of the patient’s urine. Sibutramine or its metabolites could be detected. Other weight loss drugs—such as fenfluramine or dinitrophenol—were not detectable in urine.
The most marked symptoms developed in two cases from the unnoticed combination of sibutramine with SSRI or with methylphenidate plus atomoxetine. It was impossible for us to establish whether the prior medication had been prescribed or taken by the patient independently.
In the other 15 cases, there was no evidence that drugs, substances or diseases had triggered the symptoms complained of.
It was demonstrated in 2005 that the slimming capsules declared as being herbal contained sibutramine and sibutramine metabolites were identified in the patient’s urine (12, 25). The Freiburg poison center then informed the monitoring authorities, who released several warnings in the media (11).
In the same year, studies performed by the Official Food Control Laboratory in Rhineland-Palatinate (Landesuntersuchungsamt Rheinland-Pfalz) showed that slimming capsules distributed over the Internet under another name—"Evolution Slim & Slender"—also contain sibutramine (11).
In 2007, there were also reports from Poland of allegedly herbal Chinese slimming capsules—"Meizitanc"—that contained 10 mg sibutramine each (e9). Eight women took these capsules and contacted the poison emergency center in Gdansk because of symptoms such as palpitations, headache, dizziness, sensation of heat, and nervousness. In the published cases (25, e9), the symptoms regressed after the medication had been discontinued.
A current Internet search showed that slimming pills with the same name as that which had drawn attention in Poland were still being advertised by several suppliers in different countries (21– 23).
It was striking that by 1 July in 2008 the poison emergency centers in Göttingen and Freiburg had already registered five enquiries about clinical signs of poisoning after taking Chinese slimming capsules supplied over the Internet. For the whole of 2007, only five enquiries had been registered, so that it must be feared that the rate of enquiries will be doubled for the current year of 2008.
The data mentioned on the consumption of herbal food supplements imply that use might be much higher (1– 3). Moreover there are many cases of poisoning in which the medical services are not contacted, the causal connection is not made, and a poison information center is not consulted.
As the dose of the undeclared ingredient is also much greater in the most recently analyzed sample than the dose in the licensed prescription drugs or in the products examined in Poland, it must be expected that the rate of adverse effects will rise, particularly if the activity is enhanced by concomitant medication with CNS-active substances (as mentioned above).
It is becoming clear that a medical history must include herbal products and the so-called lifestyle drugs, if clinical symptoms are to be properly evaluated. This information is also essential at the start of pharmacotherapy, to avoid interactions and to provide patients with individual rational advice, allowing for their predispositions (age, weight, pregnancy, prior diseases or special metabolic features) (boxes 1 and 2).
Box 1. Side effects of sibutramine.
-
Very common (>1/10)
Constipation
Dry mouth
Insomnia
-
Common (<1/10, >1/100)
Tachycardia
Palpitations
Increase in blood pressure
Vasodilatation
Nausea
Light-headedness
Paresthesias
Headache
Anxiety
Sweating
Anomalies of taste
Box 2. Contraindications to sibutramine (24).
Known hypersensitivity to sibutramine hydrochloride monohydrate or to one of the other components
Organic causes of obesity
Severe eating disorders in the medical history
Psychiatric diseases
Gilles de la Tourette syndrome
Simultaneous treatment or treatment within the previous two weeks with monoamine oxidase inhibitors (MAO inhibitors), antidepressants, neuroleptics, drugs to reduce weight, or tryptophan for insomnia
Coronary heart disease, heart failure, tachycardia, peripheral arterial occlusive disease, cardiac arrthymia or cerebrovascular disease (stroke or TIA) in the medical history
Inadequately controlled hypertension
Thyrotoxicosis
Severe hepatic disorders
Severe renal disorders
Benign prostatic hyperplasia with residual urine formation
Pheochromocytoma
Narrow angle glaucoma
Drug, narcotic or alcohol abuse in the medical history
Pregnancy or breast feeding
Children and adolescents under 18 years of age (inadequate experience)
Patients over 65 years of age (inadequate experience)
It would be desirable to implement the obligation to declare ingredients and dose. The marketability of the product must be checked, particularly in countries in which the ingredients are classified as prescription drugs and in which a pharmaceutically defined medicine is available.
The study shows that poison information centers register trends in poisoning and can draw attention to risks from the inadequate declaration of ingredients which can lead to faulty assessment of the risk (e10),
We wish to thank Dr. Horn of the Drug Investigation Institute North [Arzneimitteluntersuchungsinstitut-Nord], Bremen, and Mr. H. Neurath of the Pharmacological and Toxicological Service Center, Faculty of Medicine, Göttingen University, for investigating the stored samples.
Key messages.
Herbal products are not inherently harmless.
The ingredients have not been tested and labeled in all products.
Untested herbal products may contain substances which are pharmacologically or toxicologically relevant. These may originate during cultivation or processing or have been added as chemically defined substances.
Patients often expect no side effects or interactions from herbal products. An objective discussion is desirable.
Before drug therapy is started, the responsible physician should take a medical history, including specific questions about self-medication with freely available "health products." Rational advice should consider the individual disposition of the patient.
Acknowledgments
Translated from the original German by Rodney A. Yeates, M.A., Ph.D.
Footnotes
Conflict of interest statement
The authors declare that there is no conflict of interest in the sense of the International Committee of Medical Journal Editors.
References
- 1.Morris CA, Avorn J. Internet marketing of herbal products. JAMA. 2003;290:1505–1509. doi: 10.1001/jama.290.11.1505. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States 1990-1997: results of a follow-up national survey. JAMA. 1998;280:1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 3.Tindle HA, Davis RB, Phillips RS, Eisenberg DM. Trends in use of complementary and alternative medicine by US adults: 1997-2002. Altern Ther Health Med. 2005;11:42–49. [PubMed] [Google Scholar]
- 4.De Smet PA. Health risks of herbal remedies: an update. Clin Pharmacol Ther. 2004;76:1–17. doi: 10.1016/j.clpt.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Löbell-Behrends S, Maixner S, Kratz E, Kohl-Himmelseher M, Bauer-Aymanns H, Marx G. Borderlineprodukte. Kontrolle des Internethandels mit Anti-Aging und Schlankheitsmitteln. Deutsche Lebensmittel Rundschau. 2008;104:265–270. [Google Scholar]
- 6.Routledge PA. The European Herbal Medicines Directive: could it have saved the lives of Romeo and Juliet? Drug Saf. 2008;3:416–418. doi: 10.2165/00002018-200831050-00006. [DOI] [PubMed] [Google Scholar]
- 7.Foster BC, Arnason JT, Briggs CJ. Natural health products and drug deposition. Annu Rev Pharmacol Toxicol. 2005;45:203–226. doi: 10.1146/annurev.pharmtox.45.120403.095950. [DOI] [PubMed] [Google Scholar]
- 8.Ernst E. Adulteration of Chinese herbal medicines with synthetic drugs: a systematic review. J Intern Med. 2002;252:107–113. doi: 10.1046/j.1365-2796.2002.00999.x. [DOI] [PubMed] [Google Scholar]
- 9.Ernst E. Toxic heavy metals and undeclared drugs in asian herbal medicines. Trends Pharmacol Sci. 2002;23:136–139. doi: 10.1016/S0165-6147(00)01972-6. [DOI] [PubMed] [Google Scholar]
- 10.Vanherweghem JL, Depierreux M, Tielemans C, et al. Rapidly progressive interstitial renal fibrosis in young women: association with slimming regimen including chinese herbs. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 11.Landesuntersuchungsamt Rheinland-Pfalz. Jahresbericht 2005, Kap2. www.lua.rlp.de/navigation/Jahresberichte/Jahresbericht_2005/_2005/JB-2005-eKap-II-Lebensmittel.pdf. (7. 7. 2008)
- 12.Vidal C, Quandte S. Identification of a sibutramine-metabolite in patient urine after intake of a „pure herbal“ Chinese slimming product. Ther Drug Monit. 2006;28:690–692. doi: 10.1097/01.ftd.0000245392.33305.b0. [DOI] [PubMed] [Google Scholar]
- 13.Miller GM, Stripp R. A study of western pharmaceuticals contained within samples of Chinese herbal/patent medicines collected from New York City`s Chinatown. Leg Med (Tokyo) 2007;9:258–264. doi: 10.1016/j.legalmed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Corns C, Metcalfe K. Risks associated with herbal slimming remedies. J R Soc Health. 2002;122:213–219. doi: 10.1177/146642400212200407. [DOI] [PubMed] [Google Scholar]
- 15.Yuen YP, Lai CK. Adulteration of over the counter slimming products. Hong Kong Med J. 2007;13:216–220. [PubMed] [Google Scholar]
- 16.Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastroenterol Hepatol. 2008;23:366–373. doi: 10.1111/j.1440-1746.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 17.FDA announces major initiatives for dietary supplements. United States Food and Drug Administration, Department of Health and Human Services, FDA News P04-101. www.fda.gov/bbs/topics/news/2004/NEW01130.html. (23. 7. 2008)
- 18.WHO. Medical Products and the internet. A Guide to find reliable information. www.tga.gov.au/docs/html/whointer.htm. (24. 7. 2008)
- 19. http://europa.eu/agencies/community_agencies/emea/index_de.htm. (16. 11. 2008)
- 20. www.emea.europa.eu/htms/general/contacts/CHMP/CHMP.html. (16. 11. 2008)
- 21. www.lida-daidaihua.info/100-Boxes-Lida-Daidaihua-Slimming-Capsule-from-kmdali-Wholesale-only-5.2EUR%2fbox-%2c-8.1USD%2fbox-*-100-Authentic_P97803/ (27. 7. 2008)
- 22. www.lida24.com/index.php?sid=produkt_info&pid=1. (8. 11. 2008)
- 23. www.lida24.ru/ (8. 11. 2008)
- 24.Fachinfo Reductil, Stand November 2005, Abbott GmbH & Co. KG, Wiesbaden [Google Scholar]
- 25.Jung J, Hermanns-Clausen M, Weinmann W. Anorectic sibutramine detected in a Chinese herbal drug for weight loss. Forensic Sci Int. 2006;161:221–222. doi: 10.1016/j.forsciint.2006.02.052. [DOI] [PubMed] [Google Scholar]
- e1.Berichtigung der Verordnung (EG) Nr. 882/2004 des Europäischen Parlaments und des Rates vom 29. April 2004 über amtliche Kontrollen zur Überprüfung der Einhaltung des Lebensmittel- und Futtermittelrechts sowie der Bestimmungen über Tiergesundheit und Tierschutz: ABI EU L191, 1-52 (2004) [Google Scholar]
- e2.Baselt RC. Disposition of toxic drugs and chemicals in man. 7th ed. Foster City, California: Biomedical Publications; 2004. Sibutramine; pp. 1023–1024. [Google Scholar]
- e3.Klasco RK, editor. DRUGDEX® Evaluation: Monograph Sibutramine. DRUGDEX®System (electronic version) Greenwood Village, Colorado, USA.: Thomson Micromedex; www.thomsonhc.com (19. 6. 2008) [Google Scholar]
- e4.Luque CA, Rey JA. Sibutramine: a serotonin-norepinephrine reuptake inhibitor for the treatment of obesity. Ann Pharmacother. 1999;33:968–978. doi: 10.1345/aph.18319. [DOI] [PubMed] [Google Scholar]
- e5.Klasco RK, editor. POISINDEX® System (electronic version) Greenwood Village, Colorado, USA.: Thomson Micromedex; www.thomsonhc.com (24. 7. 2008) [Google Scholar]
- e6.Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry. 2000;157:2057–2058. doi: 10.1176/appi.ajp.157.12.2057-a. [DOI] [PubMed] [Google Scholar]
- e7.Persson HE, Sjoberg GK, Haines JA, de Garbino Pronczuk J. Poisoning severity score. Grading of acute poisoning. Clin Toxicol. 1998;36:205–213. doi: 10.3109/15563659809028940. [DOI] [PubMed] [Google Scholar]
- e8.Jordan MA, Haywood T. Evaluation of internet websites marketing herbal weight-loss supplements to consumers. J Altern Complement Med. 2007;13:1035–1043. doi: 10.1089/acm.2007.7197. [DOI] [PubMed] [Google Scholar]
- e9.Anand J Sein, Chodorowski Z. Side effects after the usage of Chinese dieting product Meizitanc. Przegl Lek. 2007;64:346–347. [PubMed] [Google Scholar]
- e10.Descotes J, Testud F. Toxicovigilance: a new approach for the hazard identification and risk assessment of toxicants in human beings. Toxicol Appl Pharmacol. 2005;207(2 Suppl):599–603. doi: 10.1016/j.taap.2005.02.019. [DOI] [PubMed] [Google Scholar]