Abstract
Introduction
The incidence of acute diseases of the aorta will continue to rise as our population ages.
Methods
Selective literature review.
Results/Discussion
At centers specializing in maximum care, it is important that a specific strategy should be established in cases of clinically suspected, acute aortic diseases, and non-invasive diagnostic measures should be taken without delay. If an acute aortic dissection is diagnosed, the necessary treatment must be provided immediately. As this disease is life-threatening, a cooperating network of referring physicians and institutions should be set up in order to ensure optimal treatment for these desperately ill patients. Acute type A aortic dissections generally require surgery, while the primary treatment of uncomplicated type B dissections is conservative and complicated type B dissections can be treated primarily with stent-graft implantation. The referring medical specialists and institutions should follow the patients closely after treatment so that any problems that may develop can be recognized and treated in timely fashion.
Keywords: aortic dissection, classification, diagnosis, endovascular treatment, surgical treatment
Cardiovascular diseases have become the leading cause of death in countries with high medical standards. Acute aortic syndromes—particularly acute aortic dissection—are playing an increasingly important part in this development (1). Acute aortic dissection has an extremely high mortality rate after the occurrence of an initial manifestation, so a standardized treatment algorithm is crucial for patient survival. On the basis of selected recent publications, the aim of this study was to review the current status of the management of aortic dissection and formulate recommendations for treatment.
Etiology
Mechanisms that weaken the layers of the aortic wall may lead to aortic dilatation, resulting in increased stress on the wall and eventually to hemorrhage into the wall or dissection (box 1). Among the acquired causes, arterial hypertension (72.1%) plays a leading role (2). Further risk factors (2–4) are atherosclerosis (31%) and diabetes mellitus (5.1%). Iatrogenic aortic dissection occasionally occurs as a result of invasive catheter procedures or after surgical interventions (box 1).
Box 1. Etiology of aortic dissection.
Arterial hypertension
-
Inherited fibrillinopathies
Anuloaortic ectasia
Marfan syndrome (MFS)
Ehlers-Danlos syndrome (EDS)
-
Hereditary vasculopathies
Aortic isthmus stenosis
Bicuspid aortic valve
-
Inflammatory vascular diseases
Giant-cell arteriitis
Takayasu arteriitis
Rheumatic aortitis
Systemic lupus erythematosus
Behçet’s disease
Syphilis
Mycotic aortitis
Ormond’s disease (retroperitoneal fibrosis)
-
Iatrogenic
Catheter procedures
-
Aortic/aortic valve surgery
Vascular clamp
Aortotomy
Graft anastomosis
Modified from (e10)
The most important inherited connective tissue diseases involving the aortic wall are Marfan syndrome (MFS) and Ehlers-Danlos syndrome (EDS), along with the familial types of thoracic aortic aneurysm and aortic dissection (5). MFS is the most frequent congenital connective tissue disorder, occurring in 1 in 7000 births with autosomal dominant inheritance (box 1). Diagnosis of MFS rests on clinical and molecular genetic criteria (6, e1). The term EDS embraces a number of inherited connective tissue diseases that are all characterized by increased mobility of joints and tissue fragility. The precise incidence of the 11 forms of EDS is unknown. The most frequent form is the autosomal dominant type 4 ("vascular type") (7).
Pathogenesis and classification
Acute aortic dissection entails laceration of the intima, sometimes extending to the media (entry tear), with division between the layers. In most cases the innermost layer peels off in the direction of the blood flow. There thus arises a false lumen, separated from the true lumen by a dissection membrane. The two lumina and the dissection membrane often run spirally in craniocaudal direction, corresponding to the blood flow in the aorta (8).
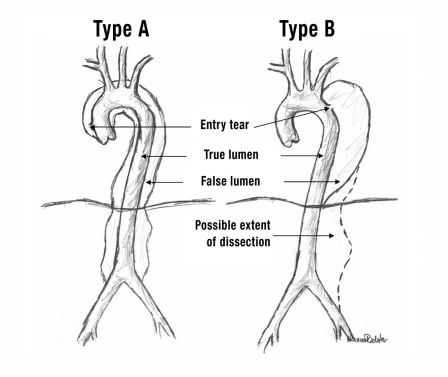
The Stanford classification (figure 1) distinguishes between type A aortic dissection, affecting the ascending aorta, and type B aortic dissection, in which the entry tear is located distal to the outlet of the left subclavian artery so the ascending aorta is, by definition, not involved. This categorization ignores the location of re-entry (the more distal tear where the blood from the false lumen flows back into the true lumen). The Stanford classification takes no account of the precursors of aortic dissection, eg, intramural hematoma, penetrating ulcer, and localized intimal detachment (9, 10).
Figure 1.
Stanford classification.
Type A:
dissections that involve the ascending aorta.
Type B:
dissections that do not involve the ascending aorta
Natural course and prognosis
Type A aortic dissection
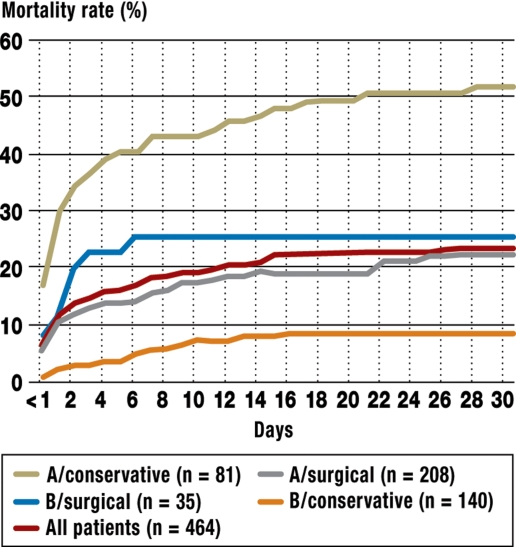
Untreated, a patient with acute type A aortic dissection has a 40% to 60% risk of dying within 48 hours of the event (11) (figure 2). For this reason it is essential to operate immediately. Untreated patients usually die of a rupture of the false lumen or from the consequences of acute pericardial tamponade. Further complications are acute high-grade aortic valve insufficiency and malperfusion of the aortic branches. The latter can lead to acute myocardial infarction, cerebral ischemic insult, paraplegia, acute visceral or renal ischemia, or acute peripheral ischemia in the extremities (11–13).
Figure 2.
The 30-day mortality of conservatively and surgically treated patients with type A and type B aortic dissection (modified from [2])
Type B aortic dissection
The 30-day mortality rate of conservatively treated patients with type B aortic dissection is about 10%. In contrast, patients with complications that would previously have been managed by open surgery have a mortality rate of about 20% after two days and about 30% after a month (figure 2). Advanced age, shock, and malperfusion predispose to higher early mortality (2, 14, 15). In the long term, the weakened aortic wall can favor the occurrence of aneurysms, usually arising from the false lumen. Moreover, there may be redissection or progression of the original dissection, or the aorta may rupture.
Diagnostic strategy
Clinical symptoms
Acute aortic dissection is often accompanied by sudden stabbing back pain (53%), which sometimes follows the course of the aorta in craniocaudal direction (17%). If aortic dissection is suspected, the patient must immediately be taken to a cardiovascular surgery center with an emergency physician in attendance. The necessity of rapid action means that the diagnostic work-up on arrival at the hospital should be restricted to confirmation of the diagnosis of aortic dissection.
The symptoms of malperfusion depend on the involvement of branches of the aorta. Some patients with type A aortic dissection have syncope (13%) following pericardial tamponade with circulatory collapse (13%), or neurological symptoms (6.1%) if the carotid artery is affected. Development of cardiac insufficiency (8.8%) after the initial pain may be underlain by acute aortic valve insufficiency (44%), cardiac infarct from coronary dissection, or pericardial tamponade (13%). Peripheral ischemic syndromes (19%) result from occlusion of branching arteries by the dissection membrane.
Paraplegia can develop if a critical number of intercostal arteries are compromised by the dissection membrane. Occlusion of the celiac trunk and particularly the superior mesenteric artery must be suspected if sudden abdominal pain (30%) occurs. In the event of acute renal failure one should consider whether the dissection might be impairing flow in the renal arteries. Pulse defects (19%) or lateral differences in pulse quality are important because they indicate the extension of the aortic dissection and correlate with prognosis.
A diastolic murmur as expression of aortic valve insufficiency (44%) may indicate acute type A aortic dissection. Clinical signs of pericardial effusion or pericardial tamponade (13%), in the form of congested cervical veins, paradoxical pulse, and arterial hypotension (12%), point to type A aortic dissection and must be managed surgically immediately after confirmation of the diagnosis by means of transthoracic echocardiography or CT.
Aortic dissection should always be considered as a differential diagnosis in patients with unexplained syncope (13%), pain in the chest (61%) or back (53%), abdominal pain (30%), stroke (4.7%), or acute heart failure (6.6%). Particular suspicion should be aroused by lateral differences in pulse (15%) or signs of malperfusion (2, 15–17).
Diagnostic imaging
Thoracic aortic dissection may still be present even if a thoracic X-ray is normal, and ECG is not very helpful in this regard. Therefore, in the absence of specific chemical biomarkers rapid differential diagnosis by imaging is required. Confirmation of a membrane separating two lumina in the aorta proves the presence of aortic dissection. Transesophageal echocardiography, CT, and MRI are all swifter and more accurate than angiography (18). If aortic dissection is suspected, the patient should immediately be transferred to a center offering both surgical and interventional treatment.
Treatment
There is currently no level A or B evidence for the comparative merits of pharmacotherapy and conventional open surgery in acute type B aortic dissection. The same is true for conventional open surgery versus endovascular stent-graft implantation in this group of patients; thus, the consensus paper of the Endovascular Surgery Task Force of the Society of Thoracic Surgeons currently gives the best overview of treatment recommendations (e2). The final decision on the timing and type of aortic intervention falls to the treating physician. The situation is similar with regard to evidence for the treatment recommendations for type A aortic dissection (the German Society for Thoracic and Cardiovascular Surgery is currently drawing up guidelines). Since endovascular treatment with stent-graft implantation is not an option for this location, the investigations to date, mostly retrospective, have compared pharmacotherapy and conventional open surgery. These studies show a clear advantage for operative treatment over pharmacotherapy, particularly if the surgery takes place soon after the onset of symptoms. Thus, further prospective randomized trials on this topic would not be justifiable (2, 11, e3, e4).
Pharmacotherapy
Pain relief and systolic blood pressure of 100 to 120 mm Hg can be achieved with administration of morphine and beta-blockers in combination with vasodilators or ACE inhibitors. Contraindications must of course be taken into account in the choice of antihypertensive agents. Monotherapy rarely suffices; massive hypertension requires the combination of several antihypertensives.
Surgery
The surgical treatment of type A aortic dissection has the goal of preventing a rupture or the development of pericardial tamponade and must be performed without delay. The occurrence of acute aortic valve or coronary insufficiency in confirmed aortic dissection permits no postponement of surgery (box 2).
Box 2. Treatment of aortic dissection.
-
Surgical treatment
Type A aortic dissection
-
Type B aortic dissection with complications
Retrograde extension into the ascending aorta
Aortic dissection in Marfan syndrome
Development of a thoracoabdominal aneurysm
-
Endovascular treatment
-
Complicated type B aortic dissection
(eg, malperfusion)
-
-
Conservative treatment
Uncomplicated type B aortic dissection
Modified from (e8)
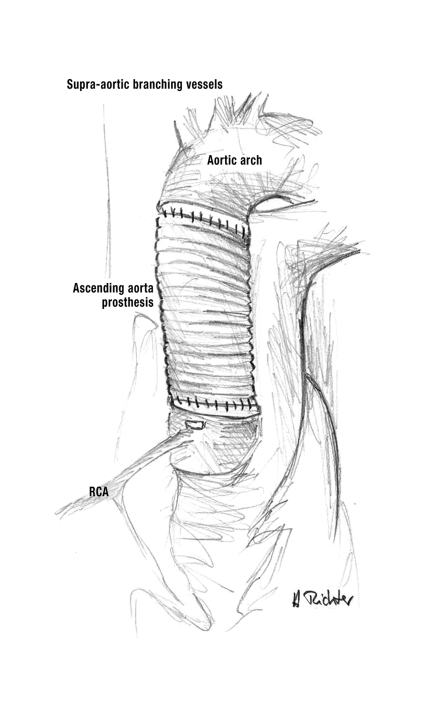
Operations on the ascending aorta—In type A aortic dissection the primary aim of surgery is to furnish the affected segment of the aorta with a vascular prosthesis or with a composite graft bearing a valve (19). If only the ascending aorta is involved, without anuloaortic ectasia, and if the aortic valve is sufficient and not stenosed, a supracoronary graft can be inserted. The dissecting aortic wall must first be repaired, then a vascular prosthesis of appropriate diameter is interposed above the coronary ostia (figure 3). If the aortic valve is sclerotic and stenosed, it can be replaced by a biological or mechanical valve. If the aortic valve is stenosed and there is also ectasia of the aortic root, a valve-bearing composite graft can be interposed. In this case the composite graft is anchored in the anulus of the aortic valve, two holes are made in the prosthesis at the level of the two coronary ostia, and the coronary ostia are reimplanted into the windowed portion of the graft.
Figure 3.
Supracoronary ascending aorta prosthesis.
RCA, right coronary artery
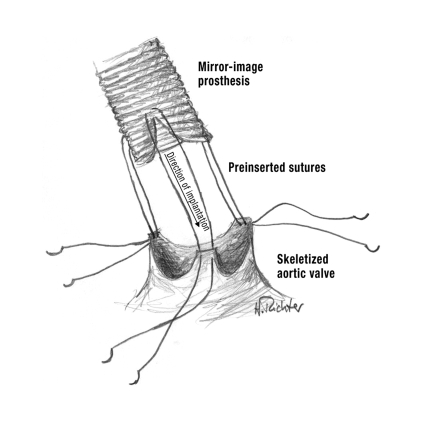
If the semilunar cusps of the aortic valve are no longer capable of coaptation owing to aortic root ectasia and the aortic valve has become insufficient, one should set out to perform a valve-sparing aortic root reconstruction according to Yacoub or David. These operations have a lower long-term complication rate than procedures involving replacement of the aortic valve (20–22). It is advantageous that the physiological function of the aortic valve is retained and that oral anticoagulation, with its adverse effects, is unnecessary.
Yacoub described the technique of remodeling the aortic root as follows: The aortic wall in the region of the three aortic sinuses of valsalvae is cut back to the transition zone between aortic valve and left ventricle. Next, the defect so created is covered by a mirror-image Dacron prosthesis (figure 4). The coronary arteries are then reimplanted orthotopically into the prosthetic aortic graft (20, 21, 23). The procedure described by David starts with skeletization of the native aortic valve, which is then reattached to the internal surface of a graft fitted over the aortic root (reimplantation technique). In this procedure too, the coronary arteries are reimplanted orthotopically (22, 23).
Figure 4.
Valve-sparing aortic root reconstruction according to Yacoub
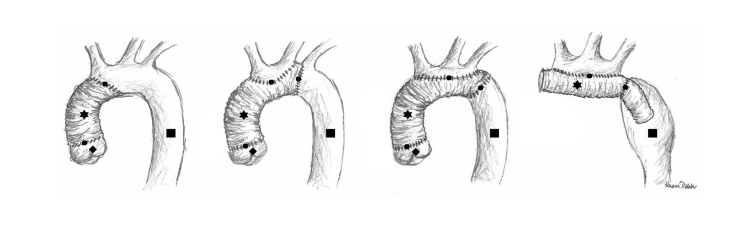
Operations on the aortic arch—Most type A aortic dissections affect the ascending aorta and the aortic arch. The supra-aortic vessels may also be involved. The aortic arch should be explored for further tears. If none are found, it suffices to fashion a distal anastomosis between the graft and all layers of the wall of the distal ascending aorta. If further lacerations are present in the aortic arch, however, partial or complete aortic arch replacement is required. Depending on the extent of dissection, the operation of choice may be proximal, partial, or total replacement of the aortic arch, or the elephant trunk technique (figure 5). Often the anastomosis can be laid diagonally across the arch, avoiding the need for separate reattachment of the supra-aortic vessels.
Figure 5.
Aortic arch replacement. From left to right: implant replacing supracoronary ascending aorta and proximal portion of arch; supracoronary ascending aorta and partial arch replacement; supracoronary ascending aorta and total arch replacement; elephant trunk technique.
Star = vascular prosthesis; circle = suture line; diamond = aortic root with three aortic sinuses of valsalvae and native aortic valve (coronary arteries not depicted); ssquare = native aorta
If total replacement of the arch is necessary, the distal anastomosis is located in the proximal descending aorta. An oval segment of the convex surface of the prosthetic aortic graft is cut away and the supra-aortic vessels are all reimplanted together (figure 5). If the whole length of the descending aorta is affected by the dissection, the elephant trunk technique is recommended. On primary arch replacement the distal end of the aortic arch graft is advanced into the descending aorta and left to float freely (figure 5). In a later operation (descending or thoracoabdominal aortic replacement), this prosthesis is then connected to a second prosthesis.
Treatment of type B aortic dissection
Patients with uncomplicated acute type B aortic dissection require close monitoring in the intensive care unit (box 2). Control of blood pressure is important. Rapid interventional or, seldom, surgical treatment may be considered in the case of acute complications of type B aortic dissection. The most frequent immediate complications of type B aortic dissection are visceral ischemia, renal ischemia, and ischemia of the extremities.
Earlier it was usual to perform surgical fenestration. Following laparotomy, the dissection membrane in the infrarenal aorta was partially excised, achieving communication between the true and the false lumen and assuring perfusion of all branches of the aorta. On the same principle, the dissection membrane can be perforated by interventional means. However, there is no evidence that either technique affects the prognosis.
Endovascular stent-graft implantation
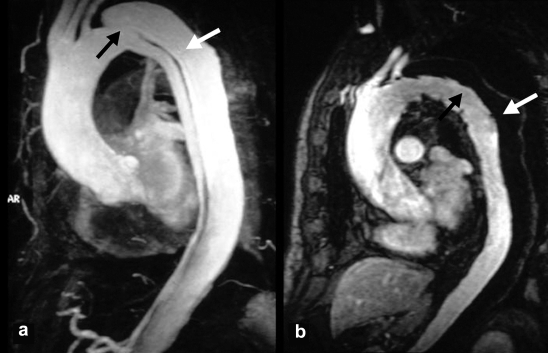
Today endovascular placement of stent-grafts is the preferred means of dealing with distal malperfusion or imminent aortic rupture in acute type B aortic dissection with complications (box 2) (24, 25, e5). Through a surgical incision, a synthetic covered stent is advanced via the common femoral artery into the aorta to cover the entry tear (figure 6). This improves the perfusion of the true lumen and the side branches immediately. The aim of this procedure is to eliminate compression of the true lumen by the false lumen, seal the proximal entry tear, and thus initiate thrombosis of the false lumen. In about two thirds of cases the dissected aorta can be reconstructed by this means (figure 6).
Figure 6.
a) MR angiogram showing a typical type B aortic dissection with a large entry tear (black arrow) and an extensive perfused false lumen (white arrow).
b) Induced thrombosis of the false lumen (white arrow) after sealing of the entry tear by a stent-graft; good perfusion of the true lumen (black arrow)
A further proposed use for stent-grafts is prevention of late complications of subacute or chronic type B aortic dissections, specifically aneurysm formation in the false lumen (e6). There is no evidence for the efficacy of this approach, so further randomized prospective trials are needed to prove the benefit of stent-graft treatment in this indication (e2). In view of the absence of controlled efficacy data, the Society of Thoracic Surgeons’ Endovascular Surgery Task Force has formulated the following treatment recommendations in a consensus paper:
"…stent-grafting as a therapeutic option for high surgical risk patients with subacute or chronic aortic dissection may be considered for those who have a patent false lumen and an identifiable, proximal entry tear that can be covered by stent-graft implantation in association with (1) a maximal thoracic aorta diameter greater than 5.5 cm, (2) documented increase of aortic diameter of more than 1.0 cm within 1 year, (3) resistant hypertension despite antihypertensive combination therapy associated with a small true lumen or renal malperfusion, or (4) recurrent episodes of chest/back pain that cannot be attributed to other causes. In younger, healthier patients, open surgical repair should be considered" (e2).
Endovascular procedures require a specialized team of interventionists and surgeons. With experience, the 30-day mortality can be reduced to less than 6%. Provided the stent-graft does not exceed 20 cm in length, paraplegia occurs in no more than 1% of cases and the one-year survival rate is over 90% (25, e5, e7).
Postoperative care
The long-term care after successful initial treatment is based on the appreciation of aortic dissection as a chronic disease that may, in the course of time, lead to vascular complications such as progression of the dissection, aneurysm formation, or rupture. The diameter of the aorta, the biological age, untreated arterial hypertension, and a perfused false lumen are all risk factors for these events. Particularly patients with MFS or EDS are in danger of vascular complications. Therefore, all patients who have had an aortic dissection must receive long-term antihypertensive treatment.
A crucial component of the pharmacological treatment is effective beta-blockade to lower the blood pressure to under 120/80 mm Hg. Especially in young patients with MFS, this has been found to improve the prognosis. Another important aspect of follow-up is regular cross-sectional imaging (CT or MRI). The reason for this is that one third of all patients who survive an acute aortic dissection either suffer progression of the disease or aortic rupture, or require further surgical or interventional treatment, within 5 years. Follow-up is recommended 3 and 9 months after onset and then at annual intervals. The aorta must always be depicted in its entirety, and comparison with previous findings is indispensable. Particular attention must be paid to progression of residual dissection, changes in aortic diameter, evidence of a contained aortic rupture, newly arisen intramural hematomas, and the behavior of the anastomoses.
Patients with reimplanted coronary arteries should undergo ergometric monitoring at regular intervals, and if there is any clinical suspicion of stenosis in the vicinity of the coronary anastomoses then coronary angiography should be performed. Patients with reconstructed valves should be submitted to annual echocardiography.
If the aortic diameter increases to more than 5.5 cm, endovascular or open surgical reconstruction is indicated (e2, e8). In patients with MFS the best results are achieved with open surgical reconstruction when the aorta reaches a diameter of 4 to 4.5 cm (e9).
Acknowledgments
The authors thank Hanna Richter for the illustrations (figures 1, 3, 4, and 5).
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Prof. Nienaber has received lecture fees and reimbursement of travel costs from Medtronic and COOK Medical.
Dr. Rehders has received lecture fees and reimbursement of travel costs from Medtronic.
PD Dr. Weigang, Prof. Ince, Prof Vahl, and Prof. Beyersdorf declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.von Kodolitsch Y, Baumgart D, Eggebrecht H, et al. Das akute Aortensyndrom. Dtsch Arztebl. 2003;100(6):A 326–A 333. [Google Scholar]
- 2.Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 3.Reed D, Reed C, Stemmermann G, Hayashi T. Are aortic aneurysms caused by atherosclerosis? Circulation. 1992;85:205–211. doi: 10.1161/01.cir.85.1.205. [DOI] [PubMed] [Google Scholar]
- 4.Stefanadis CI, Karayannacos PE, Boudoulas HK, et al. Medial necrosis and acute alterations in aortic distensibility following removal of the vasa vasorum of canine ascending aorta. Cardiovasc Res. 1993;27:951–956. doi: 10.1093/cvr/27.6.951. [DOI] [PubMed] [Google Scholar]
- 5.Weigang E, Chang X, Munk-Schulenburg S, et al. Actual management of patients with familial ascending aortic aneurysms and type-A aortic dissections. Thorac Cardiovasc Surg. 2007;55:19–23. doi: 10.1055/s-2006-924575. [DOI] [PubMed] [Google Scholar]
- 6.Weigang E, Chang X, Ghanem N, et al. Evaluation of three different measurement methods for dural ectasia in Marfan syndrome. Clin Radiol. 2006;61:969–976. doi: 10.1016/j.crad.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Steinmann B, Royce P, Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce PM, Steinmann B, editors. Connective tissue and its heritable disorders. New York: Wiley-Liss Inc; 1993. pp. 351–407. [Google Scholar]
- 8.Markl M, Harloff A, Bley TA, et al. Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging. 2007;25:824–831. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 9.Nienaber CA, Sievers HH. Intramural hematoma in acute aortic syndrome - more than one variant of dissection? Circulation. 2002;106:284–285. doi: 10.1161/01.cir.0000023453.90533.82. [DOI] [PubMed] [Google Scholar]
- 10.Ganaha F, Miller DC, Sugimoto K, et al. The prognosis of aortic intramural hematoma with and without penetrating atherosclerose ulcer: a clinical and radiological analysis. Circulation. 2002;106:342–348. doi: 10.1161/01.cir.0000022164.26075.5a. [DOI] [PubMed] [Google Scholar]
- 11.Heinemann MK, Ziemer G. Krankheiten der thorakalen Aorta. In: Hombach V, editor. Interventionelle Kardiologie, Angiologie und Kardiovaskularchirurgie: Technik, Klinik, Therapie. Stuttgart, New York: Schattauer; 2001. pp. 649–665. [Google Scholar]
- 12.Laas J, Albes J. Aortendissektion. In: Alexander K, editor. Gefäßkrank-heiten. München, Wien, Baltimore: Urban & Schwarzenberg; 1994. pp. 579–585. [Google Scholar]
- 13.Richter GM, Allenberg JR, Schumacher H, Hansmann J, Vahl C, Hagl S. Aortic dissection - when operative treatment, when endoluminal therapy? Radiologe. 2001;41:660–667. doi: 10.1007/s001170170115. [DOI] [PubMed] [Google Scholar]
- 14.Von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160:2977–2982. doi: 10.1001/archinte.160.19.2977. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era - Lessons learned from the International Registry of Aortic Dissection (IRAD) Circulation. 2003;108:II312–II317. doi: 10.1161/01.cir.0000087386.07204.09. [DOI] [PubMed] [Google Scholar]
- 16.Nallamothu BK, Mehta RH, Saint S, et al. Syncope in acute aortic dissection: Diagnostic, prognostic and clinical implications. Am J Med. 2002;113:468–471. doi: 10.1016/s0002-9343(02)01254-8. [DOI] [PubMed] [Google Scholar]
- 17.Park SW, Hutchison S, Mehta RH, et al. Association of painless acute aortic dissection with increased mortality. Mayo Clin Proc. 2004;79:1252–1257. doi: 10.4065/79.10.1252. [DOI] [PubMed] [Google Scholar]
- 18.Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328:1–9. doi: 10.1056/NEJM199301073280101. [DOI] [PubMed] [Google Scholar]
- 19.Borst HG. Aneurysma und Dissektion der Aorta ascendens und des Aortenbogens. In: Borst HG, Klinner W, Oelert H, editors. Herzchirurgie: Die Eingriffe am Herzen und an den herznahen Gefäßen. Berlin, Heidelberg, New York: Springer; 1991. pp. 434–463. [Google Scholar]
- 20.Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair - from structure to function: part I. Circulation. 2004;109:942–950. doi: 10.1161/01.CIR.0000115633.19829.5E. [DOI] [PubMed] [Google Scholar]
- 21.Yacoub MH, Cohn LH. Novel approaches to cardiac valve repair - from structure to function: part II. Circulation. 2004;109:1064–1072. doi: 10.1161/01.CIR.0000115634.66549.4D. [DOI] [PubMed] [Google Scholar]
- 22.David TE, Ivanov J, Armstrong S, Feindel CM, Webb GD. Aortic valve-sparing operations in patients with aneurysms of the aortic root or ascending aorta. Ann Thorac Surg. 2002;74:1758–1761. doi: 10.1016/s0003-4975(02)04135-8. [DOI] [PubMed] [Google Scholar]
- 23.Schafers HJ, Boehm M. Ursachen und Behandlungsstrategien der Aortenklappeninsuffizienz. Dtsch Arztebl. 2004;101(37):A2475–A2479. [Google Scholar]
- 24.Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340:1539–1545. doi: 10.1056/NEJM199905203402003. [DOI] [PubMed] [Google Scholar]
- 25.Eggebrecht H, Nienaber CA, Neuhäuser M, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27:489–498. doi: 10.1093/eurheartj/ehi493. [DOI] [PubMed] [Google Scholar]
- e1.Arslan-Kirchner M, von Kodolitsch Y, Schmidtke J. Genetische Diagnostik beim Marfan-Syndrom und verwandten Erkrankungen: Bedeutung des klinischen Managements. Dtsch Arztebl. 2008;105(27):483–491. doi: 10.3238/arztebl.2008.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Svensson LG, Kouchoukos NT, Miller DC, et al. Society of Thoracic Surgeons, Endovascular Surgery Task Force. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
- e3.Anagnostopoulos CE, Prabhakar MJ, Kittle CF. Aortic dissection and dissecting aneurysms. Am J Cardiol. 1972;30:263–273. doi: 10.1016/0002-9149(72)90070-7. [DOI] [PubMed] [Google Scholar]
- e4.Kirklin JW, Barratt-Boyes BG. Cardiac surgery. Morphology, diagnostic criteria, natural history, techniques, results and indications. 2nd edition. Vol. 2. New York: Churchill Livingstone; 1993. [Google Scholar]
- e5.Vogl TJ, Moritz A, Figuth HG, Doss M, Thalhammer A, Balzer JO. Endovaskuläre Therapie von thorakalen Aortenläsionen. Dtsch Arztebl. 2005;102(14):A987–A992. [Google Scholar]
- e6.Nienaber CA, Zannetti S, Barbieri B, Kische S, Schareck W, Rehders TC INSTEAD study collaborators. INvestigation of STEnt grafts in patients with type B Aortic Dissection: design of the INSTEAD trial - a prospective, multi-center, European randomized trial. Am Heart J. 2005;149:592–599. doi: 10.1016/j.ahj.2004.05.060. [DOI] [PubMed] [Google Scholar]
- e7.Eggebrecht H, Herold U, Kuhnt O, et al. Endovascular stent-graft treatment of aortic dissection: determinants of post-interventional outcome. Eur Heart J. 2005;26:489–497. doi: 10.1093/eurheartj/ehi099. [DOI] [PubMed] [Google Scholar]
- e8.Nienaber CA, Eagle KA. Aortic dissection: New Frontiers in diagnosis and management: Part II: Therapeutic management and follow-up. Circulation. 2003;108:772–778. doi: 10.1161/01.CIR.0000087400.48663.19. [DOI] [PubMed] [Google Scholar]
- e9.Gott VL, Greene PS, Alejo DE, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med. 1999;340:1307–1313. doi: 10.1056/NEJM199904293401702. [DOI] [PubMed] [Google Scholar]
- e10.Nienaber CA, Eagle KA. Aortic dissection: New frontiers in diagnosis and management: Part I: From etiology to diagnostic strategies. Circulation. 2003;108:628–635. doi: 10.1161/01.CIR.0000087009.16755.E4. [DOI] [PubMed] [Google Scholar]