Abstract
Introduction
The last few years have seen the rapid development of new image-guided interventions for the local treatment of malignant tumors. The goal of this article is to provide an overview of the techniques that are most commonly used today in interventional oncology.
Methods
Selective literature review on the current state of image-guided interventional techniques for local tumor therapy.
Results
While surgery, radiation oncology, and systemic chemotherapy are still the three main pillars of tumor therapy, a broad range of minimally invasive, image-guided techniques for local tumor treatment is now available. These may be categorized as percutaneous injection of a toxic substance, transarterial embolization, thermal ablation, and internal radiotherapy. The choice of treatment depends on the type, location, and size of tumor. The greatest amount of clinical experience to date has been gathered in the treatment of primary and secondary hepatic malignancy, but there are interventional treatment options for virtually all regions of the body. At present, the utility of this form of treatment is limited for very large or multiple tumors; novel therapeutic options for these situations are now being studied.
Discussion
The outcome of treatment depends on a judicious determination of the indication for it. The indication should be established by interdisciplinary consensus after all treatment options have been considered.
Keywords: surgical treatment, minimally invasive treatment, cancer treatment, adjuvant treatment, malignant melanoma
Malignant neoplasms are the second most common cause of death in Germany; every fourth German dies of a malignant tumor (e1). Surgery, radiotherapy, and systemic chemotherapy are the established cornerstones of tumor treatment. Minimally invasive, image-guided procedures have also come into widespread use in recent years. These techniques, previously largely focused on the devascularization of tumor vessels, have now been extended to include a large number of direct ablative methods as well as methods to reduce pain and stabilize bone. The term "interventional oncology" has come into use in the English-speaking world for these types of procedures (e2). However, except for the treatment of hepatocellular carcinoma (HCC), there are no uniform guidelines to date regarding the appropriate indications for these procedures. The goal of this article, based on a selective review of the literature, is to provide a summary of the local ablative techniques that are most widely used at present, and of their results.
Techniques and Indications
The local interventional treatments can be classified into four groups according to their mechanisms of action (table):
TABLE. Overview of currently available image-guided interventional techniques for local tumor therapy and their possible indications (source: the authors).
| Technique | Organ | Indications* | |
| Percutaneous direct injection | PEI/PAI | Liver | HCC ≤ 3 cm (4) |
| Other | Decision in individual cases (obsolete) | ||
| Transarterial embolization | TACE/TAE | Liver | Inoperable HCC (4), cholangiocellular carcinoma, neuroendocrine tumors, uveal melanoma (colorectal carcinoma, carcinoma of the breast) |
| Bones | (Preoperative) | ||
| Kidney | Tumor hemorrhage, before thermoablation, (preoperative) | ||
| Other | Decision in individual cases | ||
| Thermoablation | RFA | Liver | Inoperable HCC with ≤ 3 foci (4), multifocal metastases ≤ 3.5 cm, unifocal metastasis ≤ 5 cm (e85) |
| Lung | ≤ 3 metastases per lung, all of which are ≤ 3 cm (inoperable non-small-cell lung cancer) | ||
| Kidney | Tumor ≤ 5 cm in a single kidney, von Hippel-Lindau disease, multiple tumors | ||
| Bones | Osteoid osteoma, symptomatic bony metastases | ||
| Other | Decision in individual cases/experimental | ||
| LITT | Liver | See RFA | |
| Other | Decision in individual cases/experimental | ||
| Microwave ablation | All | Experimental | |
| Cryotherapy | All | Experimental | |
| Focused ultrasound | All | Experimental | |
| Internal radiotherapy | SIRT | Liver | After failure or exclusion of all other treatment options (salvage) |
| Interstitial brachytherapy | All | Experimental | |
*Indications in parentheses are currently debated.
PAI, percutaneous acetic acid injection; TAE, transarterial embolization; PEI, percutaneous ethanol injection; HCC, hepatocellular carcinoma; TACE, transarterial chomoembolization; RFA, radiofrequency ablation; LITT, laser-induced thermotherapy; SIRT, selective internal radiotherapy
Percutaneous direct injection of toxic substances
Transarterial embolization
Percutaneous delivery of thermal energy
Internal radiotherapy.
A further mode of treatment is image-guided stereotactic radiotherapy with modern methods of respiratory synchronization (respiratory gating and tracking). This is not, strictly speaking, an interventional treatment and therefore will not be discussed further in this article (e3).
Percutaneous direct injection
Direct percutaneous ethanol injection (PEI) is the oldest percutaneous method of treating tumors that remains in use (e4). A fine needle is positioned in the tumor under image guidance and pure ethanol is injected through it (figure 1). This results in dehydration and necrosis of the tumor cells. For complete tumor ablation, all parts of the tumor must be reached. This can be achieved either by multiple repositioning of a single needle or by the use of multiple needles. A high dose of alcohol can be given in one treatment session, or else the tumor can be treated in multiple sessions spaced
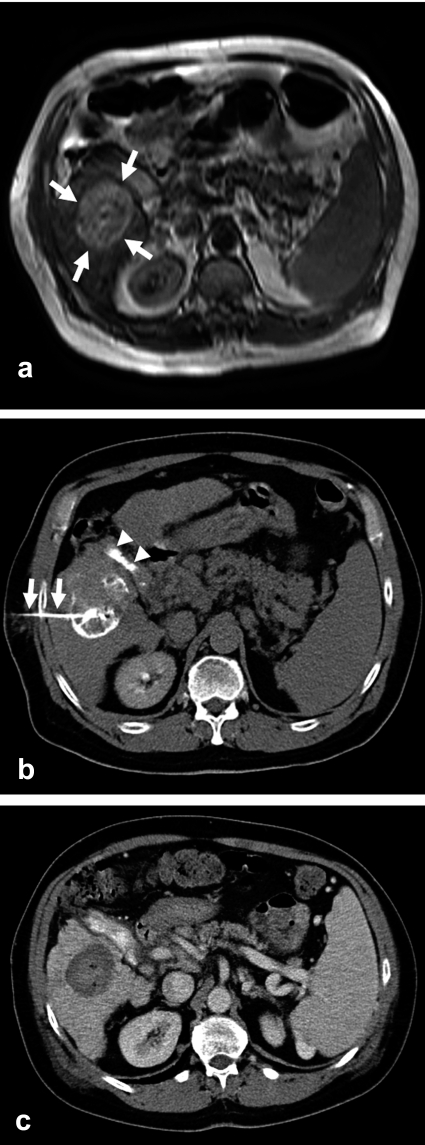
Figure 1.
The MRI scan of this 56-year-old man with longstanding hepatic cirrhosis due to hepatitis B and histologically confirmed hepatocellular carcinoma (HCC) reveals, in segments V/VI, a 4.6 cm sized tumor with nodular growth (a; arrows). The CT after tumor puncture with a dedicated 20 gauge needle with side holes (b; arrows) and injection of a mixture of ethanol and contrast medium shows a good distribution of contrast throughout the tumor. Gallstones are also seen (b;arrowheads). A CT obtained three months later shows the fully necrotic tumor as a parenchymal defect that does not take up contrast medium (c).
several days apart with a lower dose of alcohol per session. Acetic acid can also be used instead of alcohol. Because the distribution of injected fluid in the hepatic parenchyma is unpredictable, this technique is only applicable to (pseudo-)encapsulated tumors. It has not been found to be useful in the treatment of tumors that grow by infiltration, such as metastases.
Studies on the treatment of histologically verified hepatocellular carcinoma (HCC) with PEI have shown good local tumor control. Total tumor necrosis is regularly achieved in tumors measuring 2 cm in diameter or less (e5) and in two-thirds of tumors measuring 3cm (e6, e7). The median survival rates after 3 and 5 years are 50% to 80% and 28% to 48%, respectively (1, 2). The use of acetic acid seems to be advantageous for local effectiveness (e8, 3). PEI is not as good as thermoablation with respect to local tumor control and survival (e9) but has a lower complication rate (e10). Current guidelines support the use of PEI to treat up to 3 foci of HCC measuring up to 3 cm and when comorbidity contraindicates surgical treatment (4). PEI is currently undergoing a renaissance, as it is being used in combination with transarterial chemoembolization (TACE) or radiofrequency ablation (RFA) with a resulting prolongation of survival (5, 6).
Typical adverse effects include local pain, post-interventional fever, and transient alcohol intoxication. The more common complications are hemorrhage and tumor cell seeding, with overall complication rates ranging from 1.7% to 3.2% (1, e11).
Transarterial Embolization
In transarterial chemoembolization (TACE), the administration of chemotherapeutic drugs is combined with embolization. In comparison to arterial chemoperfusion, TACE results in a higher concentration of active drug in the tumor, in addition to tumor ischemia. Residual levels of the active drug can be detected in the tumor up to one month after treatment (e12, e13).
Guidelines support the routine use of TACE in the treatment of advanced HCC (figure 2) (4). Another accepted indication is for tumor control during the waiting period before liver transplantation (e14). In a randomized controlled study, the 1- and 2-year survival rates after TACE for HCC were 82% and 63%, significantly better than the results obtained with conservative treatment (63% and 27%, respectively) (7). These positive findings have been confirmed in meta-analyses (8, 9). Nodular tumors have a more favorable prognosis than diffusely infiltrative ones when treated with TACE (e15). Repetition of the procedure improves survival (e16). Transarterial embolization (TAE) without any chemotherapeutic agent is also occasionally performed (e17). Although randomized controlled trials have not yet shown any statistically significant difference between TACE and TAE with respect to survival, a trend toward prolonged overall survival has been found after TACE (9).
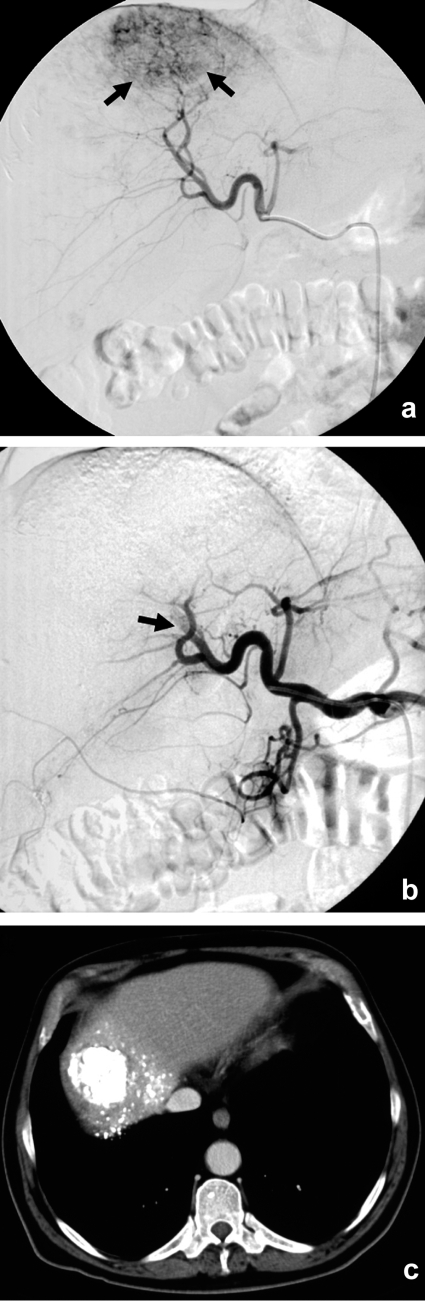
Figure 2.
The angiogram of this 63-year-old man with histologically confirmed hepatocellular carcinoma shows a 5.6 cm sized, markedly hypervascularized tumor in the right hepatic lobe (a; arrows). The tumor is no longer seen in a second angiogram performed immediately after selective transarterial chemoembolization (TACE) of the tumor-bearing hepatic segment with a mixture of doxorubicin and an oil-based contrast medium. The feeding vessel of the tumor is occluded (b; arrow). A noncontrast CT obtained the following day confirms successful embolization with good coverage of the tumor by radio-opaque embolization material (c).
Positive results are available for the treatment of hepatic metastases of neuroendocrine tumors (e18), uveal melanoma (e19), carcinoma of the breast (e20), and cholangiocellular carcinoma (e21). On the other hand, TACE appears to be indicated for the treatment of metastases of colorectal carcinoma only in patients who have already been maximally treated with chemotherapy (e22, e23).
Drug-releasing particles for use in TACE are currently being clinically tested (e24). Initial data regarding the use of doxorubicin-releasing particles to treat HCC show a radiological treatment response in 75% of patients (e25). Initial trials with irinotecan-releasing particles are now being performed for the treatment of hepatic metastases of colorectal carcinoma (e26).
In principle, embolization can be performed in any other area of the body as well. Particularly in the kidneys and bones, this technique can be performed preoperatively or as a palliative measure (e27–e29). There is some debate about the results at present, however (e30), so the use of embolization in these ways remains restricted to selected, individual cases.
Thermoablation
Hyperthermic ablation techniques have come to be used much more widely in recent years. These techniques involve the deposition of thermal energy (ie, heat) in tumor tissue under image guidance. Local heating of tissue to 60B0C to 100B0C causes protein denaturation and coagulation necrosis.
Radiofrequency ablation
In radiofrequency ablation (RFA), a high-frequency alternating current is applied to the tumor tissue through probes placed under image guidance. The current causes movement of ions, resulting in frictional heat that spreads through the tissue in a pattern determined by the design of the applicator (e31). Thus, the shape and size of the heat-induced necrosis can be controlled (e31, e32). As in all thermal techniques, local cooling effects occur, particularly at the periphery of the heated zone (e33). Therefore, tumor tissue bordering on large vessels may be inadequately heated, so that there is a locally elevated risk of recurrence. The latter risk can be reduced by the performance of embolization before RFA. Since the use of RFA for tumor ablation in animal experiments was first described in 1992, the technique has developed into the currently most commonly used thermal ablation technique (e34).
RFA was initially established as a useful method of treating inoperable primary malignancies of the liver. The indications for the procedure were rapidly extended. It is now also used to bridge the waiting period before liver transplantation, and even, in some cases, as a competing alternative to surgery. Guidelines recommend the use of RFA (just as they recommend the use of PEI) to treat up to 3 HCC foci measuring up to 3 cm in diameter when comorbid problems contraindicate surgical treatment (4). The 1-, 3-, and 5-year survival times are 87% to 97%, 51% to 67%, and 27% to 54% (10, 11, e35). Long-term survival depends on an initially complete ablation with an adequate safety margin around it (e36). The former improves 5-year survival from 27% to 42% (e35). In randomized controlled studies, the results of RFA did not differ significantly from those of surgery for small (<5 cm) HCC tumors: the 1-year survival rates were 94% to 96% vs 91% to 93%, and the3-year survival rates were 71% to 87% vs 73% to 86% (e37, e38).
Because the liver is often the first and only site of metastasis of colorectal tumors, local therapy has the potential to prolong life. In one study, RFA treatment of inoperable patients with fewer than 5 hepatic metastases measuring less than 5 cm in diameter and without any extrahepatic metastases resulted in a 5-year survival rate of 30% (12, 13, e39). Important parameters influencing the success of treatment include the pre-interventional level of carcinoembryonic antigen (CEA) and the number and size of metastases (e40). Positive results have also been reported for the treatment of neuroendocrine tumors with RFA. The rate of local tumor control has been reported to be 75% to 90% after 21 to 38 months of follow-up (e41, e42).
The complication rate of hepatic RFA is 2% to 10%, with a mortality of 0.09% to 1.4% (e43, e44). The more common complications are hemorrhage, pleural effusion, and hepatic abscess. The major drawback of RFA at present is the lack of prospective, randomized clinical trials with clearly defined patient groups and study protocols.
After the liver, the lung is the second most common site for metastases (e45). Pulmonary RFA has been in increasing use for local tumor therapy since its initial publication in 2000 (e46). When RFA, as opposed to surgical resection, is used to treat primary lung tumors, mediastinal lymphadenectomy cannot be performed. It follows that curative RFA is only possible if there is no lymphogenous metastatic disease (N0) and if the tumor spread is no more than local (stage Ia/Ib). Thus, RFA should be used to treat a primary lung tumor only if the patient’s impaired pulmonary function or comorbid conditions contraindicate surgery. The reported survival rates after RFA in this patient group with an a priori worse prognosis are 78% at 1 year, 36% at 3 years, and 27% at 5 years (14). Another study yielded comparable results, with an overall survival of 71% and a tumor-free survival of 34% at 18 months (e47).
The most common indication for pulmonary RFA is the treatment of pulmonary metastases (figure 3). Reported survival rates of RFA for pulmonary metastases of colorectal carcinoma are 85% to 87% at 1 year and 46% to 57% at 3 years, with a median survival time of 27.5 to 33 months (14, e48, e49). Repeated procedures after incomplete ablation are thought to carry a worse prognosis (e49).
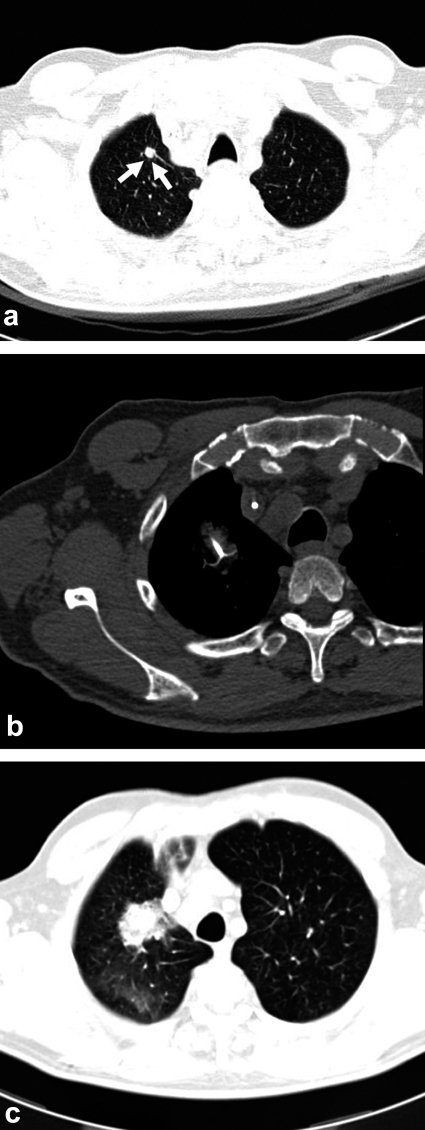
Figure 3.
CT images of a 72-year-old man with hepatic and pulmonary metastases of colorectal carcinoma. After repeated radiofrequency ablation (RFA) of the liver with local tumor control, a new pulmonary metastasis is found in the right upper lobe (a; arrow). A shielded electrode is placed in the tumor under CT guidance for RFA (b). The post-interventional CT shows an infiltrate surrounding the tumor, documenting the success of treatment. Because of the intratumoral hemorrhage that is typically produced by the intervention, the tumor appears larger than it was before treatment for the first six months after RFA (c).
The most common complications are fever and pneumothorax (e50). The major drawback of pulmonary RFA is the lack of clear indications, so that the decision to perform this procedure must be made on an interdisciplinary basis and only in selected, individual cases.
Many studies have demonstrated the effectiveness of RFA in the treatment of renal tumors (15, 16, e51). Typical indications are multiple tumors, single kidneys, or von Hippel-Lindau disease. Exophytic tumors and those measuring 3 cm or less can regularly be totally ablated (16). Repetition of the procedure may be necessary for local control of 3-cm tumors. Repeatability without the problem of a more difficult surgical approach is a major advantage of RFA, particularly when multiple interventions can be expected to be needed, as in von Hippel-Lindau disease. The procedure can be made more effective by a TAE performed beforehand; a combination of embolization and RFA is recommended for 3-cm renal tumors (e52). Residual and recurrent tumors were encountered more frequently in earlier clinical series because of technical inadequacies; in recent series, local tumor control was achieved in nearly all cases (15, e53, e54). This is confirmed by initial long-term results after more than 4 years of follow-up without any recurrence of tumor (17).
RFA has also become an important means of treating symptomatic bony tumors and indeed the treatment of choice for benign osteoid osteoma (e55). For bone metastases, RFA is an effective method of relieving pain with palliative intent (e56). In particular, RFA combined with osteoplasty is a minimally invasive way of relieving pain rapidly while simultaneously stabilizing the bone (e57, 58).
RFA has also been reported in a few cases of tumors of the adrenal gland, pancreas, lymph nodes, thyroid gland, and breast (e59–e63). These uses are still considered experimental.
Laser-induced thermotherapy
In laser-induced thermotherapy (LITT), thermal energy is applied to a tumor in the form of laser light delivered through interventionally inserted light guides. Photon absorption generates heat within the tissue that then spreads by conduction. When large tumors are treated, the laser applicator must be repositioned, or else multiple applicators must be used. The intervention can be guided by ultrasonography, computerized tomography (CT), or magnetic resonance imaging (MRI). MRI offers the additional opportunity to monitor the temperature in the treated tissue continuously and noninvasively (e64, e65).
The successful use of LITT has been shown in many different organ systems (e66–e68). The reported survival rates after LITT for hepatic metastases of colorectal tumors are 94% at 1 year, 56% at 3 years, and 37% at 5years (18). Comparable figures have been published for LITT for hepatic metastases of breast carcinoma: 96%, 63%, and 41% (e69). There are no available studies comparing LITT to surgical treatment. Despite these favorable results, LITT is used in no more than a few centers because of the sophisticated equipment that it requires and its correspondingly high cost.
Other thermal ablation techniques
Microwave ablation and focused ultrasound are two further hyperthermic ablation techniques (19, 20). Microwave ablation is still in an early phase of clinical application; to date, it is used mainly to treat small HCC tumors (e70, e71). Focused ultrasound, although it has yielded encouraging results in the treatment of breast carcinoma, HCC, and osteosarcoma (20, e72), is now used only to treat prostate cancer, and only in rare cases (e73). Percutaneous cryotherapy (a hypothermic technique) is now used almost exclusively to ablate small renal tumors (21).
Internal radiotherapy
Another class of interventional tumor therapies involves radiotherapy delivered from within the body. Selective internal radiotherapy (SIRT) and image-guided interstitial brachytherapy are treatments of this type. Both of these are among the newer treatment methods for which only phase I and phase II trials have been published to date.
Selective internal radiotherapy
In selective internal radiotherapy (SIRT), arterial microembolization is combined with high-dose interstitial radiation therapy. Particles bearing radioactive 90yttrium are applied for this purpose by way of the hepatic artery (22, 23). A meticulous preinterventional diagnostic evaluation is essential for the success of treatment: this includes invasive catheter angiography of the hepatic arteries as well as scintigraphy after the intra-arterial application of 99mtechnetium-labeled albumin particles for quantitative prediction of the distribution of the 90yttrium spheres and of the hepatopulmonary shunt volume. The applied radiation dose of no more than 3 GBq is individually adapted to the tumor volume and the hepatopulmonary shunt volume (e74). Typical adverse effects and complications of SIRT include nausea,vomiting, pain, and fever. A few cases of hyperbilirubinemia, duodenal and gastric ulcers, and necrosis of the peripheral biliary ducts have been reported (e75, e76).
SIRT is considered to be indicated for the treatment of primary or secondary hepatic tumors for which all other treatment options have failed or cannot be applied. The presence of extrahepatic tumor is considered to be a relative contraindication. Also, SIRT is occasionally used when hepatic involvement is held to be a prognosis-limiting factor (23). Initial data show that this form of treatment can delay local tumor progression for 6 to 19 months (23, e75, e77). Chemotherapy with 5-fluorouracil and leucovorin combined with SIRT hasbeen found both to delay tumor progression significantly (18.6 vs 3.6 months) and to prolong overall survival (29.4 vs 12.8 months) (e78). It should be noted that more effective chemotherapeutic treatments have become available in the meantime. On the basis of the currently available data, therefore, SIRT should be regarded as a so-called salvage option.
Image-guided interstitial brachytherapy
The image-guided insertion of treatment catheters is the underlying principle of image-guided interstitial brachytherapy and the methodological factor that distinguishes it from percutaneous radiotherapy. The radiation source for single-shot radiotherapy is brought into position by way of the treatment catheters. 192Iridium sources have been used in the studies published to date. After insertion of the catheters, a CT scan of the area to be treated is obtained. The image data are then used to plan the radiation treatment based on the position of the catheters. A target dose of 20 Gy is desired at the tumor edge (e79). The radiation exposure to neighboring radiosensitive organs must be kept as low as possible (e80); the applied dose is adjusted locally for this purpose.
Publications regarding image-guided interstitial brachytherapy have focused to date on hepatic and pulmonary tumors (24, 25). Its use at other sites has only been sporadically reported (e81). Interstitial brachytherapy seems to be particularly suitable for the treatment of large tumors. Although no data are yet available on long-term survival, initial case series show that local control, even of large tumors, can be achieved for up to 18 months (e82).
Combined treatment
The additive effect of combinations of these techniques has barely been studied to date. The available initial findings on combinations of different types of local treatment are promising. The combinations of RFA with TACE, PEI with TACE, and RFA with PEI have all been found to be superior to any of these therapies used alone to treat hepatocellular carcinoma (5, 6, e9, e83). Hardly any data are available on combinations of systemic and local treatment options. The CLOCC trial comparing chemotherapy alone to a combination of RFA and chemotherapy is the only EORTC study on this subject and was concluded in June 2007 (www.cancer.gov/clinicaltrials/EORTC-40004). Further studies on combined treatments, eg, chemotherapy with RFA, are currently in the recruiting phase (www.clinicaltrials.gov/ct/gui/show/NCT00183885?order=1). Preliminary data on a combination of TACE with angiogenesis inhibitors show improved local control of HCC (e84). This concept seems plausible because embolization raises the level of vascular-endothelial growth factor (VEGF) and is currently being studied ina number of trials for the treatment of HCC (www.clinicaltrials.gov/ct/gui/show/NCT00055692).
Conclusion
Many image-guided techniques for local tumor therapy in various organs are now available. The choice of treatment depends on the size and location of the tumor. Combinations of multiple techniques are an important prospect for further development. Although this review centers on local therapeutic techniques, the key to the success of interventional tumor therapy lies in the interdisciplinary determination of the treatment plan resulting in the gradated application of systemic, radiotherapeutic, surgical, and interventional treatment options.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Professor Mahnken has received lecture and consulting fees from Siemens AG, Schering AG, GE, Boston Scientific, and Celon AG.
Dr. Bruners and Professor Günther declare that they have no conflict of interest as defined by the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: longterm results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 2.Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for smallsized hepatocellular carcinoma: a retrospective and nation wide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224–1229. doi: 10.1053/jhep.2000.20456. [DOI] [PubMed] [Google Scholar]
- 3.Huo TI, Huang YH, Wu JC, Lee PC, Chang FY, Lee SD. Comparison of percutaneous acetic acid injection and percutaneous ethanol injection for hepatocellular carcinoma in cirrhotic patients: a prospective study. Scand J Gastroenterol. 2003;38:770–778. doi: 10.1080/00365520310003048. [DOI] [PubMed] [Google Scholar]
- 4.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 5.Kurokohchi K, Watanabe S, Masaki T, et al. Combined use of percutaneous ethanol injection and radiofrequency ablation for the effective treatment of hepatocelluar carcinoma. Int J Oncol. 2002;21:841–846. doi: 10.3892/ijo.21.4.841. [DOI] [PubMed] [Google Scholar]
- 6.Dettmer A, Kirchhoff TD, Gebel M, et al. Combination of repeated single-session percutaneous ethanol injection and transarterial chemoembolisation compared to repeated single-session percutaneous ethanol injection in patients with non-resectable hepato-cellular carcinoma. World J Gastroenterol. 2006;12:3707–3715. doi: 10.3748/wjg.v12.i23.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 8.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 9.Camma C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: Metaanalysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Cioni D, Crocetti L, et al. Early-Stage Hepatocellular carcinoma in patients with cirrhosis: Longterm results of percutaneous imageguided radiofrequency ablation. Radiology. 2005;234:961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- 11.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 12.Gillams AR, Lees WR. Radio-frequency ablation of colorectal liver metastases in 167 patients. Eur Radiol. 2004;14:2261–2267. doi: 10.1007/s00330-004-2416-z. [DOI] [PubMed] [Google Scholar]
- 13.Machi J, Oishi AJ, Sumida K, et al. Long-term outcome of radiofrequency ablation for unresectable liver metastases from colorectal cancer: evaluation of prognostic factors and effectiveness in first- and second-line management. Cancer J. 2006;12:318–326. doi: 10.1097/00130404-200607000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 15.Breen DJ, Rutherford EE, Stedman B, et al. Management of renal tumors by image-guided radiofrequency ablation: experience in 105 tumors. Cardiovasc Intervent Radiol. 2007;30:936–942. doi: 10.1007/s00270-007-9090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1. Indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005;185:64–71. doi: 10.2214/ajr.185.1.01850064. [DOI] [PubMed] [Google Scholar]
- 17.McDougal WS, Gervais DA, McGovern FJ, Mueller PR. Long-term followup of patients with renal cell carcinoma treated with radiofrequency ablation with curative intent. J Urol. 2005;174:61–63. doi: 10.1097/01.ju.0000162046.45024.2b. [DOI] [PubMed] [Google Scholar]
- 18.Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy-local tumor control rate and survival data. Radiology. 2004;230:450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 19.Yu NC, Lu DS, Raman SS, et al. Hepatocellular carcinoma: microwave ablation with multiple straight and loop antenna clusters-pilot comparison with pathologic findings. Radiology. 2006;239:269–275. doi: 10.1148/radiol.2383041592. [DOI] [PubMed] [Google Scholar]
- 20.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: an overview. Ultrason Sonochem. 2004;11:149–154. doi: 10.1016/j.ultsonch.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Atwell TD, Farrell MA, Callstrom MR, et al. Percutaneous cryoablation of 40 solid renal tumors with US guidance and CT monitoring: Initial experience. Radiology. 2007;243:276–283. doi: 10.1148/radiol.2431052133. [DOI] [PubMed] [Google Scholar]
- 22.Salem R, Lewandowski RJ, Atassi B, et al. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627–1639. doi: 10.1097/01.RVI.0000184594.01661.81. [DOI] [PubMed] [Google Scholar]
- 23.Jakobs TF, Hoffmann RT, Poepperl G, et al. Mid-term results in otherwise treatment refractory primary or secondary liver confined tumours treated with selective internal radiation therapy (SIRT) using (90)Yttrium resin-microspheres. Eur Radiol. 2007;17:1320–1330. doi: 10.1007/s00330-006-0508-7. [DOI] [PubMed] [Google Scholar]
- 24.Ricke J, Wust P, Wieners G, et al. CT-guided interstitial single-fraction brachytherapy of lung tumors: phase I results of a novel technique. Chest. 2005;127:2237–2242. doi: 10.1378/chest.127.6.2237. [DOI] [PubMed] [Google Scholar]
- 25.Ricke J, Wust P, Stohlmann A, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys. 2004;58:1496–1505. doi: 10.1016/j.ijrobp.2003.09.024. [DOI] [PubMed] [Google Scholar]
- e1.Schelhase T, Rübenach S. Wirtschaft und Statistik 6/2006. Wiesbaden: Statistisches Bundesamt; 2006. Die Todesursachenstatistik - Methodik und Ergebnisse 2004; pp. 614–629. [Google Scholar]
- e2.Rundback JH, Dorfman G, Safriel Y, et al. Development of a research agenda for interventional oncology: proceedings from an interdisciplinary consensus panel. J Vasc Interv Radiol. 2004;15:7–12. doi: 10.1097/01.rvi.0000106389.63463.d3. [DOI] [PubMed] [Google Scholar]
- e3.Giraud P, Yorke E, Jiang S, Simon L, Rosenzweig K, Mageras G. Reduction of organ motion effects in IMRT and conformal 3D radiation delivery by using gating and tracking techniques. Cancer Radiother. 2006;10:269–82. doi: 10.1016/j.canrad.2006.05.009. [DOI] [PubMed] [Google Scholar]
- e4.Sugiura N, Takara K, Ohto M, Okuda K, Hirooka N. Percutaneous intratumoral injection of ethanol under ultrasound imaging for treatment of small hepatocellular carcinoma. Acta Hepatol Jpn. 1983;21:920. [Google Scholar]
- e5.Vilana R, Bruix J, Bru C, et al. Tumor size determines the efficacy of percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Hepatology. 1992;16:353–357. doi: 10.1002/hep.1840160212. [DOI] [PubMed] [Google Scholar]
- e6.Shina S, Tagawa K, Unuma T, et al. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68:1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- e7.Castroagudin JF, Delgado M, Martinez SM, et al. Prospective histopathological analysis of hepatocellular carcinoma treated with percutaneous ethanol injection in patients on the waiting list for liver transplantation. Transplant Proc. 2005;37:1477–1479. doi: 10.1016/j.transproceed.2005.02.025. [DOI] [PubMed] [Google Scholar]
- e8.Ohnishi K, Yoshioka H, Ito S, Fujiwara K. Prospective randomized controlled trial comparing percutaneous acetic acid injection and percutaneous ethanol injection for small hepatocellular carcinoma. Hepatology. 1998;27:67–72. doi: 10.1002/hep.510270112. [DOI] [PubMed] [Google Scholar]
- e9.Luo BM, Wen YL, Yang HY, et al. Percutaneous ethanol injection, radiofrequency and their combination in treatment of hepatocellular carcinoma. World J Gastroenterol. 2005;11:6277–6280. doi: 10.3748/wjg.v11.i40.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e10.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Di Stasi M, Buscarini L, Livraghi T, et al. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma. A multicenter survey of evaluation practices and complication rates. Scand J Gastroenterol. 1997;32:1168–1173. doi: 10.3109/00365529709002998. [DOI] [PubMed] [Google Scholar]
- e12.Horiguchi Y, Itoh M, Takagawa H, et al. Assessment of chemoembolization therapy for primary liver cancer using a stabilized adriamycin-lipiodol suspension. Cancer Chemother Pharmacol. 1992;31:60–64. doi: 10.1007/BF00687107. [DOI] [PubMed] [Google Scholar]
- e13.Soulen MC. Chemoembolization of hepatic malignancies. Oncology. 1994;8:77–90. [PubMed] [Google Scholar]
- e14.Sotiropoulos GC, Malago M, Molmenti E, et al. Efficacy of transarterial chemoembolization prior to liver transplantation for hepatocellular carcinoma as found in pathology. Hepatogastroenterology. 2005;52:329–332. [PubMed] [Google Scholar]
- e15.Huppert PE, Lauchart W, Duda SH, et al. Chemoembolisation des hepatozellulären Karzinoms: Welche Faktoren bestimmen Therapieansprechen und Überleben? Rofo. 2004;176:375–385. doi: 10.1055/s-2004-812776. [DOI] [PubMed] [Google Scholar]
- e16.Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma. An analysis by the Cox proportional hazard model. Cancer. 1991;68:2150–2154. doi: 10.1002/1097-0142(19911115)68:10<2150::aid-cncr2820681011>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- e17.Brown KT, Nevins AB, Getrajdman GI, et al. Particle embolization for hepatocellular carcinoma. J Vasc Interv Radiol. 1998;9:822–888. doi: 10.1016/s1051-0443(98)70398-7. [DOI] [PubMed] [Google Scholar]
- e18.Gupta S, Johnson MM, Murthy R. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors. Cancer. 2005;104:1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]
- e19.Vogl T, Eichler K, Zangos S, et al. Preliminary experience with transarterial chemoembolization (TACE) in liver metastases of uveal malignant melanoma: local tumor control and survival. J Cancer Res Clin Oncol. 2007;133:177–184. doi: 10.1007/s00432-006-0155-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e20.Giroux MF, Baum RA, Soulen MC. Chemoembolization of liver metastasis from breast carcinoma. J Vasc Interv Radiol. 2004;15:289–291. doi: 10.1097/01.rvi.0000116190.44877.04. [DOI] [PubMed] [Google Scholar]
- e21.Burger I, Hong K, Schulick R, et al. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: Initial experience in a single institution. J Vasc Interv Radiol. 2005;16:353–361. doi: 10.1097/01.RVI.0000143768.60751.78. [DOI] [PubMed] [Google Scholar]
- e22.Geschwind JF, Hong K, Georgiades C. Utility of transcatheter arterial chemoembolization for liverdominant colorectal metastatic adenocarcinoma in the salvage setting. Presented at 2006 American Society of Clinical Oncology Gastrointestinal Cancers Symposium, San Francisco, CA, January 26-28. 2006 [Google Scholar]
- e23.Lang EK, Brown CL. Colorectal metastases to the liver: Selective chemoembolization. Radiology. 1993;189:417–422. doi: 10.1148/radiology.189.2.8210369. [DOI] [PubMed] [Google Scholar]
- e24.Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- e25.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- e26.Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res. 2006;26:3793–3795. [PubMed] [Google Scholar]
- e27.Wirbel RJ, Roth R, Schulte M, Kramann B, Mutschler W. Preoperative embolization in spinal and pelvic metastases. J Orthop Sci. 2005;10:253–257. doi: 10.1007/s00776-005-0900-1. [DOI] [PubMed] [Google Scholar]
- e28.Radeleff B, Eiers M, Lopez-Benitez R, et al. Transarterial embolization of primary and secondary tumors of the skeletal system. Eur J Radiol. 2006;58:68–75. doi: 10.1016/j.ejrad.2005.12.008. [DOI] [PubMed] [Google Scholar]
- e29.Munro NP, Woodhams S, Nawrocki JD, Fletcher MS, Thomas PJ. The role of transarterial embolization in the treatment of renal cell carcinoma. BJU Int. 2003;92:240–244. doi: 10.1046/j.1464-410x.2003.04314.x. [DOI] [PubMed] [Google Scholar]
- e30.Kalman D, Varenhorst E. The role of arterial embolization in renal cell carcinoma. Scand J Urol Nephrol. 1999;33:162–170. doi: 10.1080/003655999750015934. [DOI] [PubMed] [Google Scholar]
- e31.Pereira PL, Trübenbach J, Schmidt D. Radiofrequenzablation: Grundlagen, Techniken und Herausforderungen. Rofo. 2003;175:20–27. doi: 10.1055/s-2003-36612. [DOI] [PubMed] [Google Scholar]
- e32.Mulier S, Miao Y, Mulier P, et al. Electrodes and multiple electrode systems for radiofrequency ablation: a proposal for updated terminology. Eur Radiol. 2005;15:798–808. doi: 10.1007/s00330-004-2584-x. [DOI] [PubMed] [Google Scholar]
- e33.Goldberg Nahum S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities - part I. J Vasc Interv Radiol. 2001;12:1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- e34.McGahan JP, Brock JM, Tesluk H, Gu WZ, Schneider P, Browning PD. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J Vasc Interv Radiol. 1992;3:291–297. doi: 10.1016/s1051-0443(92)72028-4. [DOI] [PubMed] [Google Scholar]
- e35.Sala M, Llovet JM, Vilana R, et al. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352–1360. doi: 10.1002/hep.20465. [DOI] [PubMed] [Google Scholar]
- e36.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- e37.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e38.Lü MD, Kuang M, Liang LJ, et al. Surgical resection versus percutaneous thermal ablation for early-stage hepatocellular carcinoma: a randomized clinical trial. Zhonghua Yi Xue Za Zhi. 2006;86:801–805. [Chinese] [PubMed] [Google Scholar]
- e39.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous RF ablation of hepatic metastases from colorectal cancer: long term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- e40.Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358–1364. doi: 10.1200/JCO.2005.12.039. [DOI] [PubMed] [Google Scholar]
- e41.Gillams A, Cassoni A, Conway G, Lees W. Radiofrequency ablation of neuroendocrine liver metastases: the Middlesex experience. Abdom Imaging. 2005;30:435–441. doi: 10.1007/s00261-004-0258-4. [DOI] [PubMed] [Google Scholar]
- e42.Elvin A, Skogseid B, Hellman P. Radiofrequency ablation of neuroendocrine liver metastases. Abdom Imaging. 2005;30:427–434. doi: 10.1007/s00261-004-0257-5. [DOI] [PubMed] [Google Scholar]
- e43.Curley SA, Marra P, Beaty K, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e44.de Baere T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgnol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- e45.Dienemann H, Hof H, Deus J, et al. Lungenmetastasen. Onkologe. 2004;10:458–473. [Google Scholar]
- e46.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR Am J Roentgenol. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- e47.de Baere T, Palussiere J, Auperin A, et al. Midterm local efficacy and survival after radiofrequency ablation of lung tumors with minimum follow-up of 1 year: prospective evaluation. Radiology. 2006;240:587–596. doi: 10.1148/radiol.2402050807. [DOI] [PubMed] [Google Scholar]
- e48.Thanos L, Mylona S, Pomoni M, et al. Percutaneous radiofrequency thermal ablation of primary and metastatic lung tumors. Eur J Cardiothorac Surg. 2006;30:797–800. doi: 10.1016/j.ejcts.2006.08.015. [DOI] [PubMed] [Google Scholar]
- e49.Yan TD, King J, Sjarif A, et al. Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol. 2007;14:1718–1726. doi: 10.1245/s10434-006-9271-x. [DOI] [PubMed] [Google Scholar]
- e50.Yasui K, Kanazawa S, Sano Y, et al. Thoracic tumors treated with CT-guided radiofrequency ablation: initial experience. Radiology. 2004;231:850–857. doi: 10.1148/radiol.2313030347. [DOI] [PubMed] [Google Scholar]
- e51.Varkarakis IM, Allaf ME, Inagaki T, et al. Percutaneous radio frequency ablation of renal masses: results at a 2-year mean followup. J Urol. 2005;174:456–460. doi: 10.1097/01.ju.0000165655.91152.c5. [DOI] [PubMed] [Google Scholar]
- e52.Mahnken A, Rohde D, Brkovic D, Günther RW, Tacke J. Percutaneous radiofrequency ablation of renal cell carcinoma: preliminary results. Acta Radiol. 2005;46:208–214. doi: 10.1080/02841850510015938. [DOI] [PubMed] [Google Scholar]
- e53.Matsumoto ED, Johnson DB, Ogan K, et al. Short-term efficacy of temperature-based radiofrequency ablation of small renal tumors. Urology. 2005;65:877–881. doi: 10.1016/j.urology.2004.12.011. [DOI] [PubMed] [Google Scholar]
- e54.Arima K, Yamakado K, Kinbara H, Nakatsuka A, Takeda K, Sugimura Y. Percutaneous radiofrequency ablation with transarterial embolization is useful for treatment of stage 1 renal cell carcinoma with surgical risk: results at 2-year mean follow up. Int J Urol. 2007;14:585–590. doi: 10.1111/j.1442-2042.2007.01740.x. [DOI] [PubMed] [Google Scholar]
- e55.Mahnken AH, Tacke J, Wildberger JE, Günther RW. Radiofrequenzablation zur Behandlung des Osteoid-Osteoms. Dtsch Arztebl. 2006;103:A 1227–A 1232. [Google Scholar]
- e56.Callstrom MR, Charboneau JW, Goetz MP, et al. Painful metastases involving bone: feasibility of percutaneous CT- and US-guided radiofrequency ablation. Radiology. 2002;224:87–97. doi: 10.1148/radiol.2241011613. [DOI] [PubMed] [Google Scholar]
- e57.Nakatsuka A, Yamakado K, Maeda M, et al. Radiofrequency ablation combined with bone cement injection for the treatment of bone malignancies. J Vasc Interv Radiol. 2004;15:707–712. doi: 10.1097/01.rvi.0000133507.40193.e4. [DOI] [PubMed] [Google Scholar]
- e58.Toyota N, Naito A, Kakizawa H, et al. Radiofrequency ablation therapy combined with cementoplasty for painful bone metastases: initial experience. Cardiovasc Intervent Radiol. 2005;28:578–583. doi: 10.1007/s00270-004-0208-0. [DOI] [PubMed] [Google Scholar]
- e59.Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation - preliminary results. Radiology. 2004;231:225–230. doi: 10.1148/radiol.2311031007. [DOI] [PubMed] [Google Scholar]
- e60.Varshney S, Sewkani A, Sharma S, et al. Radiofrequency ablation of unresectable pancreatic carcinoma: feasibility, efficacy and safety. JOP. 2006;7:74–78. [PubMed] [Google Scholar]
- e61.Hiraki T, Yasui K, Mimura H, et al. Radiofrequency ablation of metastatic mediastinal lymph nodes during cooling and temperature monitoring of the tracheal mucosa to prevent thermal tracheal damage: initial experience. Radiology. 2005;237:1068–1074. doi: 10.1148/radiol.2373050234. [DOI] [PubMed] [Google Scholar]
- e62.Kim YS, Rhim H, Tae K, Park DW, Kim ST. Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid. 2006;16:361–367. doi: 10.1089/thy.2006.16.361. [DOI] [PubMed] [Google Scholar]
- e63.Oura S, Tamaki T, Hirai I, et al. Radiofrequency ablation therapy in patients with breast cancers two centimetres or less in size. Breast Cancer. 2007;14:48–54. doi: 10.2325/jbcs.14.48. [DOI] [PubMed] [Google Scholar]
- e64.Dickinson RJ, Hall AS, Hind AJ, Young IR. Measurement of changes in tissue temperature using MR imaging. J Comp Assist Tomogr. 1986;10:468–472. [PubMed] [Google Scholar]
- e65.Wlodarczyk W, Hentschel M, Wust P, et al. Comparison of four magnetic resonance methods for mapping small temperature changes. Phys Med Biol. 1999;44:607–624. doi: 10.1088/0031-9155/44/2/022. [DOI] [PubMed] [Google Scholar]
- e66.Hosten N, Stier A, Weigel C, et al. Laser-induzierte Thermotherapie (LITT) von Lungenmetastasen: Beschreibung eines miniaturisierten Applikators, Optimierung und erste Patientenbehandlungen. Fortschr Rontgenstr. 2003;175:393–400. doi: 10.1055/s-2003-37830. [DOI] [PubMed] [Google Scholar]
- e67.Witt JD, Hall-Craggs MA, Ripley P, Cobb JP, Bown SG. Interstitial laser photocoagulation for the treatment of osteoid osteoma. J Bone Joint Surg. 2000;82:1125–1128. doi: 10.1302/0301-620x.82b8.11307. [DOI] [PubMed] [Google Scholar]
- e68.Vogl TJ, Lehnert T, Eichler K, Proschek D, FlF6ter J, Mack MG. Adrenal metastases: CT-guided and MR-thermometry-controlled laserinduced interstitial thermotherapy. Eur Radiol. 2007;17:2020–2027. doi: 10.1007/s00330-006-0516-7. [DOI] [PubMed] [Google Scholar]
- e69.Mack MG, Straub R, Eichler K, Sollner O, Lehnert T, Vogl TJ. Breast cancer metastases in liver: laser-induced interstitial thermotherapy - local tumor control rate and survival data. Radiology. 2004;233:400–409. doi: 10.1148/radiol.2332030454. [DOI] [PubMed] [Google Scholar]
- e70.Shibata T, Iimuro Y, Yamamoto Y, et al. Small hepatocellular carcinoma: comparison of radiofrequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- e71.Lu MD, Chen JW, Xie XY, et al. Hepatocellular carcinoma: US-guided percutaneous microwave coagulation therapy. Radiology. 2001;221:167–172. doi: 10.1148/radiol.2211001783. [DOI] [PubMed] [Google Scholar]
- e72.Wu F, Wang ZB, Zhu H, et al. Extracorporeal high intensity focused ultrasound treatment for patients with breast cancer. Breast Cancer Res Treat. 2005;92:51–60. doi: 10.1007/s10549-004-5778-7. [DOI] [PubMed] [Google Scholar]
- e73.Poissonnier L, Chapelon JY, Rouviere O, et al. Control of prostate cancer by transrectal HIFU in 227 patients. Eur Urol. 2007;51:381–387. doi: 10.1016/j.eururo.2006.04.012. [DOI] [PubMed] [Google Scholar]
- e74.Welsh JS, Kennedy AS, Thomadsen B. Selective Internal Radiation Therapy (SIRT) for liver metastases secondary to colorectal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:62–73. doi: 10.1016/j.ijrobp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- e75.Bangash AK, Atassi B, Kaklamani V, et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18:621–628. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]
- e76.Murthy R, Brown DB, Salem R, et al. Gastrointestinal complications associated with hepatic arterial Yttrium-90 microsphere therapy. J Vasc Interv Radiol. 2007;18:553–561. doi: 10.1016/j.jvir.2007.02.002. [DOI] [PubMed] [Google Scholar]
- e77.Gray BN, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- e78.Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- e79.Ricke J, Wust P, Stohlmann A, et al. CT-gesteuerte Brachytherapie. Eine neue perkutane Technik zur interstitiellen Ablation von Lebermetastasen. Strahlenther Onkol. 2004;180:274–280. doi: 10.1007/s00066-004-1179-4. [DOI] [PubMed] [Google Scholar]
- e80.Streitparth F, Pech M, Bohmig M, et al. In vivo assessment of the gastric mucosal tolerance dose after single fraction, small volume irradiation of liver malignancies by computed tomography-guided, high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2006;65:1479–1486. doi: 10.1016/j.ijrobp.2006.02.052. [DOI] [PubMed] [Google Scholar]
- e81.Wieners G, Pech M, Rudzinska M, et al. CT-guided interstitial brachytherapy in the local treatment of extrahepatic, extrapulmonary secondary malignancies. Eur Radiol. 2006;16:2586–2593. doi: 10.1007/s00330-006-0241-2. [DOI] [PubMed] [Google Scholar]
- e82.Ricke J, Wust P, Wieners G, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol. 2004;15:1279–1286. doi: 10.1097/01.RVI.0000141343.43441.06. [DOI] [PubMed] [Google Scholar]
- e83.Kitamoto M, Imagawa M, Yamada H, et al. Radiofrequency ablation in the treatment of small hepatocellular carcinomas: Comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol. 2003;181:997–1003. doi: 10.2214/ajr.181.4.1810997. [DOI] [PubMed] [Google Scholar]
- e84.Britten C, Finn RS, Gomes AS, et al. A pilot study of IV bevacizumab in hepatocellular carcinoma patients undergoing chemoembolization. J Clin Oncol. 2005;23 342s (Abstract 4138) [Google Scholar]
- e85.Pereira PL, Clasen S, Boss A, et al. Radiofrequenzablation von Lebermetastasen. Radiologe. 2004;44:347–357. doi: 10.1007/s00117-004-1033-9. [DOI] [PubMed] [Google Scholar]