Abstract
Tagging a small molecule ubiquitin to a protein substrate, or protein ubiquitination, plays an important role in the immune responses. This process is catalyzed by a cascade of enzymatic reactions, with the E3 ubiquitin ligases being the critical enzymes that determine the specificity of substrate recognition. The E3 ligase Itch was identified from a mutant mouse which displays skin scratching and abnormal immune disorders. In the past few years, much progress has been made in our understanding of Itch-promoted protein ubiquitination, modulation of its ligase activity by upstream kinases, and the kinase-ligase interaction in T cell differentiation and tolerance induction.
Keywords: Itch, ubiquitin, E3 ligase, T helper type 2, Jun proteins, anergy, asthma
1. Introduction
The thymus-derived T lymphocytes play a central role in mounting effective immune responses against invading pathogens from small viruses to multicellular parasites. Depending on the types of pathogens, naive CD4+ T cells can divide into two distinct subsets: T cell helper type 1 (Th1) cells and Th2 cells. Th1 cells secrete interferon (IFN)-γ, and mediate cellular immunity against viruses and intracellular bacteria, whereas Th2 cells produce interleukin (IL)-4 and are involved in the elimination of parasitic worms [1]. On the other hand, the T cell-mediated immune response is tightly controlled, since excessive reaction could result in the damage to self-tissues, leading to the development of autoimmunity, or allergy. The immune tolerance is defined as the process by which the immune system does not attack self and involves multiple mechanisms such as the generation of suppressive T regulatory T cells, or T cell anergy [2, 3].
T cell activation is initiated by the recognition of the pathogenic peptide presented by antigen presenting cells. Engagement of the T cell antigen receptor by the antigenic peptide triggers a cascade of signaling pathways including the phosphorylation of kinases and adaptor proteins, protein complex formation, subcellular relocation of signal molecules and transcription factors, which eventually leads to the gene transcription and the development from naïve T cells to effector T cells [4]. The signaling turn-on process is balanced by turn-off mechanisms such as the de-phosphorylation of signaling molecules. One recently well-recognized mechanism to terminate signal transduction is the process called protein ubiquitination [5]. The tagging of the 76 amino acid molecule to a protein substrate provides a signal for degradation via the garbage disposal machinery or for functional modification.
Protein ubiquitination is achieved by sequential enzymatic reactions, with E3 ubiquitin ligase enzymes being the critical components which determine the specificity of the ubiqutin system by specifically recruiting protein substrates [6, 7]. The importance of protein ubiquitination in T cell regulation is realized with the identification of the Cbl family of adaptor proteins as E3 ubqiuitin ligases [8, 9]. In this work, I will focus on a novel E3 ligase Itch that has been implicated in T cell activation, differentiation, and tolerance induction.
2. Discovery of Itch
Itch was originally described by Neal G. Copeland and Nancy A. Jenkins’ group from studies on mouse coat color alterations [10]. Mutations in the agouti locus on mouse chromosome 2 that either up-regulate or down-regulate the expression of agouti protein cause the color changes in the hair shaft. One of these mutations, a18H, results from the decreasing expression of agouti, which leads to darker than normal coats. Interestingly, unlike mutations in other alleles, this mutation also causes a skin (in the back and neck) scratching phenotype and immunological disorders, manifested by hyperplasia of lymphoid organs and inflammation in the lung and digestive tract. Due to the constant itching in the skin, the mutant mice were also called itchy mice. It was hypothesized that in addition to the agouti gene, there was another mutation in the a18H allele, which is responsible for the itchy and immunological abnormality.
Subsequent genetic studies by the same group confirmed this hypothesis and revealed that the a18H mutation results from a chromosomal inversion that deletes 18 and 20 base pairs from the proximal and distal inversion breaks, respectively [11]. This inversion affects the expression of agouti and disrupts the expression of a novel gene, named Itch. Sequencing of the Itch cDNAs identified an open reading frame of 2,562 base pairs, which encodes 854 amino acids with a molecular mass of approximately 113 kDa. Homology alignments of the predicted amino acid sequences showed that the Itch protein contains a carboxyl-terminal E3 ligase domain, proceeded by four protein-interacting WW domains, with high homology with E3 ligases such as the yeast Rsp5 or the mammalian Nedd4 proteins. This genetic study suggests for the first time that Itch may act as an E3 ubiquitin ligase in regulating immune responses.
3. Structural features of Itch
The process of ubiquitin conjugation is carried by a cascade of enzymatic reactions via a ubiquitin activating enzyme E1, a ubiquitin conjugating enzyme E2, and a ubiquitin-protein ligase E3 [6]. The E1 enzyme activates ubiquitin to form a thioester bond between the active site cysteine of the E1 and the C-terminal glycine residue of the Ub in an ATP-dependent manner. The activated ubiquitin is then transferred to the active site cysteine of one of the E2 enzymes to form a similar thiolester linkage. The E3 ubiquitin ligases recruit both the E2-ubiquitin complex and a protein substrate and facilitate the transfer of ubiquitin from the E2 to a lysine residue of the substrate or to another ubiquitin of a growing poly-ubiquitin chain.
The E3 ubiquitin ligases can be divided into two families: the RING (really interesting new gene) family, and the HECT (homologous to the E6-associated protein C-terminus) family. Both the RING domain and the HECT domain share similar surface structures in E2 binding [6], but use distinct mechanisms for substrate ubiquitination. The RING-type E3s act as scaffolds that bring the E2-ubiquitin and the substrate within the vicinity of each other to allow a direct attack of the substrate lysine residue. In contrast, the HECT-type E3s have intrinsic enzymatic activity, in which the ubiqutin is first transferred to the active site cysteine in the HECT domain, and then to the target protein.
Itch belongs to HECT domain-containing E3 ubiquitin ligases that, with a few exceptions, contain an N-terminal C2 domain, followed by 2 to 4 protein-interacting WW domains, and a C-terminal HECT catalytic domain (Fig. 1). The protein kinase C-related C2 domain is present in a variety of proteins with diverse biological functions [12]. The C2 domain, which is about 116 amino acid residues, forms an eight-stranded antiparallel beta sandwich, at the end of which constitutes a loop region for binding of Ca2+ and phospholipid. This domain is thought to play a role in membrane targeting or subcellular location. Study of the C2 domain of Rsp5, a yeast HECT-type E3, indicated that this domain binds specifically to some forms of phospholipids and directs the sorting of endosomal cargo proteins [13]. The C2 domain of PKCδ also acts as a phosphotyrosine binding domain [14]. The function of the Itch C2 domain remains unclear.
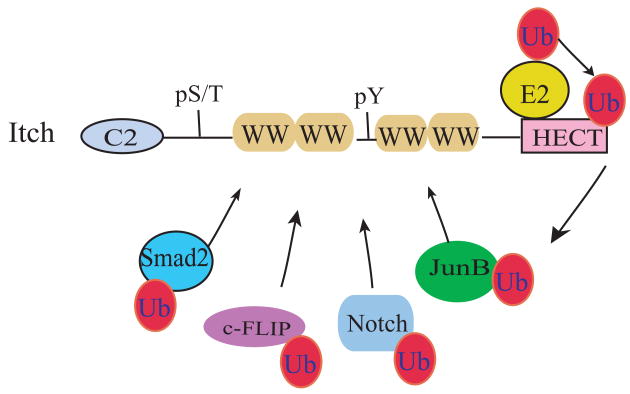
Figure 1.
A schematic representation of Itch and its potential substrates.
Itch consists of an N-terminal protein-kinase C-related C2 domain, followed by four protein-interacting WW domains, and a C-terminal HECT E3 ligase domain. Positions for serine/threonine (pS/T) or tyrosine phosphorylation (pY) are also indicated. The HECT ligase domain binds to E2-ubiquitin complex and transfers the activated ubiquitin to an active site cysteine residue at its C-terminus. Itch catalyzes the ubiqutin conjugaton to protein substrates by facilitating the ubiquitin transfer from the HECT domain to the lysine residues of the substrates. Several protein substrates that have been characterized for the WW domain interaction are shown.
The WW domain is a short domain of approximately 40 amino acids and is so named due to the presence of two signature tryptophan residues that are spaced 20 to 23 amino acids apart and is involved in interaction with proteins containing PPXY or phospho-serine/threonine motifs [15]. As further described below, the WW domains of Itch are shown to interact with several PPXY motif-containing proteins such as JunB, as well as with proteins without PPXY motifs such as Notch [16, 17].
Structural studies of the HECT domain from E6-AP, the first identified HECT-type E3 ligase, revealed that it consists of two lobes, with the N-terminal lobe complexing with E2-ubiquitin, and the C-terminal lobe harboring the active site cysteine residue [18]. A subsequent analysis of the crystal structure of the WWP1 HECT domain showed a hinge region between the two lobes. This confers conformational flexibility for a rotation of the C-lobe to a closer position for the ubiquitin transfer from the E2 to the active site cysteine in the C-lobe [19]. These structural analyses suggest that the ligase activity of HECT-type E3s is subjected to regulation via conformation modifications.
4. Itch in T cell differentiation
In younger itchy (or Itch−/− as annotated thereafter) mice (4 to 8 week-old), before the appearing of skin scarring, the most obvious change is the enlarged spleen and lymph nodes, which may suggest that loss of Itch affects T cell development and activation. However, a detailed analysis of thymocytes and peripheral mature T cells did not reveal discernable alterations in either thymocytes or mature T cells from spleen or lymph nodes in Itch−/− mice [16]. Stimulation of primary T cells by anti-CD3 antibody plus anti-CD28 in vitro showed only a modest increase in proliferation and a slight increase in IL-2 production. However, under the same stimulation conditions, IL-4 but not IFN-γ production was increased in Itch−/− T cells. The increased IL-4 production became much more obvious when Itch−/− CD4 T cells were differentiated into Th2 cells. The results suggest that Itch may play a role in T cell differentiation without much effect on the initial activation process after T cell receptor engagement.
The immunological phenotype in Itch−/− mice is quite similar to the previously described motheaten (me) mice, which also display skin itching and immunological diseases caused by the deficiency of a protein tyrosine phosphatase, SHP1 [20]. Given such similarity, it was hypothesized that Itch may regulate the tyrosine phosphorylation of intracellular signaling molecules in T cells. However, a careful examination of the tyrosine phosphorylation profile in Itch−/− T cells revealed normal phosphorylation patterns, together with normal activation of downstream mitogen-activated protein kinases (MAPKs) such as the phosphorylation of Erk, JNK, and p38 [16]. The relatively normal activation of intracellular signaling molecules in the mutant T cells prompted an examination of further downstream transcription factors including nuclear factor at activated T cells (NFAT), c-Fos, c-Jun, and NFκB, plus other transcription factors which have been implicated in the differentiation of Th2 cells such as c-Maf, and GATA-3 [21]. The study was facilitated by the knowledge that WW domains interact with proteins containing PPXY motifs [15]. It was hypothesized that Itch recruits its substrate(s) via recognizing such PPXY motifs. By using GST-WW fusion protein as bait, it was demonstrated that the WW domain pulled down c-Jun and JunB, but not JunD or other transcription factors [16]. Sequence scanning of the Jun proteins revealed the presence of a PPXY motif in c-Jun and Jun-B, but not JunD. Indeed, Itch interacts with c-Jun and JunB and promotes the Ub conjugation to them. The stability of JunB was enhanced in Itch−/− T cells, in addition to the increased DNA-binding activity.
Following antigen exposure, naïve CD4 T cells differentiate into two distinct subsets of Th cells, Th1 and Th2, based on their cytokine profiles and different effector functions [1]. In addition to the critical transcription factors such as T-bet in IFN-γ gene expression, and c-Maf and GATA-3 in IL-4 expression, JunB was implicated in the Th2 differentiation in JunB transgenic studies [22], which was further supported by the observation that loss of JunB in T cells reduced the production of Th2 cytokines and decreased Th2-mediated immune responses [23]. It should be noted that the serum concentrations of Th2-related immunoglobulin G1 (IgG1) and IgE subclasses were much higher in Itch−/− mice than in control littermates. These studies are consistent with the idea that Itch modulates Th2 function by promoting ubiquitination of Jun proteins.
5. Regulation of Itch by phosphorylation
5.1. Upregulation of Itch ligase activity by MEKK1-JNK kinases
To study the role of JNK pathway in T cell function, Karin’s group generated mice expressing a mutant MAPK kinase kinase, MEKK1, which lacks the catalytic domain. Interestingly, the mutant T cells showed increased production of Th2 cytokines without affecting much of IL-2 and IFN-γ production [24]. Similar to Itch−/− T cells, the protein amounts of c-Jun and JunB were augmented in MEKK1 mutant T cells, whereas their mRNA levels remained unchanged, suggesting that MEKK1 may affect the stability of Jun proteins via post-translational modification.
Biochemical studies showed that MEKK1 enhances Itch-promoted ubiquitination of Jun proteins, which is dependent on the kinase activity of MEKK1 [24]. Loss of MEKK1 activity in the mutant T cells resulted in decreased c-Jun ubiquitination. It seems that MEKK1 functions via the activation of downstream JNK1, since activated JNK1 had similar activity as MEKK1 in increasing Itch-mediated Jun ubiquitination. Indeed, JNK1 directly induces the phosphorylation of Itch, which enhances its self-ligase activity, whereas loss of JNK1 in T cells leads to reduced Itch phosphorylation and increased Jun stability. It should be noted that c-Jun phosphorylation is not related to Itch-mediated ubiquitin conjugation to c-Jun, since mutations at the potential phosphorylation sites in c-Jun did not affect its ubiquitination by Itch. These results clearly suggest a novel pathway in which MEKK1-JNK1 signaling modulates the Th2 cytokine production via phosphorylating and activating the E3 ligase Itch (Fig. 2). The results are also consistent with previous observations that T cells lacking JNK proteins display increased Th2 cytokine production [25, 26].
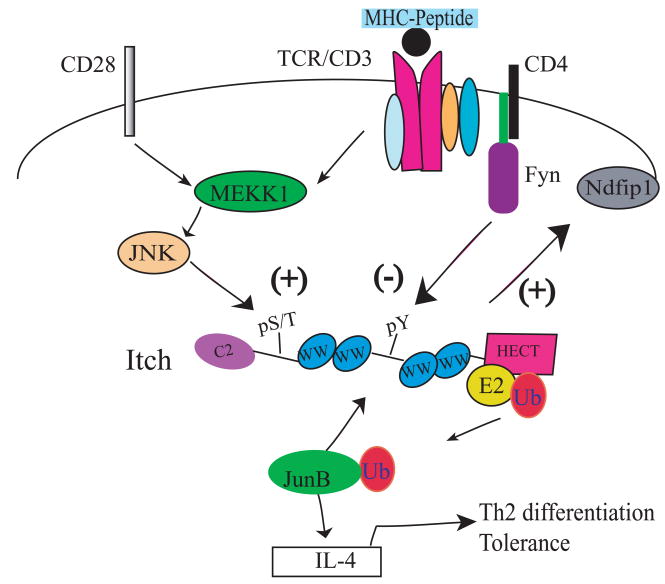
Figure 2.
Regulation mechanisms for Itch-mediated Th2 differentiation.
TCR triggering plus CD28-mediated costimulation results in the activation of MEKK1-JNK signaling pathway, which induces the serine/threonine phosphorylation of Itch. This phosphorylation event causes the increase of Itch ligase activity and the subsequent ubiquitination and degradation of JunB, a transcription factor involved in the production of a Th2 cytokine, IL-4. On the other hand, T cell stimulation also activates the Src kinase Fyn and induces Fyn-mediated tyrosine phosphosphorylation of Itch. Phosphorylation of Itch on the tyrosine residue inhibits the interaction between Itch WW domain and JunB, thus negatively regulating Itch-promoted JunB ubiquitination. Ndfip1 is a membrane-bound protein which may change the subcellular localization of Itch.
The JNK1-induced serine/threonine phosphorylation sites in Itch were mapped in a proline-rich region located between the Itch N-terminal C2 domain and the WW domains [27]. Mutations at the potential phosphorylation sites abolished Itch phosphorylation and decreased its self-ligase activity normally induced by activated JNK1. JNK1 associates directly with a MAPK-binding D domain in the proximal region of the Itch HECT domain and mutations at this D domain inhibits Itch phosphorylation to a similar degree as the mutations in the serine/threonine sites. More importantly, Itch seems to form intra-molecular interactions between the WW and the HECT domains and this intra-molecular interaction results in a closed inactive conformation. This study leads to a model in that binding of JNK1 to the Itch D domain induces Itch phosphorylation at the proline-rich region, which reduces the binding affinity of the WW domains to the HECT domain, releases the WW domain for the recruiting of JunB substrate, and increases the catalytic activity of the HECT domain.
It is well established that protein ubiquitination is subjected to regulation via protein phosphorylation [28]. More often, this is carried out by the phosphorylation of the substrates, which creates a binding site for the recognition by the E3 ligases. One example is the ligand-induced tyrosine phosphorylation of receptor tyrosine kinases, which results in the binding of Cbl E3 ligase via its phospho-tyrosine binding domain and the subsequent Ub transfer from the Ub-E2-Cbl RING finger complex to the activated receptor [9]. In the case of Itch-promoted Jun ubiquitination, JNK1 directly induces the serine/threonine phosphorylation and the subsequent activation of the E3 ligase Itch, whereas the JNK1-induced phosphorylation of its authentic substrates, the Jun proteins, becomes irrelevant, thus pointing out a novel regulatory mechanism for the ubiquitin system.
It has to be mentioned that such a regulatory mechanism may be more complicated than a linear MEKK1-JNK1-Itch pathway. In addition to being an upstream kinase, MEKK1 itself has E3 ligase activity, that either causes self-ubiquitination or ubiquitination of a downstream target Erk [29, 30]. MEKK1 contains an N-terminal plant homeodomain (PHD) region that is highly related to the RING finger domain. Mutations at the conserved cysteine residues in the PHD domain disrupt its self-ligase activity and its effect on Erk ubiqutination and phosphorylation. Since the MEKK1 mutant T cells only lack the kinase domain while the PHD domain remains, it is not clear whether the PHD domain of MEKK1 plays a role in Th2 differentiation.
5.2. Negative regulation of Itch by tyrosine phosphorylation
Engagement of the TCR with antigenic peptide in the context of the major histocompatibility complex results in the activation of the Src kinases Lck and Fyn and the subsequent tyrosine phosphorylation of TCR complexes, leading to the recruitment and activation of Zap-70 and the initiation of intracellular signaling cascades to T cell proliferation and cytokine production [31]. Under resting T cells, the Src kinases are in a closed conformation imposed by the interaction of the Src SH2 domain with a C-terminal phosphorylated tyrosine residue. Upon T cell activation, the regulatory tyrosine residue is dephosphorylated, which renders the kinases into an open structure essential for their activation. In addition to the tight control by tyrosine phosphorylation and dephosphorylation, Src kinases are also subjected to ubiquitination and degradation. An early study demonstrated that E6-AP, a HECT-type E3 ligase, associates with the Src kinases Lck and Blk and promotes their ubiquitination [32]. Particularly, the activated form of Blk is easily degraded via the proteasome-dependent pathway. In another study, it was shown that the latent membrane protein 2A of Epstein-Barr virus serves as a scaffold to recruit the Src kinase Lyn and AIP4, the human homologue of mouse Itch, and facilitates AIP4-mediated Lyn ubiquitination and subsequent degradation [33, 34].
Although initial preliminary studies suggested that Itch associates with Fyn, a detailed analysis of Itch−/− T cells did not reveal an obvious effect of Itch on the activation status and protein stability of the Src kinases Lck and Fyn in T cells. Yet, co-expression of Fyn with Itch in cultured cells reproducibly resulted in the tyrosine phosphorylation of Itch [35]. By using a systematic proteomic approach, a small peptide fragment containing the phosphorylated tyrosine-371 was identified. Mutation of this tyrosine residue caused a marked reduction of Itch tyrosine phosphorylation by Fyn. It seems that Fyn associates with Itch via the SH3 domain of Fyn and a region near the third WW domain of Itch. This interaction is not dependent on the tyrosine phosphorylation of Itch. Importantly, the level of Itch tyrosine phosphorylation was reduced to a larger degree in Fyn−/− T cells upon TCR stimulation, suggesting a physiological role of Fyn kinase in the Itch phosphorylation event.
Unlike MEKK1-JNK1-induced serine/threonine phosphorylation, Itch tyrosine phosphorylation does not affect its self-ligase activity, nor does it increase its activity in promoting JunB ubiquitination [35]. Rather, a mutation at tyrosine 371 augments Itch-mediated JunB ubiquitination, which is further supported by the reduction in JunB ubiquitination with the expression of constitutively active Fyn. It turns out that phosphorylation at tyrosine 371, which is located close to the 3rd WW domain, decreases its association with JunB, and loss of Fyn increases Itch-JunB interaction, which results in lesser JunB stability, as revealed in Fyn−/− T cells. Thus, in contrast to serine/threonine phosphorylation, tyrosine phosphorylation of Itch plays a negative role in Itch-mediated JunB ubiquitination (Fig. 2).
Although an early study showed that Fyn plays a negative role in the differentiation of naïve CD4 T cells into Th2 cells [36], two recent studies convincingly demonstrate that Fyn is required for production of Th2 cytokines [37, 38]. In addition to the TCR complex, Fyn is also recruited to the SLAM family receptors via the adaptor protein SAP and induces SLAM phosphorylation and subsequent complex formation with other signaling molecules such as PKCθ. Like Fyn−/− T cells, T cells lacking either SLAM or SAP display reduced Th2 cytokine production [37, 38]. Consistent with these genetic findings, T cells lacking PKCθ are also defective in Th2 cytokine production and Th2-mediated allergic immune responses [39]. It seems that Fyn functions in both the TCR-mediated and SLAM-mediated signal pathways to modulate Th2 cytokine production, though the details of the downstream events remain unclear. The finding of Fyn-mediated Itch tyrosine phoshporylation and JunB ubiquitination is in agreement with the notion that Fyn is a positive regulator in Th2 differentiation. The demonstration of the Fyn-Itch-JunB pathway may provide an explanation for the reduced IL-4 production in Fyn−/− T cells.
5.3. Regulation of Itch by Ndfip1
A recent study documented a novel mechanism of Itch regulation via a binding partner, Nedd4 family interacting protein 1 (Ndfip1) [40]. Mice deficient in Ndfip1 display similar phenotype as Itch−/− mice, including severe skin and lung inflammation, increased Th2 cytokine production, and upregulated serum IgE amounts. Ndfip1 was initially reported to be a binding protein for WW domain-containing E3 ligases including Nedd4, WWP1, and Itch [41, 42]. Indeed, Itch is co-immunoprecipited with Ndfip1 and co-localized in T cells [40]. More importantly, the protein stability of JunB is augmented in Ndfip1−/− T cells, further supporting that Ndfip1 functions in a similar pathway as Itch. Since Ndfip1 is a membrane-bound protein, it is hypothesized that it may modify the subcellular localization of Itch, and thus Itch-mediated ubiquitination of its target proteins. The findings suggest that multiple mechanisms exist to regulate the biological function of Itch.
6. Itch in T cell tolerance
6.1. T cell tolerance
Mature T cells are capable of mounting robust immune responses against invading pathogens, but at the same time, are tolerant of self-tissues. The induction of T cell tolerance involves many mechanisms at different stages of T cell development. At first, self-reactive T cells are eliminated during thymocyte maturation via negative selection, a process called central tolerance. In addition to thymus-derived antigens, many antigens from other tissues or organs are expressed in the thymus antigen presenting cells, which causes clonal deletion of T cells specific for self-peptide-MHC complexes. Evidence supporting the central tolerance mechanism includes the finding of AIRE, a transcription factor as well as an E3 ubiquitin ligase, which promotes the expression of many peripheral tissue antigens in thymus medullary epithelial cells [43].
However, the central tolerance mechanism is not sufficient, since autoreactive T cells can escape into the secondary lymphoid organs, where the peripheral tolerance mechanisms take effect to keep them under control. Several mechanisms have been proposed to account for tperipheral T cell tolerance to self-antigens, which include ignorance, activation-induced cell death, and suppression by T regulatory T cells [44]. T cell anergy represents another peripheral tolerance mechanism, in which T cells lose the ability to proliferate and produce IL-2 [3]. Effective activation of mature T cells requires the engagement of two signals: the TCR-mediated signal induced by the triggering of TCR complex by the antigen peptide-MHC complex (signal 1) and the costimulatory signal through the binding of costimulatory molecules, such as CD28 on T cells, with their ligands on antigen presenting cells (signal 2). Stimulation of TCR-mediated signal 1 alone in the absence of costimulatory signal 2 renders T cell into a non-responsive anergic state even upon restimulation with both the TCR and the costimulatory receptors.
6.2. E3 ligases in T cell anergy
Although the phenomenon of T cell anergy has been reproducibly observed in both in vitro and in vivo models, it has intrigued scientists for quite a while how T cell anergy occurs. Early studies have documented that T cell anergy is due to defective TCR signal transduction starting from partial or reduced phosphorylation of upstream Src kinases, decreased Erk phosphorylation, or diminished activation of AP-1 transcription factors [45–47]. Recent studies have shown that E3 ubiquitin ligases such as GRAIL, Cbl-b, and Itch, play a critical role in the process of T cell anergy induction [48–50]. A strong and persistent Ca2+ signal without a concomitant activation of the AP-1 signal causes the transcription of anergy inducing genes, with some of these genes encoding E3 ubiquitin ligases or Ub-binding proteins [49]. Upregulation of these E3 ligases results in the downmodulation of critical signal molecules such as PLC-γ1 or PKCθ, that blocks T cell activation even upon effective stimulation.
Previous genetic studies have shown that loss of Cbl-b uncouples T cells from CD28-dependent co-stimulation [51, 52]. The direct in vivo evidence of Cbl-b in T cell anergy induction was demonstrated in a collagen-induced arthritis model in which Cbl−/− mice developed autoimmune arthritis with the antigen injection alone in the absence of adjuvant co-injection [50]. In a MHC class-I-restricted tolerance model, tolerizing viral peptide could not induce the tolerance of Cbl-b−/− CD8+ T cells. Importantly, Cbl−/− mice developed excessive immune responses to the tolerizing viral peptide, which resulted in the acute death of the mice. These studies clearly indicate the importance of the E3 ligase Cbl-b in T cell anergy.
The exact mechanisms by which anergizing stimuli induce the upregualtion of E3 ligases remain unclear. However, recent studies have shown that un-balanced T cell stimulation causes the expression of the transcription factors Egr-2 and Egr-3, which account for the upregulation of Cbl-b [53]. Indeed, T cells deficient in Egr-3 have lower levels of Cbl-b expression and are resistant to in vivo peptide antigen-induced tolerance.
Although both genetic and biochemical evidence supports a critical role of E3 ubiquitin ligases in T cell anergy, it should be noted that the molecular events during T cell anergy induction are more complex and cannot be solely explained by the effects of E3 ubiquitin ligases. One recent study showed that the phosphorylation of LAT, an adaptor linker protein for activated T cells, is selectively inhibited in anergic T cells [54]. The palmitoylation of LAT is impaired in those cells, which results in a defective localization of LAT to the immunological synapse. Notably, Cbl-b neither affects the phosphorylation event of LAT in anergic T cells, nor its protein stability. The new study suggests that in addition to ubiquitination, protein palmitoylation is also very important in T cell anergy induction.
6.3. Itch in Th2 tolerance
The implication of Itch in T cell anergy induction in the in vitro studies raised an issue of whether such observations are physiologically relevant. To address this issue, in vivo mouse models were established in which mice were injected with high-dose tolerizing soluble antigen to induce T cell tolerance [50]. To elicit the immune response, mice were immunized concurrently with regular amounts of the same antigen conjugated with the adjuvant CFA to induce a Th1 response, or with another adjuvant Alum to induce a Th2 response. It was found that Itch−/− T cells are more resistant to tolerance induction under Th2 conditions, as reflected in normal T cell proliferation and IL-2 production [55]. This finding is further supported by an in vitro study in which in vitro differentiated, Itch−/− Th2 cells showed almost complete rescue in cell proliferation and IL-2 production in ionomycin-anergized cells, whereas Itch−/− Th1 cells displayed only a partial rescue. A marked increase of IL-4 production was also observed in both in vivo antigen- and in vitro ionomycin-tolerized Itch−/− T cells. These results suggest that Itch plays a more important role in Th2 tolerance.
One of the critical questions in studying T cell anergy is whether the anergized T cells maintain an unresponsive state. Jenkin’s group developed an elegant adoptive transfer system to examine whether in vivo tolerized T cells remain anergic in response to antigen restimulation in vivo [56]. By using a similar protocol, it was shown that in vivo antigen-tolerized CD4 T cells from Itch−/− mice divided in a relatively normal manner in the syngeneic recipient mice following antigen injection [55].
Given the previous observations that MEKK1-JNK signaling acts via the phosphorylation and activation of Itch [24], it was then asked whether T cells deficient in MEKK1 or JNK1 are also resistant to T cell anergy induction. By using the same in vitro and in vivo tolerance protocols, it was demonstrated that both MEKK1 and JNK1 mutant T cells are resistant to the tolerance induction under the Th2 conditions [55]. Mechanistically, the MEKK1-JNK1 pathway functions by promoting Itch-mediated JunB ubiquitination. Downregulation of JunB protein in Itch−/− Th2 cells by the use of the siRNA technique restored the ability of these cells to become anergic following ionomycin treatment.
It has been a controversial issue whether Th2 cells can be tolerized. Previous studies have mostly used Th1 cells for anergy induction, with only a few addressing Th2 cell anergy. Evidence even suggests that anergic Th1 cells behave like Th2 cells, due to the production of IL-4 and IL-10 [57]. The study using mice deficient in MEKK1, JNK1, or Itch clearly indicates that there exists a genetic pathway that is detrimental in governing the tolerance induction of Th2 cells.
Excessive Th2 cytokine production is linked to the development of allergic asthma that is characterized by the chronic lung inflammation, serum IgE elevation, and airway hyper-responsiveness. The physiological role of Itch-regulated Th2 tolerance in airway inflammation was tested in an in vivo system that combines the soluble antigen-induced Th2 tolerance protocol with the classical antigen-induced asthma model [55]. Injection of high dose soluble antigen blocked lung inflammation and serum IgE increases, both of which are normally observed in aerosol antigen-challenged mice. However, Itch−/− mice still developed massive infiltration of eosinophils, accompanied by augmented production of Th2 cytokines. The results are consistent with the original observations of the increased serum IgE levels and excessive lung inflammation in older Itch−/− mice [16]. Loss of Itch breaks the tolerance of Th2 cells and hence the development of allergic responses.
6.4. Tregs in Th2 tolerance
Historically, instead of a systematic antigen injection as described above, oral or nasal antigen administration has been used to induce Th2 tolerance [58]. Unlike the Th2 tolerance induced by high-dose antigen injection, tolerance via the oral or nasal route has been shown to result from the generation of Tregs [59, 60]. Tregs are a subset of CD4+ T cells which are characterized by the expression of the high affinity IL-2 receptor alpha chain (CD25) and the capacity to inhibit the function of effector T cells via still unclear mechanisms [61]. CD4+CD25+ Tregs are either thymus-derived naturally occurring Tregs, or generated from CD4+CD25− T cells or adaptive Tregs [62]. One critical transcription factor, Foxp3, has been implicated in the suppressing activity of Tregs, since deletion of the Foxp3 gene abolished Treg differentiation and function [63].
Repeated exposure to inhaled low-dose antigen results in the generation of Foxp3+CD4+ Tregs that also express membrane bound TGF-β [60]. These Tregs from the tolerized mice inhibit the proliferation of normal CD4+ T cells and suppress Th2-mediated allergic responses when adoptively transferred into a naïve host. A more recent study suggests that antigen-specific Tregs could be generated via oral tolerance in the absence of naturally occurring Tregs [59]. Like the inhaled antigen-induced Tregs, the oral tolerance-generated Tregs suppress CD4+ T cell proliferation in vitro and inhibit IgE production and lung inflammation in vivo. In both studies, TGF-β was shown to be involved in the proper function of Treg-mediated suppression of the Th2 response, since administration of anti-TGF-β antibody abrogated the tolerance induction and hence the restoration of allergic responses.
TGF-β has been previously shown to convert CD4+CD25− normal T cells into CD4+CD25+ Tregs, which acts via the induction of Foxp3 [64]. Consistently, loss of TGF-β signaling results in excessive immune responses [65]. In the high-dose soluble antigen-induced Th2 tolerance model, Itch deficiency did not affect the generation of Tregs and the Tregs from both wild-type and Itch−/− mice displayed similar inhibiting activity [55]. It is possible that differences in the tolerance protocols lead to different tolerance mechanisms: one directly inducing the tolerance of Th2 cells via high-dose soluble injection, and the other indirectly inducing the generation of Tregs, which then inhibit Th2 responses. It should be noted that Itch is implicated in the regulation of TGF-β signaling via the ubiquitination of the signal transducer Smad2 and Itch−/− fibroblasts are resistant to TGF-β-induced cell growth arrest [66]. Whether Itch regulates Th2 tolerance by interfering with TGF-β signaling and/or TGF-β-mediated Treg development and function awaits further investigation.
7. Itch regulation of Notch signaling
Notch proteins are a family of transmembrane receptors that play an important role in cell fate decisions via evolutionarily conserved mechanisms in many cell types from C. elegans to humans [67]. Activation of Notch involves a series of proteolytic cleavage processes which result in the release of Notch intracellular domain and its translocation into the nucleus, where it associates with DNA-binding transcription regulators and participates in the transcriptional activation of its target genes. Previous studies have documented that Notch is essential in several stages of T cell development including the early pre-T cell development, or CD4 vs. CD8 T cell differentiation in the thymus [68]. In addition, Notch is also implicated in peripheral T cell function such as T cell activation, Th1 vs. Th2 differentiation, and the generation of Tregs [69].
An early study in Drosophila identified a suppressor of deltex, Su(DX), as a HECT type E3 ligase and a close homologue of mouse Itch protein, which negatively regulates Notch-mediated fly wing development [70]. Biochemical studies in Jurkat T cells demonstrated that Itch associates with Notch and induces its ubiquitination [17]. It seems that Notch is also regulated by E3 ubiquitin ligases such as Sel-10 or Cbl [71–74]. However, evidence to support a physiological role of Itch or other E3 ligases in Notch-regulated T cell function remains lacking.
A recent study from Jenkins’ group provides strong evidence that Itch genetically interacts with Notch, which modulates T cell activation and tolerance. In their study, mice carrying an activated form of Notch intracellular domain are bred onto Itch−/− mice (Matesic and Jenkins, personal communication). The double mutant mice display excessive autoimmune phenotypes including inflammation in multiple tissues or organs and production of anti-self antibodies. The thymocyte development is largely perturbed, and thymocytes show resistance to apopotosis induction, a phenotype not observed in single mutant mice. The study suggests that Itch-Notch interaction plays an important role in central, and probably in peripheral, tolerance induction.
8. Other target proteins for Itch
In addition to Jun proteins, Smad2, and Notch, as described above, Itch also promotes the ubiquitination of other proteins. One recently identified substrate is p73, a member of the p53 family of transcription factors [75]. Itch specifically binds to p73, but not p53, via the association of Itch WW domains with a PPPY motif in the C-terminus of p73 (not present in p53) and induces the ubiquitination and degradation of p73. This selective function of Itch is implicated in the responses to DNA damage and in cell cycle control.
In addition to the observation that JNK1 regulates Itch-mediated Jun protein turnover [24], a more recent study showed that JNK-mediated Itch activation also serves as an important regulator in c-FLIP-mediated cell death [76]. The proinflammatory cytokine TNFα induces both cell survival via the activation of NFκB signaling, and cell death via JNK activation [77]. However, the mechanisms that determine the death or survival remain unclear. In JNK1−/− liver cells, the TNFα-induced cell death is reduced, accompanied by the upregulation of the protein amounts of an anti-apoptotic molecule, c-FLIPL, which is induced by NFκB signaling. It was found that Itch directly associates with c-FLIPL and promotes its ubiquitination and proteasome-dependent degradation. As in T cells, JNK1 functions via phosphorylating and activating Itch, which in turn affects the stability of c-FLIPL. Thus, by controlling the amounts of c-FLIPL, the two pathways induced by JNK and NFκB interact with each other, which determine the final outcome of TNFα signaling.
Another study showed that the Itch human homologue AIP4 promotes the ubiquitination of the chemokine receptor CXCR4 and in collaboration with Hrs, an endocytic adaptor protein, induces the downmodulation of CXCR4 via the endosome-lysosomal pathway [78]. Itch was also shown to bind to a tight-junction protein, Occludin, and to regulate its stability via ubiquitin conjugation to it [79]. Additional studies have shown that Itch acts as an E3 ligase for a transcription factor NF-E2 [80], Bcl10 [81], and endophilin [82].
9. Concluding remarks
Significant progress has been made in understanding the biology of Itch E3 ubiquitin ligase, since the initial description of Itchy mice. Biochemical studies allow us to identify several target proteins as substrates, and analysis of Itch−/− T cells provides us with insight into the mechanisms by which Itch regulates T cell activation, differentiation, and tolerance induction. In addition, we have gained a decent amount of knowledge on the regulation of Itch by upstream kinases via Itch phosphorylation. However, we are still facing many issues in directly linking the skin-scratching and immunological phenotype with Itch-mediated protein ubiquitination. For example, we still don’t know whether the Th2-prone characteristic of Itch−/− T cells could fully explain the immunological disorder in the mutant mice, whether other cell types than T cells are also dysfunctional due to Itch deficiency, whether there are other mechanisms than phosphorylation that are involved in the regulation of Itch function, whether similar or different mechanisms operates in diverse signaling pathways induced by different stimuli, or whether Itch E3 ligase cooperates with other E3 ligases. These and other potential questions will allow us to further explore the basic biology of this E3 ligase, and such knowledge will be beneficial in developing novel therapeutic approaches in treating immunological abnormalities such as autoimmune diseases or allergic asthma.
Acknowledgments
I would like to thank former and current lab members, and collaborators for their contribution to the Itch project, and A. Teng for assistance. This work is based on a lecture presented at the 1st summer workshop of the Research Center for Allergy and Immunology at RIKEN, Yokohama, Japan and is supported by NIH funding.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–34. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 4.Samelson LE. Signal transduction mediated by the T cell antigen receptor: the role of adapter proteins. Annu Rev Immunol. 2002;20:371–94. doi: 10.1146/annurev.immunol.20.092601.111357. [DOI] [PubMed] [Google Scholar]
- 5.Liu YC, Penninger J, Karin M. Immunity by ubiquitylation: a reversible process of modification. Nat Rev Immunol. 2005;5:941–52. doi: 10.1038/nri1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33. doi: 10.1146/annurev.biochem.70.1.503. [DOI] [PubMed] [Google Scholar]
- 7.Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–78. doi: 10.1038/35056563. [DOI] [PubMed] [Google Scholar]
- 8.Fang D, Liu Y-C. Proteolysis-independent regulation of phosphatidylinositol 3-kinase by Cbl-b-mediated ubiquitination in T cells. Nature Immunol. 2001;2:870–5. doi: 10.1038/ni0901-870. [DOI] [PubMed] [Google Scholar]
- 9.Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science. 1999;286:309–12. doi: 10.1126/science.286.5438.309. [DOI] [PubMed] [Google Scholar]
- 10.Hustad CM, Perry WL, Siracusa LD, et al. Molecular genetic characterization of six recessive viable alleles of the mouse agouti locus. Genetics. 1995;140:255–65. doi: 10.1093/genetics/140.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry WL, Hustad CM, Swing DA, O’Sullivan TN, Jenkins NA, Copeland NG. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice [see comments] Nat Genet. 1998;18:143–6. doi: 10.1038/ng0298-143. [DOI] [PubMed] [Google Scholar]
- 12.Rizo J, Sudhof TC. C2-domains, structure and function of a universal Ca2+-binding domain. J Biol Chem. 1998;273:15879–82. doi: 10.1074/jbc.273.26.15879. [DOI] [PubMed] [Google Scholar]
- 13.Dunn R, Klos DA, Adler AS, Hicke L. The C2 domain of the Rsp5 ubiquitin ligase binds membrane phosphoinositides and directs ubiquitination of endosomal cargo. J Cell Biol. 2004;165:135–44. doi: 10.1083/jcb.200309026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benes CH, Wu N, Elia AE, Dharia T, Cantley LC, Soltoff SP. The C2 domain of PKCdelta is a phosphotyrosine binding domain. Cell. 2005;121:271–80. doi: 10.1016/j.cell.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 15.Sudol M. The WW module competes with the SH3 domain? Trends Biochem Sci. 1996;21:161–3. [PubMed] [Google Scholar]
- 16.Fang D, Elly C, Gao B, et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol. 2002;3:281–7. doi: 10.1038/ni763. [DOI] [PubMed] [Google Scholar]
- 17.Qiu L, Joazeiro C, Fang N, et al. Recognition and Ubiquitination of Notch by Itch, a Hect-Type E3 Ubiquitin Ligase. J Biol Chem. 2000;275:35734–7. doi: 10.1074/jbc.M007300200. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Kinnucan E, Wang G, Beaudenon S, Howley PM, Huibregtse JM, Pavletich NP. Structure of an E6AP-UbcH7 Complex: Insights into Ubiquitination by the E2-E3 Enzyme Cascade. Science. 1999;286:1321–6. doi: 10.1126/science.286.5443.1321. [DOI] [PubMed] [Google Scholar]
- 19.Verdecia MA, Joazeiro CA, Wells NJ, Ferrer JL, Bowman ME, Hunter T, Noel JP. Conformational flexibility underlies ubiquitin ligation mediated by the WWP1 HECT domain E3 ligase. Mol Cell. 2003;11:249–59. doi: 10.1016/s1097-2765(02)00774-8. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Schweitzer PA, Rajan TV, Yi T, Ihle JN, Matthews RJ, Thomas ML, Beier DR. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–54. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 21.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–44. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 22.Li B, Tournier C, Davis RJ, Flavell RA. Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. Embo J. 1999;18:420–32. doi: 10.1093/emboj/18.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartenstein B, Teurich S, Hess J, Schenkel J, Schorpp-Kistner M, Angel P. Th2 cell-specific cytokine expression and allergen-induced airway inflammation depend on JunB. Embo J. 2002;21:6321–9. doi: 10.1093/emboj/cdf648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science. 2004;306:271–5. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 25.Dong C, Yang DD, Wysk M, Whitmarsh AJ, Davis RJ, Flavell RA. Defective T cell differentiation in the absence of Jnk1. Science. 1998;282:2092–5. doi: 10.1126/science.282.5396.2092. [DOI] [PubMed] [Google Scholar]
- 26.Dong C, Yang DD, Tournier C, Whitmarsh AJ, Xu J, Davis RJ, Flavell RA. JNK is required for effector T-cell function but not for T-cell activation. Nature. 2000;405:91–4. doi: 10.1038/35011091. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher E, Gao M, Liu YC, Karin M. Activation of the E3 ubiquitin ligase Itch through a phosphorylation-induced conformational change. Proc Natl Acad Sci U S A. 2006;103:1717–22. doi: 10.1073/pnas.0510664103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 29.Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T. The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell. 2002;9:945–56. doi: 10.1016/s1097-2765(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 30.Witowsky JA, Johnson GL. Ubiquitylation of MEKK1 inhibits its phosphorylation of MKK1 and MKK4 and activation of the ERK1/2 and JNK pathways. J Biol Chem. 2003;278:1403–6. doi: 10.1074/jbc.C200616200. [DOI] [PubMed] [Google Scholar]
- 31.Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–74. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 32.Oda H, Kumar S, Howley PM. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc Natl Acad Sci U S A. 1999;96:9557–62. doi: 10.1073/pnas.96.17.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winberg G, Matskova L, Chen F, et al. Latent membrane protein 2A of epstein-barr virus binds WW domain E3 protein-ubiquitin ligases that ubiquitinate B-cell tyrosine kinases [In Process Citation] Mol Cell Biol. 2000;20:8526–35. doi: 10.1128/mcb.20.22.8526-8535.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ikeda M, Ikeda A, Longan LC, Longnecker R. The Epstein-Barr virus latent membrane protein 2A PY motif recruits WW domain-containing ubiquitin-protein ligases. Virology. 2000;268:178–91. doi: 10.1006/viro.1999.0166. [DOI] [PubMed] [Google Scholar]
- 35.Yang C, Zhou W, Jeon MS, Demydenko D, Harada Y, Zhou H, Liu YC. Negative Regulation of the E3 Ubiquitin Ligase Itch via Fyn-Mediated Tyrosine Phosphorylation. Mol Cell. 2006;21:135–41. doi: 10.1016/j.molcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Tamura T, Igarashi O, Hino A, Yamane H, Aizawa S, Kato T, Nariuchi H. Impairment in the expression and activity of Fyn during differentiation of naive CD4+ T cells into the Th2 subset. J Immunol. 2001;167:1962–9. doi: 10.4049/jimmunol.167.4.1962. [DOI] [PubMed] [Google Scholar]
- 37.Cannons JL, Yu LJ, Hill B, et al. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Davidson D, Shi X, Zhang S, et al. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in T(H)2 cytokine regulation. Immunity. 2004;21:707–17. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Salek-Ardakani S, So T, Halteman BS, Altman A, Croft M. Differential regulation of Th2 and Th1 lung inflammatory responses by protein kinase C theta. J Immunol. 2004;173:6440–7. doi: 10.4049/jimmunol.173.10.6440. [DOI] [PubMed] [Google Scholar]
- 40.Oliver PM, Cao X, Worthen GS, et al. Ndfip1 Protein Promotes the Function of Itch Ubiquitin Ligase to Prevent T Cell Activation and T Helper 2 Cell-Mediated Inflammation. Immunity. 2006 doi: 10.1016/j.immuni.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey KF, Shearwin-Whyatt LM, Fotia A, Parton RG, Kumar S. N4WBP5, a potential target for ubiquitination by the Nedd4 family of proteins, is a novel Golgi-associated protein. J Biol Chem. 2002;277:9307–17. doi: 10.1074/jbc.M110443200. [DOI] [PubMed] [Google Scholar]
- 42.Jolliffe CN, Harvey KF, Haines BP, Parasivam G, Kumar S. Identification of multiple proteins expressed in murine embryos as binding partners for the WW domains of the ubiquitin-protein ligase Nedd4. Biochem J. 2000;351(Pt 3):557–65. [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson MS, Venanzi ES, Klein L, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 44.Walker LS, Abbas AK. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat Rev Immunol. 2002;2:11–9. doi: 10.1038/nri701. [DOI] [PubMed] [Google Scholar]
- 45.Fields PE, Gajewski TF, Fitch FW. Blocked Ras activation in anergic CD4+ T cells. Science. 1996;271:1276–8. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 46.Gajewski TF, Qian D, Fields P, Fitch FW. Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci U S A. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, Whaley CD, Mondino A, Mueller DL. Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science. 1996;271:1272–6. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 48.Anandasabapathy N, Ford GS, Bloom D, et al. GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity. 2003;18:535–47. doi: 10.1016/s1074-7613(03)00084-0. [DOI] [PubMed] [Google Scholar]
- 49.Heissmeyer V, Macian F, Im SH, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 50.Jeon MS, Atfield A, Venuprasad K, et al. Essential Role of the E3 Ubiquitin Ligase Cbl-b in T Cell Anergy Induction. Immunity. 2004;21:167–77. doi: 10.1016/j.immuni.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 51.Bachmaier K, Krawczyk C, Kozieradzki I, et al. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature. 2000;403:211–6. doi: 10.1038/35003228. [DOI] [PubMed] [Google Scholar]
- 52.Chiang YJ, Kole HK, Brown K, et al. Cbl-b regulates the CD28 dependence of T-cell activation. Nature. 2000;403:216–20. doi: 10.1038/35003235. [DOI] [PubMed] [Google Scholar]
- 53.Safford M, Collins S, Lutz MA, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–80. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 54.Hundt M, Tabata H, Jeon MS, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–22. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Venuprasad K, Elly C, Gao M, et al. Convergence of Itch-induced ubiquitination with MEKK1-JNK signaling in Th2 tolerance and airway inflammation. J Clin Invest. 2006;116:1117–26. doi: 10.1172/JCI26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 57.Gajewski TF, Lancki DW, Stack R, Fitch FW. “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994;179:481–91. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMenamin C, Pimm C, McKersey M, Holt PG. Regulation of IgE responses to inhaled antigen in mice by antigen-specific gamma delta T cells. Science. 1994;265:1869–71. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 59.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–33. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance [In Process Citation] Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 62.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 63.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 64.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–81. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 66.Bai Y, Yang C, Hu K, Elly C, Liu YC. Itch E3 ligase-mediated regulation of TGF-beta signaling by modulating smad2 phosphorylation. Mol Cell. 2004;15:825–31. doi: 10.1016/j.molcel.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 67.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 68.Maillard I, Adler SH, Pear WS. Notch and the immune system. Immunity. 2003;19:781–91. doi: 10.1016/s1074-7613(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 69.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–53. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 70.Cornell M, Evans DA, Mann R, Fostier M, Flasza M, Monthatong M, Artavanis-Tsakonas S, Baron M. The Drosophila melanogaster Suppressor of deltex gene, a regulator of the Notch receptor signaling pathway, is an E3 class ubiquitin ligase. Genetics. 1999;152:567–76. doi: 10.1093/genetics/152.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta-Rossi N, Le Bail O, Gonen H, Brou C, Logeat F, Six E, Ciechanover A, Israel A. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J Biol Chem. 2001;276:34371–8. doi: 10.1074/jbc.M101343200. [DOI] [PubMed] [Google Scholar]
- 72.Jehn BM, Dittert I, Beyer S, von der Mark K, Bielke W. c-Cbl binding and ubiquitin-dependent lysosomal degradation of membrane-associated Notch1. J Biol Chem. 2002;277:8033–40. doi: 10.1074/jbc.M108552200. [DOI] [PubMed] [Google Scholar]
- 73.Oberg C, Li J, Pauley A, Wolf E, Gurney M, Lendahl U. The Notch intracellular domain is ubiquitinated and negatively regulated by the mammalian Sel-10 homolog. J Biol Chem. 2001;276:35847–53. doi: 10.1074/jbc.M103992200. [DOI] [PubMed] [Google Scholar]
- 74.Wu G, Lyapina S, Das I, et al. SEL-10 is an inhibitor of notch signaling that targets notch for ubiquitin-mediated protein degradation. Mol Cell Biol. 2001;21:7403–15. doi: 10.1128/MCB.21.21.7403-7415.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rossi M, De Laurenzi V, Munarriz E, Green DR, Liu YC, Vousden KH, Cesareni G, Melino G. The ubiquitin-protein ligase Itch regulates p73 stability. Embo J. 2005 doi: 10.1038/sj.emboj.7600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang L, Kamada H, Solinas G, Luo J, Maeda S, Venuprasad K, Liu YC, Karin M. The E3 ubiquitin ligase Itch couples JNK activation to TNFa-induced cell death by inducing c-FLIPL turnover. Cell. 2006;124:601–13. doi: 10.1016/j.cell.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 77.Muppidi JR, Tschopp J, Siegel RM. Life and death decisions: secondary complexes and lipid rafts in TNF receptor family signal transduction. Immunity. 2004;21:461–5. doi: 10.1016/j.immuni.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 78.Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3 ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G protein-coupled receptor CXCR4. Dev Cell. 2003;5:709–22. doi: 10.1016/s1534-5807(03)00321-6. [DOI] [PubMed] [Google Scholar]
- 79.Traweger A, Fang D, Liu YC, Stelzhammer W, Krizbai IA, Fresser F, Bauer HC, Bauer H. The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch. J Biol Chem. 2002;277:10201–8. doi: 10.1074/jbc.M111384200. [DOI] [PubMed] [Google Scholar]
- 80.Chen X, Wen S, Fukuda MN, Gavva NR, Hsu D, Akama TO, Yang-Feng T, Shen CK. Human ITCH is a coregulator of the hematopoietic transcription factor NF-E2. Genomics. 2001;73:238–41. doi: 10.1006/geno.2001.6512. [DOI] [PubMed] [Google Scholar]
- 81.Scharschmidt E, Wegener E, Heissmeyer V, Rao A, Krappmann D. Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappa B signaling. Mol Cell Biol. 2004;24:3860–73. doi: 10.1128/MCB.24.9.3860-3873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Angers A, Ramjaun AR, McPherson PS. The HECT domain ligase itch ubiquitinates endophilin and localizes to the trans-Golgi network and endosomal system. J Biol Chem. 2004;279:11471–9. doi: 10.1074/jbc.M309934200. [DOI] [PubMed] [Google Scholar]