Abstract
From a glycoproteomic perspective, the unambiguous localization of O-linked oligosaccharide attachment sites is fraught with analytical obstacles. Because no consensus protein sequence exists for O-glycosylation, there is potential for glycan attachment at numerous serine and threonine residues of a given protein. The well-established tendency for O-glycan attachment to occur within serine and threonine rich domains adds further complication to site-specific assignment of mucin-type glycosylation. In addition to the complexities contributed by the polypeptide chain, the O-linked carbohydrate modifications themselves are exceedingly diverse in both compositional and structural terms. This work is aimed at contributing an improved fundamental understanding of the chemistry that dictates dissociation of O-glycopeptide ions during tandem mass spectrometry (MS/MS). Infrared multiphoton dissociation (IRMPD) has been applied to an assortment of O-linked glycopeptide ions encompassing various compositions and charge states. Protonated O-glycopeptides were found to undergo a combination of glycosidic bond cleavage (complete coverage) and peptide bond cleavage (partial coverage). In contrast to previous observations of N-linked glycopeptide dissociation, the sodiated O-glycopeptides did not yield significantly different information as compared to the corresponding protonated ions. IRMPD of deprotonated O-glycosylated peptides provided informative side chain losses from non-glycosylated serine and threonine residues, which indirectly implicated sites of glycan attachment. In this manner, the combination of positive mode and negative mode MS/MS was found to provide conclusive assignment of O-glycosites.
Keywords: ESI, FT-ICR MS, glycopeptides, glycoproteomics, IRMPD, MS/MS, O-linked glycosylation, protein glycosylation, site-specific glycosylation analysis
INTRODUCTION
In order to further understand the various biological roles of proteins, it is essential to characterize their post translational modifications (PTMs) and determine the influence of these modifications on function. Glycosylation is one of the most common PTMs and affects more than fifty percent of proteins.1 Unlike other PTMs, specific molecular details on the interplay between glycosylation and protein function are only beginning to be revealed on a protein specific basis.2-5 The relatively poor understanding of structure-function relationships involving protein glycosylation is largely attributable to a dearth of analytical capability for investigation of glycosylation in both a high throughput and site-specific manner.
In recent years, site-specific glycosylation analysis using mass spectrometry (MS) has seen important methodological advancement; however, most of this work has been directed at N-linked glycoproteins.6-10 The site-specific investigation of O-linked glycosylation has seen far less progress due to additional complications not shared by N-linked glycosylation. For example, N-linked glycans are attached only to asparagine residues which occur in the context of a specific consensus sequence, whereas O-linked glycosylation can potentially occur at any serine or threonine residue. In addition, all N-linked glycans share a common core structure, whereas eight core structures exist for O-linked glycans. Several of these O-linked cores may be present on a single protein.11 Another confounding characteristic of O-glycosylation is the tendency of this modification to occur within domains rich in serine, threonine, alanine, and proline.12 The presence of multiple flanking serine and threonine residues can introduce significant complications in assigning O-glycan modification to a specific site.
Standard methods for the investigation of site-specific glycosylation involve digestion of the glycoprotein with a specific enzyme such as trypsin followed by MS analysis of the peptide and glycopeptide mixture. This method has proven somewhat useful for determining sites of N-linked glycosylation; however, this approach is far less suitable for the analysis of O-linked glycosylation. Again, O-linked oligosaccharide modification usually occurs in serine, threonine, alanine, and proline rich regions which are often void of the lysine and arginine residues necessary for tryptic cleavage. Moreover, glycosylation confers protease resistance to nearby peptide bonds, thus resulting in O-glycopeptides that can contain greater than 35 amino acid residues.13-16 In order to identify sites of glycosylation on such glycopeptides, tandem mass spectrometry (MS/MS) with very high peptide sequence coverage is essential.
Recently, the use of a protease mixture called pronase has been illustrated for site-specific glycosylation analysis of both N and O-linked glycoproteins.17 This method takes advantage of the non-specific nature of pronase to hydrolyze essentially every peptide bond of a protein, except for those around the site of glycosylation. This process eliminates nonglycosylated peptides which otherwise could prevent the detection of the lower abundance and less efficiently ionized glycopeptides. The length of the peptide tag, or “footprint,” around the glycosylation site has also been shown to be tunable (two to ten amino acid residues) via alteration of digestion time. This allows preparation of shorter glycosylated peptides harboring fewer potential glycosylation sites as compared to large tryptic glycopeptides. This approach has proven very useful for preparation of informative glycopeptides; however, there are often numerous potential glycopeptide compositions that correspond to a given mass spectral signal. This is especially true for larger glycopeptides such as those formed via specific proteolysis.18, 19 Due to the vast number of theoretical glycopeptides, conflicting glycopeptide assignments may occur even for very accurately measured glycopeptide masses.
Even with the advantages of pronase digestion, there is still a great need for MS/MS analysis in order to confirm glycopeptide composition and more importantly characterize sites of glycosylation.20 Glycoproteomic site characterization should both identify the protein and localize the attached glycans to their respective sites on that protein. It has been illustrated that low energy collision induced dissociation (CID) or infrared multiphoton dissociation (IRMPD) of multiply charged glycopeptide ions mainly results in glycosidic bond fragmentation.20, 21 By contrast, electron capture dissociation (ECD) or electron transfer dissociation (ETD) primarily induce fragmentation at peptide bonds.21, 22 Fragmentation of N-linked glycopeptide ions via IRMPD has recently been illustrated to have high dependence upon charge carrier, charge state, and overall glycopeptide composition with both glycosidic and peptide backbone cleavages being accessible depending on the chemistry of a given precursor ion.10
Early studies of O-linked glycopeptide fragmentation were conducted by Medzihradszky et al. utilizing high energy CID of BOC-Tyr derivatized glycopeptides.23 Other synthetic or modified O-linked glycopeptides have also been investigated via post source decay and more recently with CID and ECD.24-27 ECD has proven to be a useful method for the localization of glycosylation sites, but in the majority of these analyses sialic acid residues were removed to enhance ECD efficiency. This practice precludes the site-specific assignment of glycans containing sialic acid residues. Marshall and coworkers also recently showed that both ECD and activated ion ECD (AI-ECD) of a 16 amino acid O-linked glycopeptide resulted in the predominant loss of the sugar residues with no peptide backbone cleavages observed; however, a glycopeptide with 38 amino acid residues yielded up to 50% peptide sequence coverage.14
Based on these studies and others, it has become apparent that the dissociation chemistries of glycosylated peptide ions are sufficiently complex to prevent a simplistic generalization of their fragmentation behavior. Nonetheless, it should be possible to exploit the rich and varied fragmentation chemistry of glycopeptide ions to obtain more informative MS/MS data. A detailed understanding of these dissociation pathways will be essential for the further advancement of MS based glycoproteomics. This is especially true of O-glycoproteomics, which encompasses a greater diversity of carbohydrate structures and compositions, and involves glycosylation at relatively unpredictable sites. This work strives to address the need for an improved fundamental understanding of O-linked glycopeptide fragmentation, especially with regard to those glycopeptides obtained via pronase digestion.
EXPERIMENTAL SECTION
Materials and Reagents
Bovine fetuin A (BF; SwissProt accession number P12763) was purchased form CalBiochem (San Diego, CA). Bovine kappa-casein (κ-CN; SwissProt accession number P02668), pronase E, ammonium acetate, ethanolamine, hydrochloric acid, formic acid, ammonium acetate, and cyanogen bromide activated sepharose 4B beads (average particle diameter, 90 μm) were obtained from Sigma-Aldrich (St. Louis, MO). All other reagents were of analytical grade or higher.
Glycoprotein Digestion
Pronase E was immobilized on sepharose beads following a modified procedure originally described by Clowers et al.17 In short, 150 mg of cyanogen bromide activated sepharose beads were hydrated with HCl (1 mM), rinsed with phosphate buffer (100 mM, pH 7.4), combined with pronase (1 mg), and allowed to couple for 2 hours. The remaining active sites on the sepharose beads were then blocked with ethanolamine (1 M, pH 9.0), and rinsed with ammonium acetate buffer (100 mM, pH 7.4). The pronase beads were then stored in ammonium acetate buffer with sodium azide (0.05%) at 4 °C until needed. Stock glycoprotein solutions were prepared at a concentration of 50 μM in ammonium acetate buffer. For nonspecific proteolysis, the glycoprotein stock solution (100 μL) and fresh ammonium acetate buffer (200 μL) were added to the pronase coupled beads after washing with fresh ammonium acetate to remove the sodium azide. The digestion was allowed to proceed at 37 °C with gentle mixing. The supernatant was sampled at multiple time points ranging from 90 min to 24 h in order to obtain glycopeptides with varying peptide lengths. To avoid the transfer of any pronase immobilized beads, the digestion solution was centrifuged before sampling the supernatant. Glycopeptide solutions were then analyzed immediately or stored at -20 °C prior to analysis.
No sample cleanup was performed on the digestion supernatants; instead, they were diluted into an electrospray-compatible solvent system for direct MS analysis. All digests were diluted at least 10-fold to a final composition of 50% aqueous acetonitrile buffered with either formic acid (0.1%, pH 3.5) or ammonium acetate (1.0 mM, pH 7.4), depending on the desired precursor ion and/or polarity of mass spectral analysis. Multiple ionic forms of each glycopeptide were observed with each buffer system; however, the lower pH solutions favored the formation of protonated species while the higher pH solutions favored the formation of sodiated species. After removal of the supernatant, the pronase coupled beads were washed and stored for future digestions.
Instrumentation and Analysis
Mass spectral analyses were performed using a 9.4 T Fourier transform ion cyclotron resonance mass spectrometer (IonSpec QFT, Lake Forest, CA) equipped with a Picoview nano-ESI stage (New Objective, Woburn, MA). Samples were introduced via a 1.0 μl full loop injection using a NanoLC-1D microfluidic pump and injector port (Eksigent Technologies, Dublin, CA), or via direct infusion using a syringe pump. In both cases, flow rates of 100 nL/min were applied. The infused solutions were charged via a liquid junction immediately before the nano-electrospray tip and held at ±1500-2000 V with respect to the sample cone interface. Ions entered the system through a 390 micron aperture and were externally accumulated in a RF-only storage hexapole for up to 5 seconds prior to injection into the ICR cell.
Individual glycopeptide ions were selected within the ICR cell using stored-waveform inverse Fourier transform (SWIFT) isolation prior to infrared multiphoton dissociation (IRMPD). For IRMPD experiments, the infrared radiation was supplied by a 10.6 μm, 20 W CO2 laser (Parallax Laser Inc., Waltham, MA). The infrared laser beam was expanded to a diameter of 0.5 cm with an inline beam expander (Synrad Laser, Mukilteo, WA) to ensure the isolated ion cloud received maximum exposure during each infrared laser pulse. The fragmentation was optimized by varying the IRMPD laser pulse between 500 and 1500 ms. Irradiation time was increased until the majority of the precursor ion was dissociated. Up to 50 mass spectral scans were acquired and averaged to enhance signal to noise ratio. Spectra were externally calibrated using the IonSpec Omega data station. The m/z error threshold used for all glycopeptide and fragment ion assignments was 10 ppm.
RESULTS AND DISCUSSION
Multiple O-linked glycopeptides were produced via the digestion of κ-CN and BF with immobilized pronase. κ-CN is a well-characterized protein that is known to contain five glycosylation sites. Glycosylation at these sites has recently been shown to occur in a mostly ordered fashion, with only T152 occupied in the singly glycosylated glycoform, T163 and T154 becoming occupied in the doubly and triply glycosylated forms, respectively, after which sites T157 and T142 are occupied without specificity.28 BF is a glycoprotein containing both N-linked and O-linked glycans at N99, N156, N176, S271, T280, and S282 respectively.16, 23, 29, 30 The N-linked glycans are large, complex type multiply sialylated di- or triantennary glycans, whereas the O-linked glycans are a mixture of core 1 and core 2 types. Due to the nonspecific nature of pronase digestion, glycopeptides containing between four and eight amino acid residues were formed by modulating the digestion time. The nomenclature for glycopeptide fragmentation used here follows the conventions of Roepstorff and Fohlman for peptide cleavages, and the conventions of Domon and Costello for glycan cleavages.31, 32 For differentiation between peptide and glycan fragments, all peptide fragments are marked in lower case letters and all glycan fragments are marked in upper case letters. The monosaccharides are represented in the figures according to the symbolic nomenclature of the Consortium for Functional Glycomics (http://glycomics.scripps.edu/CFGnomenclature.pdf). According to this scheme, the following monosaccharides are indicated by the following respective symbols: galactose (Gal), yellow circle; mannose (Man), green circle; N-acetylgalactosamine (GalNAc), yellow square; N-acetylglucosamine (GlcNAc), blue square; and N-acetyIneuraminic acid (NeuAc), purple diamond. In the tables, the more general monosaccharide abbreviations Hex (hexose) and HexNAc (N-acetylhexosamine) are used for simplicity.
Protonated O-Linked Glycopeptides
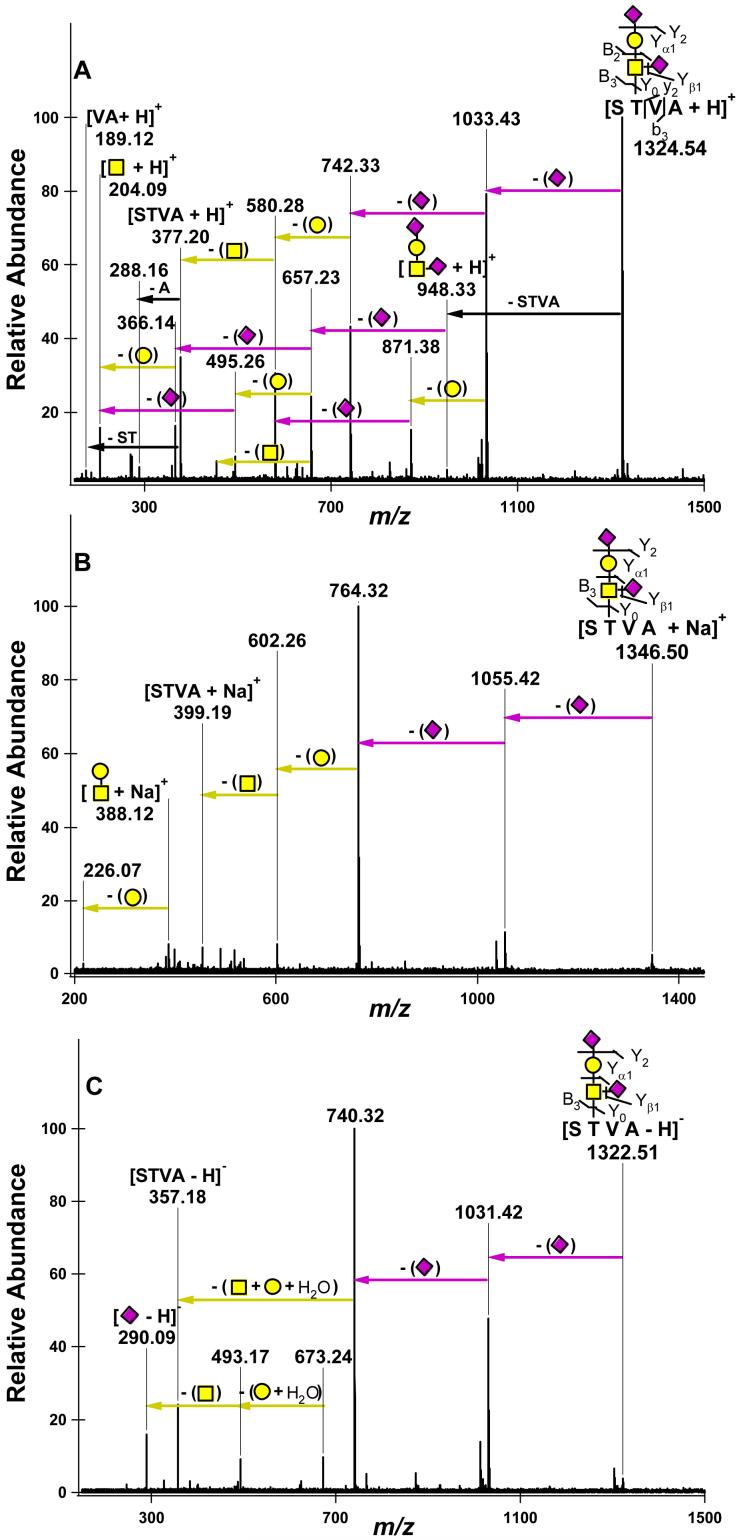
Upon investigation of multiple O-linked glycopeptide ions with IRMPD, it was observed that the charge carrier greatly affected their fragmentation behavior. This result was not unexpected, as similar dependence on both charge state and charge carrier was previously demonstrated for N-linked glycopeptides ions.10 Figure 1 shows the IRMPD spectra of the glycopeptide STVA + Hex1 HexNAc1 NeuAc2 in three different ionic forms. The singly protonated form of this glycopeptide was cleaved along both glycosidic and peptide bonds to reveal the connectivity of the molecule (Figure 1a). The most abundant fragments resulted from cleavage of the labile sialic acid residues at m/z 1033.43 and 742.33. It is unclear which sialic residue is lost first; however, due to the presence of m/z 495.26 and 871.38 it is clear that there is a sialic residue linked to the HexNAc with the other NeuAc residing on the hexose. After these losses, the remaining glycosidic bonds fragmented in a sequential manner to yield the protonated peptide. The peptide then further dissociated, allowing confirmation of the amino acid composition and sequence. The location of the glycosylation site in this case remained ambiguous due the lack of peptide fragmentation prior to glycan loss.
Figure 1.
IRMPD nano-ESI FT-ICR mass spectra of the O-glycopeptide 162ST*VA165 + Hex1 HexNAc1 NeuAc2 derived from κ-CN with multiple charge carriers: (a) singly charged, protonated glycopeptide m/z 1324.54 (10 scans); (b) singly charged, sodium coordinated glycopeptide m/z 1346.50 (15 scans); (c) singly charged, deprotonated glycopeptide m/z 1322.51 (10 scans).
The IRMPD mass spectra for the protonated glycopeptides PTST + Hex1 HexNAc1 NeuAc2 and GEPTSTPT + Hex1 HexNAc1 NeuAc2 are shown in Figure 2a and 3a, respectively. In a parallel manner to the previous example, these glycopeptides first lost the labile sialic acid residues; however, unlike the previous example, the entire glycan was not lost from the peptide prior to the cleavage of peptide bonds. The fragment ion at m/z 489.22, which was very abundant in both spectra, corresponds to PTS + HexNAc. This fragment narrows down the site of glycosylation to either the remaining serine or threonine residue. The site was conclusively assigned based on additional information gained from the negative mode spectrum (as will be discussed below). Complete sequence coverage of the short peptide PTST was obtained as shown in Figure 2a. The glycopeptide GEPTSTPT + Hex1 HexNAc1 NeuAc2 had several abundant peptide fragment ions, including y6 and b6 which both occur N-terminal to proline residues with one also C-terminal to a glutamic acid residue (Figure 3a). These were expected to be major product ions due to the well-known “proline effect” and “aspartic acid effect.”33 These fragmentation effects lead to the assignment of the before mentioned ion m/z 489.22. Based on accurate mass this ion could have been assigned to PTS + HexNAc or STP + HexNAc, however due to the proline effect the observation of STP + HexNAc is highly unlikely since cleavage N-terminal to proline is much more labile than both of the cleavages that would lead to this production.
Figure 2.
IRMPD nano-ESI FT-ICR mass spectra of the O-glycopeptide 151PT*ST154 + Hex1 HexNAc1 NeuAc2 derived from κ-CN with multiple charge carriers: (a) singly charged, protonated glycopeptide m/z 1352.53 (25 scans); (b) singly charged, sodium coordinated glycopeptide m/z 1374.50 (25 scans); (c) singly charged, deprotonated glycopeptide m/z 1350.51 (30 scans).
Figure 3.
IRMPD nano-ESI FT-ICR mass spectra of the O-glycopeptide 149GEPT*STPT156 + Hex1 HexNAc1 NeuAc2 derived from κ-CN with multiple charge carriers: (a): singly charged, protonated glycopeptide m/z 1736.67 (25 scans); (b) singly charged, sodium coordinated glycopeptide m/z 1758.67 (50 scans); (c) doubly charged, doubly deprotonated glycopeptide m/z 866.83 (10 scans).
A core 2 type glycan occurring on the protonated glycopeptide GPTPSA + Hex2 HexNAc2 NeuAc2 derived from BF fragmented in a similar manner to the core 1 glycan containing glycopeptides previously discussed (Figure 4a). The core structure was confirmed by m/z 935.42, which corresponded to the intact peptide connected to two HexNAc residues. This ion was complemented by two fragment ions at m/z 713.26 and 551.21, both of which contain two HexNAc residues after release from the peptide backbone indicating that this was a single glycan moiety with HexNAc to HexNAc connectivity. The peptide moiety of this glycopeptide dissociated to yield b3 and y3 fragment ions, both of which were N-terminal to proline residues. This glycopeptide had the lowest peptide sequence coverage of all the glycopeptides investigated.
Figure 4.
IRMPD nano-ESI FT-ICR mass spectra of the O-glycopeptide 278GPT*PSA283 + Hex2 HexNAc2 NeuAc2 derived from BF with multiple charge carriers: (a) singly charged, protonated glycopeptide m/z 1841.72 (20 scans); (b) singly charged, sodium coordinated glycopeptide m/z 1863.70 (50 scans); (c) singly charged, deprotonated glycopeptide m/z 1839.72 (20 scans).
Table 1 summarizes the fragmentation spectra of 12 distinct glycopeptide ions derived form BF and κ-CN in the protonated form, including the four specific cases detailed above. The glycosidic bond cleavages were not listed in the table because all of these glycopeptide ions dissociated to yield a complete series of either B or Y ions, which was sufficient to verify connectivity (but not linkage). The glycopeptides exhibited differing amounts of peptide fragmentation, with some yielding 100% sequence coverage and others only 20% (53% on average). Since these are relatively small peptide moieties, it is more likely that the site can be determined despite mediocre sequence coverage. This is an important contrast to glycopeptides with large peptide moieties containing many potential glycosites, which would require very high sequence coverage for site localization. Another notable feature was tendency of the peptide moiety to act as the more dominant charge carrier, as the majority of the fragments contained the peptide portion of the molecule while comparatively few fragments only contained the glycan. A multiply glycosylated glycopeptide ion GEPTSTPT + (Hex1 HexNAc1 NeuAc)2 was found to fragment in a manner similar to the singly glycosylated variant. No additional peptide fragment ions were observed for this glycopeptide; however, another series of glycan fragments was observed due to the additional glycan moiety. All fragment ions observed below m/z 1175 were identical to those shown in Figure 3a.
Table 1.
Protonated O-linked glycopeptide ions subjected to IRMPD. Asterisks appear after glycosylated residues
| m/z | Peptide | Glycan | Peptide Cleavages | Seq. Cov. |
|---|---|---|---|---|
| 1114.45 | GPT*PS | HeX1 HexNAc1 NeuAc1 | b3 | 25% |
| 1253.49 | ST*V | HeX1 HexNAc1 NeuAc2 | b2 | 50% |
| 1312.55 | APS*AVPD | HeX1 HexNAc1 NeuAc1 | y6, b3, b5 | 50% |
| 1324.54 | ST*VA | HeX1 HexNAc1 NeuAc2 | y2, b3 | 66% |
| 1352.53 | PT*ST | HeX1 HexNAc1 NeuAc2 | y3, b2, b3 | 100% |
| 1425.57 | ST*VAT | HeX1 HexNAc1 NeuAc2 | Y4, b3, b4 | 75% |
| 1445.59 | GEPT*STPT | HeX1 HexNAc1 NeuAc1 | y6, b4, b5, b6 | 57% |
| 1453.56 | EST*VA | HeX1 HexNAc1 NeuAc2 | y4 | 25% |
| 1603.65 | APS*AVPD | HeX1 HexNAc1 NeuAc2 | y6, b3, b5 | 50% |
| 1736.67 | GEPT*STPT | HeX1 HexNAc1 NeuAc2 | y6, b4, b5, b6 | 57% |
| 1841.72 | GPT*PSA | Hex2 HexNAc2 NeuAc2 | b3, y3 | 20% |
| 2101.82 | GEPT*ST*PT | (HeX1 HexNAc1 NeuAc)2 | y6, b4, b5, b6 | 57% |
Sodiated O-Linked Glycopeptides
The dissociation of the sodium coordinated STVA + Hex1 HexNAc1 NeuAc2 yielded m/z 764.32 as the most abundant fragment (Figure 1b). After these neutral losses of the two sialic acid residues, low abundance fragments were observed for both the sodiated peptide, m/z 399.19, and the sodiated glycan void of sialic acid residues, m/z 388.12 (Hex1 HexNAc1). This was the only sodiated O-glycopeptide that was found to dissociate in a manner that yielded a glycan only fragment. The sodium coordinated glycopeptide PTST + Hex1 HexNAc1 NeuAc2 demonstrated similar fragmentation, with an abundant loss of both NeuAc residues, m/z 792.31 (Figure 2b). The intact sodium coordinated peptide was also observed at m/z 427.18.
The fragmentation of sodium coordinated GEPTSTPT + Hex1 HexNAc1 NeuAc2 was found to closely mimic the behavior of the other sodiated glycopeptides analyzed, with the loss of the two sialic residues yielding the most abundant fragment (Figure 3b). The y6 peptide cleavage was also observed in multiple product ions (i.e., in combination with various glycan losses) due to the labile cleavage of the peptide between the glutamic acid and proline residues. The intact peptide was observed at m/z 811.35, as was the y6 ion at m/z 625.29. The IRMPD mass spectrum for the sodium coordinated glycopeptide ion GPTPSA + Hex2 HexNAc2 NeuAc2 showed dissociation exclusively along glycosidic bonds, with the desialylated glycopeptide, m/z 1281.51, being the most abundant fragment ion (Figure 4b). Since this glycopeptide contained the larger core 2 type glycan, it was predicted that the glycan moiety or some part thereof would be a favored product ion due to the increased ability of a large glycan to coordinate with sodium. In practice, this glycopeptide behaved in a parallel manner to the other O-linked glycopeptides discussed, as well as the additional examples listed in Table 2. In general, the sodium coordinated O-glycopeptide ions fragmented almost exclusively along glycosidic bonds, with the desialylated glycopeptide being the most abundant fragment. Sodiated peptide moieties were present to a lesser extent; hence no addition information was gained by dissociation of sodiated precursor ions as compared to the protonated precursor ions.
Table 2.
Sodium coordinated O-linked glycopeptide ions subjected to IRMPD. Asterisks appear after glycosylated residues
| m/z | Peptide | Glycan | Peptide Cleavages | Seq. Cov. |
|---|---|---|---|---|
| 1346.50 | ST*VA | Hex1 HexNAc1 NeuAc2 | 0% | |
| 1374.50 | PT*ST | Hex1 HexNAc1 NeuAc2 | 0% | |
| 1467.57 | GEPT*STPT | Hex1 HexNAc1 NeuAc1 | y6 | 14% |
| 1625.63 | APS*AVPD | Hex1 HexNAc1 NeuAc2 | 0% | |
| 1758.67 | GEPT*STPT | Hex1 HexNAc1 NeuAc2 | y6 | 14% |
| 1863.70 | GPT*PSA | Hex2 HexNAc2 NeuAc2 | 0% |
Although the fragmentation behavior of the sodium coordinated O-linked glycopeptides was distinct from that of the protonated forms, the difference in dissociation character was not as dramatic as that recently observed by Lebrilla and coworkers for N-linked glycopeptide ions.10 In that work, the N-linked chitobiose core cleavage was the dominant fragment in both ionic forms; however, in the protonated form the peptide + GlcNAc was observed while the glycan - GlcNAc was observed in the sodium coordinated form. Unlike the N-linked glycopeptides, the sodium coordinated O-linked glycopeptides investigated in this study did not exhibit a large number of glycan only fragments. This difference is proposed to be a result of two effects. Firstly, O-glycopeptides lack the labile chitobiose core, which easily divides the molecule in the N-linked case. Secondly, the smaller O-glycans appear less capable to coordinate solely with the smaller glycan moiety. Thus, part of the peptide moiety is required for stable multidentate coordination of sodium. As a result, facile separation of the glycan and peptide moieties is impeded.
Deprotonated O-Linked Glycopeptides
Upon investigation of the deprotonated glycopeptide ions, it was observed that this ionic form dissociated more readily, requiring on average 75% of the irradiation time when compared to both the protonated and sodiated forms. When subjected to IRMPD, the deprotonated glycopeptide STVA + Hex1 HexNAc1 NeuAc2 was found to dissociate exclusively along glycosidic bonds, with the desialylated glycopeptide as the most abundant fragment at m/z 740.32 (Figure 1c). Deprotonated NeuAc, m/z 290.09, was also observed in addition to other glycan only fragments at m/z 493.17 and m/z 673.24. The fragments containing NeuAc were anticipated to be more abundant due to their acidic nature and previously observed trends for sialic acid containing oligosaccharides.34, 35 These results indicate that the peptide moiety, with the acidic C-terminus, can effectively compete with sialic acid as the charge carrier in negative mode.
The deprotonated form of PTST + Hex1 HexNAc1 NeuAc2 replicated the behavior of the previous example, yielding the desailylated glycopeptide, m/z 768.31, and the glycan moiety fragment ions at m/z 290.09, m/z 493.17, and m/z 655.22 (Figure 2c). Moreover, the presence of two peptide side chain cleavages for threonine and serine add substantial site-specific information to that obtained in the positive ion mode. From all of the peptide containing fragment ions, the loss of 44.03 Da was also observed. In one case, this loss was combined with the loss of 30.01 Da for a total loss of 74.04 Da. These losses have previously been described to dominate deprotonated peptide spectra, and correspond to side chain losses with 44.03 Da and 30.01 Da corresponding to the loss of C2H4O and CH2O from the side chains of threonine and serine, respectively.36 Each native, non-glycosylated threonine residue was found to yield an abundant side chain loss (Table 3). Due to the high fidelity of threonine side chain fragmentation it was possible to both identify the number of non-glycosylated threonine residues and hence localize the site of glycosylation to either serine or threonine. For example, PTST + Hex1 HexNAc1 NeuAc2 yielded only one threonine side chain loss, thus providing evidence for glycosylation at one of the threonine residues but not the serine residue. The information gained from the presence or absence of the side chain cleavage of serine could also be used to confirm the assignment. The knowledge that the glycan occupied a threonine residue combined with the information obtained from the positive mode spectra allowed the site of glycosylation to be conclusively assigned to the threonine C-terminal to proline.
Table 3.
Deprotonated O-linked glycopeptide ions subjected to IRMPD. Asterisks appear after glycosylated residues. Informative side chain losses are listed under “other,” with -44 indicating loss of C2H4O from threonine and -30 corresponding to the loss of CH2O from serine
| m/z | Peptide | Glycan | Charge Carrier | Peptide Cleavages | Seq. Cov. | other |
|---|---|---|---|---|---|---|
| 721.28 | GEPT*STPT | Hex1 HexNAc1 NeuAc1 | [M-2H]2- | y6 | 14% | -44, -44, -30 |
| 866.83 | GEPT*STPT | Hex1 HexNAc1 NeuAc2 | [M-2H]2- | y6 | 14% | -44, -44, -30 |
| 1322.51 | ST*VA | Hex1 HexNAc1 NeuAc2 | [M-H]- | 0% | ||
| 1350.51 | PT*ST | Hex1 HexNAc1 NeuAc2 | [M-H]- | 0% | -44, -30 | |
| 1443.58 | GEPT*STPT | Hex1 HexNAc1 NeuAc1 | [M-H]- | y6 | 14% | -44, -44, -30 |
| 1734.67 | GEPT*STPT | Hex1 HexNAc1 NeuAc2 | [M-H]- | y6 | 14% | -44, -44, -30 |
| 1839.72 | GPT*PSA | Hex2 HexNAc2 NeuAc2 | [M-H]- | b3, y5 | 40% | |
| 2099.80 | GEPT*ST*PT | (Hex1 HexNAc1 NeuAc1)2 | [M-H]- | y6 | 14% | -44, -30 |
The lack of threonine side chain loss for the glycopeptide STVA + Hex1 HexNAc1 NeuAc2 was also used to assign the glycan to the threonine residue (Figure 1c). The utility of side chain losses was further illustrated upon investigation of the doubly deprotonated glycopeptide GEPTSTPT + Hex1 HexNAc1 NeuAc2 (Figure 3c). Two threonine side chain losses were clearly present and were observed in multiple series together with a single threonine side chain loss. Once again, this information localized the site of glycosylation to a threonine residue and when combined with information from the protonated spectra (Figure 3a) the site was unambiguously assigned. The majority of the fragment ions including the side chain losses were observed in the charge reduced form due to the loss of the labile sialic acid residues. The y6 ion corresponding to the loss of GE was observed as it was in both the protonated and sodium coordinated spectra. The core 2 type glycopeptide GPTPSA + Hex2 HexNAc2 NeuAc2 was also investigated as a deprotonated ion (Figure 4c). This glycopeptide also fragmented mainly along glycosidic bonds with the majority of the fragment ions containing the intact peptide moiety. Some peptide fragments ions were observed including the loss of glycine and deprotonated PT at m/z 197.06 both of which were due to cleavages N-terminal to proline.37
N-linked Glycopeptides Containing NeuAc
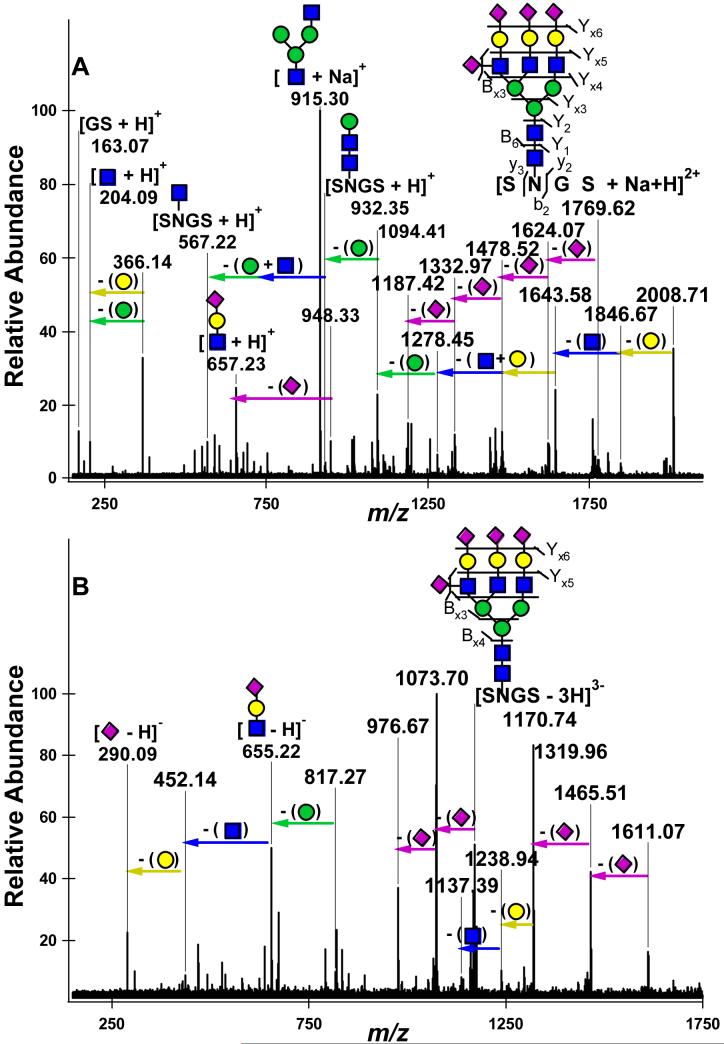
For comparison, an N-linked glycopeptide containing multiple sialic acid residues was investigated. The doubly charged protonated and sodium coordinated N-linked glycopeptide SNGS + Hex6 HexNAc5 NeuAc4 derived from BF was subjected to IRMPD (Figure 5a). From the doubly charged precursor, the loss of four sialic acid residues was observed sequentially at m/z 1624.07, m/z 1478.52, m/z 1332.97, and m/z 1187.42. After the loss of these labile residues, the rest of the glycan was cleaved in a charge reduced state. The larger glycan fragment ions were sodium coordinated, while the peptide containing fragments were mainly protonated. The most abundant fragment ion at m/z 915.30 was the result of chitobiose core cleavage combined with the loss of glycan antennae. The complement to this cleavage, the peptide moiety plus HexNAc, was also observed except with a proton as the charge carrier. This further demonstrates the lability of the chitobiose core and the preferred charge carriers for both the glycan and peptide moieties.
Figure 5.
IRMPD nano-ESI FT-ICR mass spectra of the N-glycopeptide 175SNGS178 + Hex6 HexNAc5 NeuAc4 derived from BF with multiple charge carriers: (a) doubly charged, protonated and sodium coordinated glycopeptide m/z 1769.62 (20 scans); (b) triply charged, triply deprotonated glycopeptide m/z 1170.74 (20 scans).
The triply deprotonated IRMPD mass spectra for this same glycopeptide is presented in Figure 5b. The fragment ions corresponding to the loss of one and two sialic acid residues were observed in the triply charged state at m/z 1073.70 and 976.67 and in the doubly charged state at m/z 1611.07 and 1455.51. A few fragment ions resulting from other glycan losses were observed in the doubly charged state at m/z 1319.96, m/z 1238.94, and m/z 1137.39. The neutral losses of 30.01 Da and 60.02 Da were observed, which arise from two sequential serine side chain losses. These side chain losses complement the assignment of the N-glycopeptide; however, they do not assist in the assignment of the glycosylation site since N-glycans only occur at specific glycosylation motifs which do not involve the modification of serine or threonine side chains. The only other fragment ion series observed corresponded to the deprotonated intact antennae down to the deprotonated NeuAc residue. These results further indicate that N-linked and O-linked glycopeptides fragment with distinct behaviors, and that this distinction is not solely due to the presence of labile and anionic sialic acid residues on the O-glycopeptides.
CONCLUSIONS
The characterization of O-linked glycosylation sites has lagged behind the characterization of N-linked glycosites due to the increased complexity inherent to O-linked glycosylation. The lack of a consensus sequence and the large number of serine and threonine residues present in O-glycosylated regions of proteins contribute a complicated problem. The utilization of specific enzymes such as trypsin does not alleviate these complications due the large tryptic peptides produced in these regions (greater than 40 amino acid residues is not uncommon). Such large glycopeptides with so many potential glycosylation sites necessitates almost complete peptide sequence coverage to identify glycosylation sites.
The use of nonspecific proteolysis combined with low energy tandem mass spectrometry has been illustrated to simplify this complex problem. Nonspecific proteolysis affords the tunability of peptide footprints around each glycosylation site, thus allowing for the creation of smaller and more manageable glycopeptides with reduced numbers of potential glycosylation sites. IRMPD of the deprotonated form of O-glycopeptides has been shown to produce informative side chain cleavages from unoccupied serine and threonine residues, allowing determination of whether a serine or threonine residue is glycosylated. Uniting this information with the sequence coverage produced via IRMPD of the protonated glycopeptide leads to unambiguous site identification. These dissociation chemistries inherent to O-glycopeptide ions have also been observed via CID and should be transferable for use on an LC timescale.
ACKNOWLEDGMENTS
The authors would like to acknowledge the following funding sources: California Dairy Research Foundation (06 LEC-01-NH), Dairy Management Incorporated, University of California Discovery Grant (05GEB01NHB), and the National Institutes of Health (GM 49077).
REFERENCES
- 1.Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12(4):43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- 2.Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nature Biotechnology. 2006;24(10):1241–1252. doi: 10.1038/nbt1252. [DOI] [PubMed] [Google Scholar]
- 3.Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annual Review of Immunology. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 4.Varki A. Biological Roles of Oligosaccharides - All of the Theories Are Correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291(5512):2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 6.Zhao J, Simeone DM, Heidt D, Anderson MA, Lubman DM. Comparative serum glycoproteomics using lectin selected sialic acid glycoproteins with mass spectrometric analysis: Application to pancreatic cancer serum. Journal of Proteome Research. 2006;5(7):1792–1802. doi: 10.1021/pr060034r. [DOI] [PubMed] [Google Scholar]
- 7.Wang YH, Wu SL, Hancock WS. Approaches to the study of N-linked glycoproteins in human plasma using lectin affinity chromatography and nano-HPLC coupled to electrospray linear ion trap-Fourier transform mass spectrometry. Glycobiology. 2006;16(6):514–523. doi: 10.1093/glycob/cwj091. [DOI] [PubMed] [Google Scholar]
- 8.Nakano M, Nakagawa T, Ito T, Kitada T, Hijioka T, Kasahara A, Tajiri M, Wada Y, Taniguchi N, Miyoshi E. Site-specific analysis of N-glycans on haptoglobin in sera of patients with pancreatic cancer: A novel approach for the development of tumor markers. International Journal of Cancer. 2008;122(10):2301–2309. doi: 10.1002/ijc.23364. [DOI] [PubMed] [Google Scholar]
- 9.Wada Y, Azadi P, Costello CE, Dell A, Dwek RA, Geyer H, Geyer R, Kakehi K, Karlsson NG, Kato K, Kawasaki N, Khoo KH, Kim S, Kondo A, Lattova E, Mechref Y, Miyoshi E, Nakamura K, Narimatsu H, Novotny MV, Packer NH, Perreault H, Peter-Katalinic J, Pohlentz G, Reinhold VN, Rudd PM, Suzuki A, Taniguchi N. Comparison of the methods for profiling glycoprotein glycans - HUPO Human Disease Glycomics/Proteome Initiative multi-institutional study. Glycobiology. 2007;17(4):411–422. doi: 10.1093/glycob/cwl086. [DOI] [PubMed] [Google Scholar]
- 10.Seipert RR, Dodds ED, Clowers BH, Beecroft SM, German JB, Lebrilla CB. Factors that influence fragmentation behavior of N-linked glycopeptide ions. Anal Chem. 2008;80(10):3684–92. doi: 10.1021/ac800067y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morelle W, Canis K, Chirat F, Faid V, Michalski JC. The use of mass spectrometry for the proteomic analysis of glycosylation. Proteomics. 2006;6(14):3993–4015. doi: 10.1002/pmic.200600129. [DOI] [PubMed] [Google Scholar]
- 12.Wilson IBH, Gavel Y, Vonheijne G. Amino-Acid Distributions around O-Linked Glycosylation Sites. Biochemical Journal. 1991;275:529–534. doi: 10.1042/bj2750529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Settineri CA, Medzihradszky KF, Masiarz FR, Burlingame AL, Chu C, Georgenascimento C. Characterization of O-Glycosylation Sites in Recombinant B-Chain of Platelet-Derived Growth-Factor Expressed in Yeast Using Liquid Secondary Ion Mass-Spectrometry, Tandem Mass-Spectrometry and Edman Sequence-Analysis. Biomedical and Environmental Mass Spectrometry. 1990;19(11):665–676. doi: 10.1002/bms.1200191106. [DOI] [PubMed] [Google Scholar]
- 14.Renfrow MB, Mackay CL, Chalmers MJ, Julian BA, Mestecky J, Kilian M, Poulsen K, Emmett MR, Marshall AG, Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Analytical and Bioanalytical Chemistry. 2007;389(5):1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 15.Pouria S, Corran PH, Smith AC, Smith HW, Hendry BM, Challacombe SJ, Tarelli E. Glycoform composition profiling of O-glycopeptides derived from human serum IgA1 by matrix-assisted laser desorption ionization-time of flight-mass spectrometry. Analytical Biochemistry. 2004;330(2):257–263. doi: 10.1016/j.ab.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 16.Peterman SM, Mulholland JJ. A novel approach for identification and characterization of glycoproteins using a hybrid linear ion trap/FT-ICR mass spectrometer. Journal of the American Society for Mass Spectrometry. 2006;17(2):168–179. doi: 10.1016/j.jasms.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Clowers BH, Dodds ED, Seipert RR, Lebrilla CB. Site determination of protein glycosylation based on digestion with immobilized nonspecific proteases and Fourier transform ion cyclotron resonance mass spectrometry. Journal of Proteome Research. 2007;6(10):4032–4040. doi: 10.1021/pr070317z. [DOI] [PubMed] [Google Scholar]
- 18.Ren JM, Rejtar T, Li L, Karger BL. N-Glycan structure annotation of glycopeptides using a linearized glycan structure database (GlyDB) Journal of Proteome Research. 2007;6(8):3162–73. doi: 10.1021/pr070111y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg D, Bern M, Parry S, Sutton-Smith M, Panico M, Morris HR, Dell A. Automated N-glycopeptide identification using a combination of single and tandem-MS. Journal of Proteome Research. 2007;6(10):3995–4005. doi: 10.1021/pr070239f. [DOI] [PubMed] [Google Scholar]
- 20.Wuhrer M, Catalina MI, Deelder AM, Hokke CH. Glycoproteomics based on tandem mass spectrometry of glycopeptides. Journal of Chromatography B. 2007;849(12):115–28. doi: 10.1016/j.jchromb.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 21.Hakansson K, Cooper HJ, Emmett MR, Costello CE, Marshall AG, Nilsson CL. Electron capture dissociation and infrared multiphoton dissociation MS/MS of an N-glycosylated tryptic peptide to yield complementary sequence information. Analytical Chemistry. 2001;73(18):4530–4536. doi: 10.1021/ac0103470. [DOI] [PubMed] [Google Scholar]
- 22.Wu SL, Huehmer AFR, Hao ZQ, Karger BL. On-line LC-MS approach combining collision-induced dissociation (CID), electron-transfer dissociation (ETD), and CID of an isolated charge-reduced species for the trace-level characterization of proteins with post-translational modifications. Journal of Proteome Research. 2007;6(11):4230–4244. doi: 10.1021/pr070313u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medzihradszky KF, Gillececastro BL, Settineri CA, Townsend RR, Masiarz FR, Burlingame AL. Structure Determination of O-Linked Glycopeptides by Tandem Mass-Spectrometry. Biomedical and Environmental Mass Spectrometry. 1990;19(12):777–781. doi: 10.1002/bms.1200191205. [DOI] [PubMed] [Google Scholar]
- 24.Goletz S, Leuck M, Franke P, Karsten U. Structure analysis of acetylated and non-acetylated O-linked MUC1-glycopeptides by post-source decay matrix-assisted laser desorption/ionization mass spectrometry. Rapid Communications in Mass Spectrometry. 1997;11(13):1387–1398. doi: 10.1002/(SICI)1097-0231(19970830)11:13<1387::AID-RCM28>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 25.Deguchi K, Ito H, Baba T, Hirabayashi A, Nakagawa H, Fumoto M, Hinou H, Nishimura SI. Structural analysis of O-glycopeptides employing negative-and positive-ion multi-stage mass spectra obtained by collision-induced and electron-capture dissociations in linear ion trap time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry. 2007;21(5):691–698. doi: 10.1002/rcm.2885. [DOI] [PubMed] [Google Scholar]
- 26.Mormann M, Paulsen H, Peter-Katalinic J. Electron capture dissociation of O-glycosylated peptides: radical site-induced fragmentation of glycosidic bonds. European Journal of Mass Spectrometry. 2005;11(5):497–511. doi: 10.1255/ejms.738. [DOI] [PubMed] [Google Scholar]
- 27.Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrometry Reviews. 2004;23(3):161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- 28.Holland JW, Deeth HC, Alewood PF. Analysis of O-glycosylation site occupancy in bovine kappa-casein glycoforms separated by two-dimensional gel electrophoresis. Proteomics. 2005;5(4):990–1002. doi: 10.1002/pmic.200401098. [DOI] [PubMed] [Google Scholar]
- 29.Edge ASB, Spiro RG. Presence of an O-Glycosidically Linked Hexasaccharide in Fetuin. Journal of Biological Chemistry. 1987;262(33):16135–16141. [PubMed] [Google Scholar]
- 30.Green ED, Adelt G, Baenziger JU, Wilson S, Vanhalbeek H. The Asparagine-Linked Oligosaccharides on Bovine Fetuin - Structural-Analysis of NGlycanase-Released Oligosaccharides by 500-Megahertz H-1-Nmr Spectroscopy. Journal of Biological Chemistry. 1988;263(34):18253–18268. [PubMed] [Google Scholar]
- 31.Roepstorff P, Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomedical Mass Spectrometry. 1984;11(11):601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- 32.Domon B, Costello CE. A Systematic Nomenclature for Carbohydrate Fragmentations in Fab-Ms Ms Spectra of Glycoconjugates. Glycoconjugate Journal. 1988;5(4):397–409. [Google Scholar]
- 33.Paizs B, Suhai S. Fragmentation pathways of protonated peptides. Mass Spectrometry Reviews. 2005;24(4):508–548. doi: 10.1002/mas.20024. [DOI] [PubMed] [Google Scholar]
- 34.Sagi D, Peter-Katalinic J, Conradt HS, Nimtz M. Sequencing of tri- and tetraantennary N-glycans containing sialic acid by negative mode ESI QTOF tandem MS. Journal of the American Society for Mass Spectrometry. 2002;13(9):1138–1148. doi: 10.1016/S1044-0305(02)00412-9. [DOI] [PubMed] [Google Scholar]
- 35.Harvey DJ, Martin RL, Jackson KA, Sutton CW. Fragmentation of N-linked glycans with a matrix-assisted laser desorption/ionization ion trap time-of-flight mass spectrometer. Rapid Communications in Mass Spectrometry. 2004;18(24):2997–3007. doi: 10.1002/rcm.1709. [DOI] [PubMed] [Google Scholar]
- 36.Bowie JH, Brinkworth CS, Dua S. Collision-induced fragmentations of the (M-H)(-) parent anions of underivatized peptides: An aid to structure determination and some unusual negative ion cleavages. Mass Spectrometry Reviews. 2002;21(2):87–107. doi: 10.1002/mas.10022. [DOI] [PubMed] [Google Scholar]
- 37.Harrison AG, Young AB. Fragmentation reactions of deprotonated peptides containing proline. The proline effect. Journal of Mass Spectrometry. 2005;40(9):1173–1186. doi: 10.1002/jms.891. [DOI] [PubMed] [Google Scholar]