Abstract
Molecular, cellular, and systems-level processes convert initial, labile memory representations into more permanent ones, available for continued reactivation and recall over extended periods of time. These processes of memory consolidation and reconsolidation are not all-or-none phenomena, but rather a continuing series of biological adjustments that enhance both the efficiency and utility of stored memories over time. In this chapter, we review the role of sleep in supporting these disparate but related processes.
Introduction
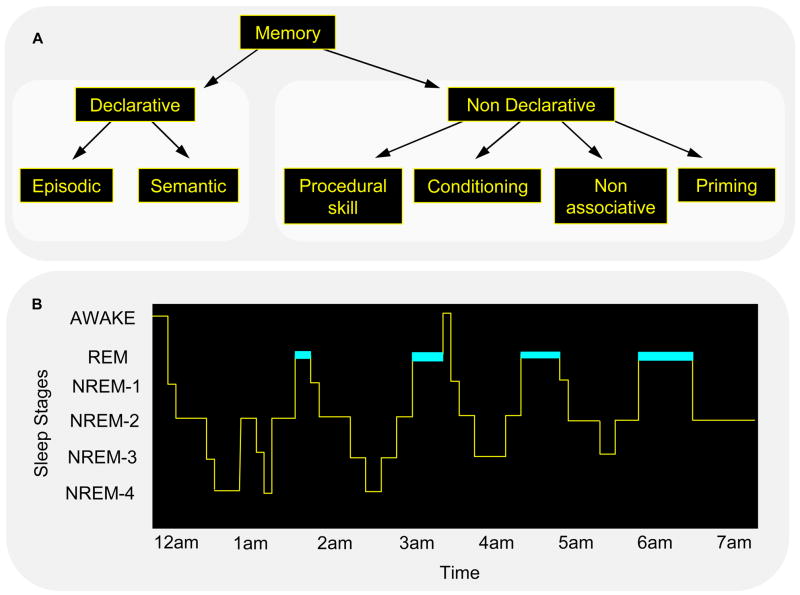
The question of “sleep-dependent memory consolidation” is a complex one. Each term in the phrase – sleep, dependent, memory, and consolidation – begs for clarification. For a start, the term “memory” covers a wide range of memory types, which differ in the kinds of information stored, the brain structures mediating this storage, and, in humans, whether the information is accessible to conscious awareness. There is no clear consensus at this time on how many such memory systems there are, and how they should be defined, either in terms of information content or brain structures involved in their storage [1]. The most widely accepted taxonomy divides human memories first into declarative and non-declarative, based on their accessibility to conscious recall, and then into finer and finer subdivisions of these basic categories (Fig. 1A) [2].
Figure 1.
Forms of memory and stages of sleep. Neither memory (A) nor sleep (B) represents a homogeneous phenomenon. (A) Declarative memory includes consciously accessible memories of fact-based information (i.e., knowing “what”), and contains several subcategories, including episodic memory (memory for events in one’s past) and semantic memory (memory for general knowledge) [110]. In contrast, non-declarative memory includes all non-conscious memories, and includes subcategories such as conditioning, implicit memory and procedural memory (i.e., knowing “how”). (B) In mammals, sleep is divided into REM and NREM sleep, and in primates and felines, NREM sleep has been divided into sub-stages 1 through 4, corresponding to increasingly deeper states of sleep [7]. The deepest NREM stages, stages 3 and 4, are collectively referred to as slow wave sleep (SWS), based on a prevalence of low frequency (0.5–4 Hz) cortical oscillations. Dramatic changes in brain electrophysiology, neurochemistry and functional anatomy occur across these sleep stages, making them biologically distinct from the waking brain, and dissociable from one another. For example, SWS is characterized by a diminution in cholinergic activity and REM sleep by a suppression of release of norepinephrine from the locus coeruleus and serotonin from the raphe nucleus. (Reproduced with permission from [8])
Similarly, the term “memory consolidation” refers to a poorly defined set of processes which take an initial, unstable memory representation and convert it into a form that is both more stable and more effective. At this time, it is unclear how memories are altered after initial encoding, and no consensus as to which of the processes contributing to this alteration should be included under the umbrella of memory consolidation. When the term was first introduced, it referred to as yet unknown processes which, over a period of minutes to hours, made learning resistant to degradation by, for example, electroconvulsive shock [3–5], but this notion of a single, relatively rapid process of memory consolidation has yielded to one including phases of stabilization, enhancement, and integration, extending over hours to years.
More recently, the concept “memory reconsolidation” has resurfaced to describe yet another aspect of post-encoding memory modification [6]. There is now evidence that when previously stabilized memories are reactivated, either by returning an animal to an earlier learning environment or by having humans briefly perform a previously learned task, the memory is destabilized and returns to a labile state in which it is again susceptible to destructive interference. Thus, the terms “consolidated” and “stabilized” must take on a more nuanced meaning, reflecting the relative consolidation and stabilization of the memory.
Many of the steps in this consolidation cascade occur preferentially or even exclusively during periods of sleep. Researchers are now focusing on identifying those types of memory, and, for each, the individual steps that show strong sleep-dependent activation. However, again, sleep does not refer to a simple unitary phenomenon, instead representing a complex array of brain states that differ in their physiology, chemistry, and phenomenological experiences. Sleep has been broadly divided into rapid eye movement (REM) sleep and non-REM (NREM) sleep, which alternate across the night, in humans in a 90-min cycle (Fig. 1B). NREM sleep is further subdivided into NREM stages 1–4 [7], which appear to differ in their contribution to sleep-dependent memory consolidation [8].
Before describing these systems of memory consolidation in greater detail, we would like to offer an overview of our perspective on memory consolidation and reconsolidation. First, all of these processes occur over time automatically, outside of awareness and without intent. Thus, they are specifically different from changes that result from conscious reminiscing or intentional rehearsal. In this respect, they are no different from molecular cascades triggered by an initial biochemical event, but while molecular cascades are normally restricted to a single cell, the cascade of events characterizing memory consolidation range from intracellular gene inductions to brain-wide, system-level reorganizations of memories representations.
Secondly, while these processes occur automatically, they are, nevertheless, modulated by other factors. Again, this is not different from what is seen with intracellular molecular cascades, but the greatly extended time course allows for different forms of modulation. As a result, the multiple components of memory consolidation and reconsolidation form a coherent whole, which functions to optimally integrate initially encoded memories into an organism’s existing informational networks, and which continues to refine and remodel these memories following reactivation, during wake and sleep. In short, memories do not simply form in the brain; they evolve.
For the purposes of this review, we use the term memory consolidation to refer to all post-encoding memory processing that is automatic and which occurs without intent or awareness under the rubric of “memory consolidation,” while those that require either conscious or behavioral rehearsal are excluded. Thus, the development of hippocampal independence as described by McClelland and colleagues [9] would be included, but improvement through actual or imagined rehearsal would not. Similarly, all post-recall memory processing that is automatic and which occurs without intent or awareness is placed under the rubric of “memory reconsolidation.”
Stages of memory consolidation and reconsolidation
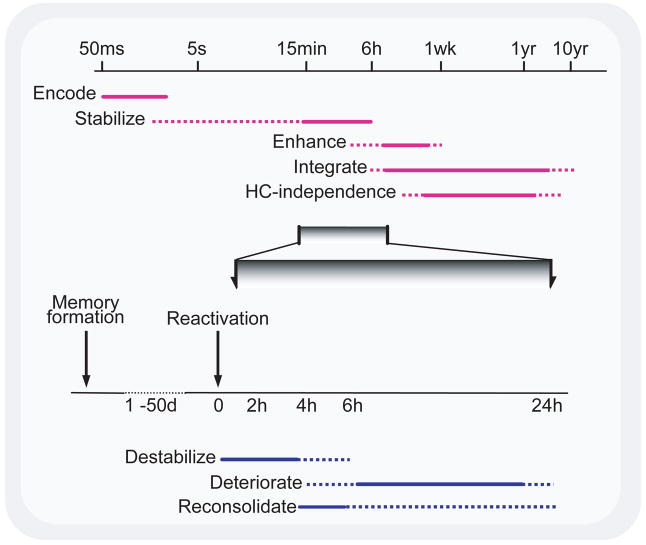
The evolution of a memory can be a long and complex process, occurring in several distinct stages (Fig. 2). While the initial encoding of a memory is a rapid (milliseconds) process, its long-term maintenance requires processes that continue to modify it over hours to years, processes that are collectively referred to as memory consolidation [10].
Figure 2.
Time course of memory processes. (Top) Memory formation and consolidation – after the initial rapid encoding of a sensory experience, the neural representation of the memory can go through a number of automatic processes, independent of rehearsal, intent, or awareness. These can stabilize and enhance a memory, making it resistant to interference and more effective to guiding behavior, and also integrating the memory into larger associative networks. The latter process is thought to permit episodic memories to be recalled without hippocampal (HC) involvement. The extent to which such processes affect different memory systems is unclear. Note logarithmic time scale. (Bottom) Memory reactivation and reconsolidation – after stabilization is complete, reactivation of a memory can lead to its return to an unstable form. Normally, such memories appear to be reconsolidated following this destabilization, but if such reconsolidation is blocked, degradation of the memory can ensue. (Reproduced with permission from [8])
Even the original view of consolidation as stabilization is now in flux. When originally proposed in 1900 [10], consolidation was defined by resistance to interference from competing memories. Animal studies in the middle of the 20th century demonstrated that such consolidation was also required for the formation of memories resistant to the more drastic actions of electroconvulsive shock (ECS) [11] and protein synthesis inhibitors [12]. More recently, the concept of memory consolidation has been simultaneously extended and challenged. Human motor skill memories have now been shown to be disrupted by training on an alternate task within the first hours after training, suggesting that such learning also requires a process of stabilization [13,14].
More importantly, these newer findings demonstrate that the time course of stabilization can be functionally significant. When initially conceptualized, consolidation was considered to be an inexorable process which, once started, continued to completion except under the most severe of insults, such as ECS. From this perspective, the length of the consolidation process could be considered irrelevant, presumably determined by idiosyncrasies of evolution. However, the finding that ecologically relevant stimuli can also interrupt consolidation suggests a functional role for this interval, where a memory is consolidated unless other similar, and competing, memories are formed shortly after the first memory. This could allow for the functional correction of inadvertently or imprecisely formed memories before they are stabilized.
The recent failure of Caithness and others to reproduce these interference effects for motor adaptation learning [15] is perhaps not surprising, since it is still unclear what the stimulus characteristics are for an effective blockade of this early consolidation. While they failed to observe interference with new learning on their tasks, it is still likely that even these memories remain sensitive to ECS and protein synthesis inhibitors for several hours [16], reflecting a required process of consolidation. Indeed, there is little objection to the view that conversion of initial memory traces into long-term memories requires protein synthesis [5].
While memory consolidation clearly serves to stabilize memories, this is far from all that it does. For a start, consolidation also enhances memories, for example, improving behavioral performance, independent of further practice [17]. Although these two phases of consolidation could reflect a single process, we believe that this is unlikely for at least two reasons [18]. First, the consolidation process leading to enhancement of a motor sequence learning task continues for up to 10 times as long as the earlier stabilizing phase [14,17] (Fig. 2, top), and for a visual discrimination process continues over at least 2–4 days [19]. Second, while stabilization of this motor sequence task occurs over six hours of wake, the enhancement phase for both tasks occurs only during sleep [17,19]. Other post-encoding stages of memory consolidation include the integration of recently encoded memories into existing memory networks (memory integration/association) [20,21], the development of hippocampal independence for declarative memories [9,22], and even the active weakening of memory representations (“memory erasure”) [23]. All of these are thought to be facilitated by sleep.
Memories can be retained for weeks to years, during which time they can be effectively recalled, but the mere act of memory recall can destabilize the memory and return it to a labile form, where it is again vulnerable to interference and degradation. Reconsolidation—the transformation of this now destabilized memory into a restabilized form—is necessary if the memory is to be retained in the face of interference [6]. Otherwise, it can degrade relatively quickly (Fig. 2, bottom). (To be more precise, it is unclear whether such degradation actually weakens the memory trace or, instead, simply makes it inaccessible to recall mechanisms, but this distinction is irrelevant for our current review.)
We noted above that memories are not simply formed, but evolve over time. This time course appears to serve two conceptually distinct functions. Some post-encoding stages of memory consolidation appear necessary simply to cope with the constraints of the brain. Thus, the molecular mechanisms which support rapid memory encoding (e.g., calcium influxes) are inadequate for long-term maintenance of synaptic changes, while those processes which support long-term maintenance (e.g., protein synthesis) cannot be accomplished quickly enough to support rapid encoding [24]. Similarly, network structures that can capture an episodic memory may be incapable of supporting dense network storage of memories [9]. A recent review of off-line memory reorganization has recently described many of these features in detail [25].
However, processes of consolidation also serve to facilitate behavior. As examples, they could (i) automate behaviors, shifting representations from declarative to procedural systems and reducing frontal demands; (ii) extract valuable details from complex episodic memories so that one can, for example, recall the sum of five plus five without recalling an episodic memory of when and where it was learned; and (iii) integrate information, so that one associates all the “addition facts” with one another. For both classes of memory consolidation processes, sleep is now thought to play an important role in meeting the demands of the organism.
Our understanding of memory reconsolidation is at a much earlier stage. Although originally reported in the 1960s [3,4], the details of memory reconsolidation have only recently come under intensive investigation [6]. Its component processes, their time courses and functions, are far less well defined, and almost no attention has been paid to their possible dependence on wake–sleep states. Likewise, there has been little discussion of the significance or possible functions of these processes.
Conceptually, there are at least four processes that a consolidated memory can undergo: (i) reactivation, leading to (ii) destabilization, which in turn leads to either (iii) degradation or (iv) reconsolidation. However, the time courses of these individual steps, the mechanisms and brain states which produce them, and even their biological functions, remain unclear.
While memory reactivation can presumably occur in a fraction of a second, the destabilizing effects of such reactivation appear to depend on longer periods of reactivation. Anywhere from 30 sec to 10 min can be required to produce destabilization (defined by memory degradation following the prevention of reconsolidation) [26–28], with longer times required when the intensity and duration of the initial training is increased. The duration of this destabilization appears to be on the order of 5–6 hr, after which the memory becomes reconsolidated and again resistant to destructive interference [26,29,30].
Once destabilized, and in the absence of subsequent reconsolidation, degradation of a memory has generally been considered a passive process, perhaps based on molecular turnover. Alternatively, it may be that the memory is not degraded at all, but its recall ability is lost. Regardless, the nature of this degradation remains unclear. Currently, degradation is defined behaviorally as diminished performance of the learned task or response. There is little data on the time course over which this reduced efficacy, let alone its molecular correlates, develops. Following reactivation and blockade of reconsolidation, previously learned behaviors are still intact 2–4 hr later [26,31,32] [27]. This makes sense, since reconsolidation appears to take at least this long, and it would be counterproductive for memories to begin to degrade before reconsolidation normally has completed. By 24 hr after reactivation, any degradation of the memory appears to be complete [32–34] (see also [35], Table 1).
While early studies using ECS or administration of protein synthesis inhibitors to block reconsolidation suggested that reconsolidation as serving no practical purpose other than preventing the inadvertent degradation of the memory, hints of these more complex mechanisms and functions come from studies showing that inhibitors of cholinergic [33] and noradrenergic [29] neuromodulation can also prevent reconsolidation. In addition, N-methyl-D-aspartate (NMDA) antagonists reportedly can block the destabilization associated with reactivation [36]. Finally, even interference from training on competing tasks have now been shown to block reconsolidation [37]. In light of these more recent findings, we propose that destabilization and reconsolidation of memories simply represent yet another sophisticated mechanism for modulating and modifying preexisting memories.
Sleep-dependent memory consolidation
Over the last 10 years, a large body of evidence has been reported, supporting a role for sleep in the offline (re)processing of memories. We have recently reviewed the evidence for the critical role of sleep in memory consolidation [38] and only briefly summarize the behavioral literature here ([for an opposing viewpoint, see 39]).
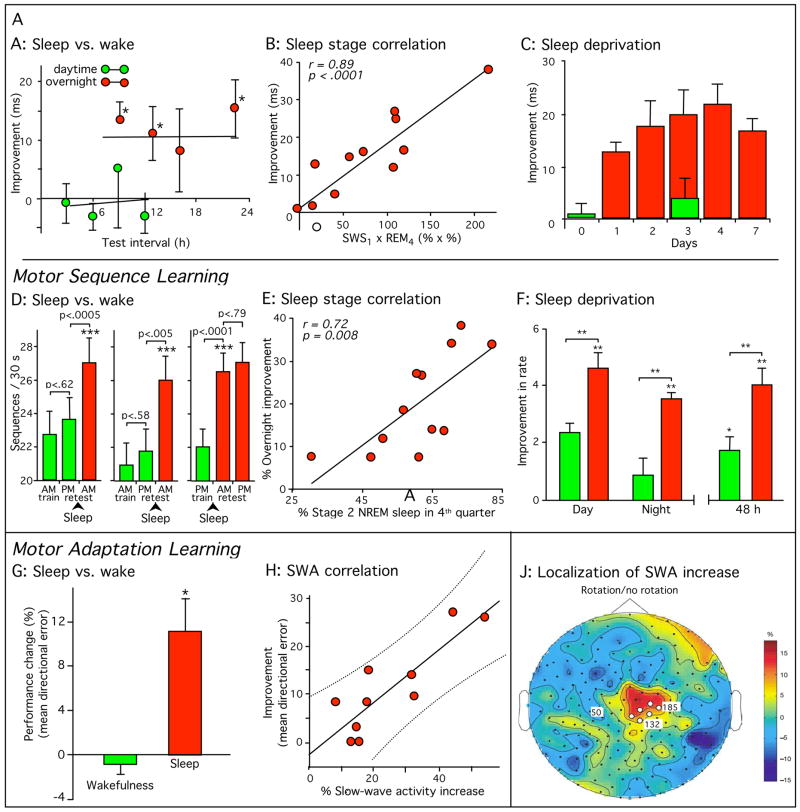
Specific stages of sleep appear to be critical for discrete steps in the consolidation of various forms of memory, while for other steps sleep appears unnecessary ([for review, see 40]). For example, stabilization of some forms of procedural motor memory can develop across 3–6 hr of wake [13,16,37]. In contrast, the offline enhancement of procedural sensory and motor memories has almost always been found to depend on overnight sleep, with equivalent periods of wake failing to produce any performance gains [14,17,19,37,41–52] (see Fig. 3 for examples). Such overnight enhancement has been seen for a variety of memory tasks, but individual tasks differ dramatically in the sleep stages or sleep characteristics required. Consolidation of motor skills has been connected to NREM sleep stages, stage 2 in some cases and slow wave sleep (SWS) in others, as well as to specific physiological characteristics of NREM [17,41,44,45,53]. In contrast, both SWS and REM sleep have been associated with the consolidation of memory for a visual texture discrimination task [19,46,47], suggesting that there may be more than a single phase of sleep-dependent consolidation [48,54].
Figure 3.
Sleep-dependent consolidation of procedural memories. (A–C) Participants in a visual texture discrimination task show improvement only after post-training sleep. Improvement correlated with early-night slow-wave sleep and late-night REM sleep. (D–F) Participants in a motor sequence finger-tapping task show similar sleep-dependent improvement, even when finger movement was suppressed with mittens during wake periods (D, right panel), improvement which correlated with late-night stage 2 non-REM sleep. (G-J) Participants in a motor adaptation task also show sleep-dependent improvement, correlated with EEG slow-wave activity in task-related regions of the cortex. All error bars represent standard error of the mean. Green bars, performance without intervening sleep or without sleep on the first post-training night. Dark red bars, performance after normal sleep. (Reproduced with permission from [8]; Panel J with permission from [45])
In some instances, the sleep dependency of consolidation for a single task can depend on subtle features of the task. The serial reaction time (SRT) task is a procedural visual-motor task. In it, subjects watch a screen with four circles displayed across the center (Fig 4). As individual circles light up, subjects press the key located immediately below it. As the lights flash one after another, subjects slowly learn the pattern in which they are flashing, commonly a sequence of at least 12 lights (i.e., each light three times in a complex order). If subjects are trained on this task and then retested 12 hr later, enhanced performance is seen, but the sleep dependency of this improvement is not straightforward. When subjects are told ahead of time that there is a pattern to the flashing of the lights, they show time-dependent improvement across a night of sleep, but not across a day when they are awake. On the other hand, if they are simply told that it is a reaction time test, and if they do not become consciously aware of the pattern during training, then they show improvement both across the day and across the night [44]. Thus, it appears that explicit knowledge of the sequence is enhanced by sleep and not by wake, while implicit knowledge is enhanced by both. However, this still fails to fully describe the sleep dependency of the learning, since when subjects are tested after the 12-hr period specifically for their knowledge of the finger sequence (e.g., right hand little finger – index finger – ring finger) or the numerical sequence (1 – 4 – 2), implicit knowledge of the finger sequence appears to improve only across the day and implicit knowledge of the numerical sequence improves only across the night [55]. Thus, the distinction between implicit and explicit knowledge might only reflect the fact that explicit knowledge is always of the numerical sequence and hence improves only across the night, while implicit knowledge is of both the numerical and motor sequences and improves across either wake or sleep. This is not the only procedural task to show improvement during wake. An auditory procedural task has been reported to show enhancement without the need for sleep [50], although a second auditory task showed sleep-dependent changes in the brain response to test stimuli [49]). While most procedural memories show sleep-dependent enhancements, in the end it remains unclear what determines whether a given procedural task will in fact show such enhancement.
Figure 4.
Serial Reaction Time Task. Four circles are presented on a computer screen and lit in a repeated, pseudorandom order. When each circle is lit, the button beneath it must be pressed. In this example, the fourth, then first, then third button are lit sequentially. To determine whether subjects are learning the finger movements (as a motor task) or the spatial positions (as a visual task), subjects are retested using their other hand. For example, subjects trained with their right hand are retested with their left hand. For the spatial sequence 4–1–3 shown here, during training subjects would press keys with the little, then index, and then ring finger of their right hand. If asked to type the same spatial sequence (4–1–3) at retest, subjects would have to use the index, little, and then middle fingers of their left hand. In contrast, to test for retention of learning of the motor sequence (little–index–ring fingers), subjects would be retested on the sequence 1–4–3 in place of 4–1–2.
The evidence for sleep-dependent consolidation of declarative memories is less consistent. First, it is difficult to separate processes of stabilization from processes of enhancement, since performance generally deteriorates across both wake and sleep, but when performance after equivalent periods of wake and sleep are compared, early studies split over a role for sleep-dependent memory consolidation ([for review, see 56]). More recently, Born and colleagues, using a word-pair associates task, have shown enhanced recall after periods of nocturnal sleep compared to similar periods without sleep. Furthermore, periods of early night sleep, rich in SWS, were particularly beneficial for this consolidation [57]. The fact that this effect is only seen during SWS-rich periods early in the night, rather than across all sleep periods, argues that the physiological state of sleep, and of particular stages of sleep, are critical for this consolidation process [38]. As further support, they have shown that daytime training can trigger changes in characteristics of early night SWS, with modifications reported in both the number of sleep spindles [58], and in the coherence of NREM slow-frequency electroencephalographic (EEG) oscillations [59]. In support of these findings, Peigneux and colleagues [60] have reported that overnight improvement on a hippocampally mediated spatial memory task is positively correlated with increased hippocampal activation during SWS, a finding that would seem to argue in favor of actual memory enhancement, and against the diminished interference model.
Memory destabilization and reconsolidation may also be facilitated by sleep. Although there is little data that directly pertains to this question, we propose that both degradation and reconsolidation processes can, and in some circumstances must, occur during sleep. Indeed, most rodent studies of reconsolidation are carried out during the light (sleep) phase of the circadian cycle, and it is likely that animals in all of these studies slept between reactivation and subsequent measurements of reconsolidation. Thus, existing evidence cannot distinguish between time-dependent and sleep-dependent reconsolidation. This is different from the situation with initial stabilization, for which there is good evidence of consolidation during wake.
Support for this comes from studies of procedural memory reconsolidation in humans [14]. Following training on a finger-tapping motor sequence task, subjects show overnight sleep-dependent gains in performance accuracy, but if subjects are taught a new competing sequence 10 min after learning the first sequence, the normal overnight improvement in accuracy is completely blocked [17]. If the time period between learning the two sequences was increased from 10 min to 6 hr, no significant interference was observed the next day.
If the original memory is reactivated through 90 sec of rehearsal 24 hr after training, however, the memory appears to be destabilized, and now training on a second sequence leads to a complete reversal of the improvement in accuracy seen across the first night, to the level seen at the end of initial training, but not to the much higher level seen at the very start of initial training. These results suggest that the deterioration in performance seen following blockade of reconsolidation might be limited to the reversal of earlier sleep-dependent consolidation.
Sleep and brain plasticity
Memory formation depends on brain “plasticity”–lasting structural and/or functional neural changes in response to stimuli (such as experiences). If sleep is to be considered a critical mediator of memory consolidation, then evidence of sleep-dependent plasticity would greatly strengthen this claim. Indeed, there is now a wealth of data describing sleep-dependent brain plasticity at a variety of different levels in both animals and humans, complimenting evidence of sleep-dependent changes in behavior.
Neuroimaging studies
Several studies have investigated whether daytime training is capable of modifying functional brain activation during subsequent sleep. Based on animal studies, neuroimaging experiments have explored whether the signature pattern of brain activity elicited while practicing a memory task actually re-emerges, that is, is “replayed”, during subsequent sleep. Using brain imaging, Maquet and colleagues have shown that patterns of brain activity expressed during training on a serial reaction time motor task reappear during subsequent REM sleep, while no such change in REM sleep brain activity occurs in subjects who received no daytime training [61]. Furthermore, the extent of learning during daytime practice exhibits a positive relationship to the amount of reactivation during REM sleep [62]. As with previously described animal studies [63], these findings suggest that it is not simply experiencing the task which modifies subsequent sleep physiology, but the process of learning itself. Similar findings have been reported using a virtual maze task. Daytime task learning is initially associated with hippocampal activity. Then, during post-training sleep, there was a re-emergence of hippocampal activation, this time specifically during SWS. Most compelling, however, the amount of SWS reactivation in the hippocampus is proportional to the amount of next-day task improvement, suggesting that this reactivation leads to off-line memory improvement[60]. Such sleep-dependent replay may potentially modify synaptic connections established within specific brain networks during practice, strengthening some synaptic circuits while potentially weakening others in the endeavor of refining the memory.
A second approach, which more directly examines sleep-dependent plasticity, compares patterns of brain activation before and after a night of sleep. In contrast to measuring changes in functional activity during sleep, this technique aims to determine whether improved performance results from an overnight, sleep-dependent re-structuring of the neural representation of the memory. Using the sleep-dependent motor-skill task, Walker and colleagues have recently used functional magnetic resonance imaging (fMRI) to investigate differences between patterns of brain activation before and after sleep [64]. Following a night of sleep, and relative to an equivalent intervening time period awake, increased activation was identified in motor control structures of the left cerebellum (Fig. 5A) and right primary motor cortex (Fig. 5B)–changes which allow more precise motor output [65] and faster mapping of intention to key-press [66]. There were also regions of increased activation in the medial prefrontal lobe and hippocampus (Fig. 5C&D), structures recently identified as supporting improved sequencing of motor movements [67–70]. In contrast, decreased activity post-sleep was identified bilaterally in the parietal cortices (Fig. 5E), possibly reflecting a reduced need for conscious spatial monitoring[71–73] due to improved task automation [43], together with regions of signal decrease throughout the limbic system (Fig. 5F–H), suggesting a decreased emotional task burden. In total, these results suggest that sleep-dependent motor learning is associated with a large-scale plastic reorganization of memory throughout several brain regions, allowing skilled motor movements to be executed more quickly, more accurately and more automatically following sleep. These findings hold important implications for understanding the brain basis of perfecting real-life skills, and may also signify a potential role for sleep in clinical rehabilitation following brain damage.
Figure 5.
Sleep-dependent motor memory reorganization in the human brain. Subjects were trained on the sleep-dependent finger-tapping motor skill task and then tested 12 hours later, either following a night of sleep or a day of wake, during a functional magnetic resonance imaging (fMRI) brain-scanning session. Scans after sleep and wake were compared (subtracted), resulting in regions showing increased fMRI activity post-sleep (in red/yellow; A–D) or decreased signal activity (in blue; E–H) post-sleep, relative to post-wake. Activation patterns are displayed on three-dimensional rendered brains (top panel of each graphic), together with corresponding coronal sections (bottom panel of each graphic). Coronal sections all show left on the left; three-dimensional renderings are reversed left for right except (A). Following sleep, regions of increased activation were identified in the left cerebellum (A), the right primary motor cortex (B), the right hippocampus (C), and the right medial prefrontal cortex (D). Regions of decreased activity post-sleep were expressed bilaterally in the parietal lobes (E), together with the left insula cortex (F), left temporal pole (G), and left frontopolar area (H), all regions of the extended limbic system. All data are displayed at a corrected threshold of p < 0.05. (Reproduced with permission from [111].
Walker and colleagues have also used fMRI to investigate whether overnight reorganization similarly occurs in sensory-perceptual systems using the sleep-dependent visual texture discrimination task described earlier [74]. Subjects were trained with or without intervening sleep. Relative to the condition without sleep, retest following sleep was associated with significantly greater activation in an area of primary visual cortex corresponding to the visual target location. However, there were also several other regions of increased post-sleep activity, throughout both the ventral object recognition (inferior parietal and occipital-temporal junction) and dorsal object location (superior parietal lobe) pathways [75], together with corresponding decreases in the right temporal pole, a region involved in emotional visual processing. Thus, a night of sleep appears to reorganize the representation not only of procedural motor but of visual skill memories as well, with greater activation throughout the visual processing streams, offering improved identification of both the stimulus form and its location in space, and with signal decreases in the temporal pole, reflecting a reduced emotional task burden that results from the overnight learning benefits.
Maquet et al.[76] have investigated the detrimental effects of sleep deprivation on underlying brain activity using a visuo-motor adaptation task–the only such study to date. Subjects were trained on the task and retested three days later, with half the subjects deprived of sleep the first night. At retest, subjects performed both the previously learned motor task and a new related task. Controls, who slept all three nights, showed both enhanced behavioral performance at retest, and a selective increase in activation in the superior temporal sulcus (a region involved in the evaluation of complex motion patterns) relative to subjects deprived of sleep the first night. In contrast, no such enhancement of either performance or brain activity was observed in these subjects, indicating that sleep deprivation had interfered with a latent process of plasticity and consolidation. This study offers an early indication that sleep deprivation not only disrupts consolidation but the underlying neural mechanisms that support it as well.
Electrophysiological studies
Throughout the sleep cycle, both REM and NREM sleep stages contain numerous distinct electrophysiological events which contribute to each unique physiological state. Many of these electrical phenomena have been implicated in processes of plasticity, either potentiating or depressing synaptic connections [77]. For example, it has been proposed that sleep spindles, seen most commonly during stage 2 NREM sleep, can provide brief trains of depolarizing inputs to targets in the neocortex, which are similar to spike trains used experimentally to induce long-term synaptic potentiation [78–81]. Indeed, Steriade and colleagues [82] have shown that experimental trains of impulses similar to those produced by sleep spindles can produce lasting changes in the responsiveness of cortical neurons. Similarly, theta waves, seen in the hippocampus during REM sleep in both humans [83] and other animals [84], greatly facilitate the induction of long-term potentiation (LTP) in the hippocampus, potentiation that is believed to be a physiological mediator of memory formation [85,86].
As noted earlier, phasic events during REM sleep, and ponto-geniculo-occipital (PGO) waves specifically, have been associated with learning. Sanford et al. [87] have demonstrated that fear conditioning in rats can increase the amplitude of elicited P-waves during REM sleep, suggesting again that they represent a homeostatically regulated component of a sleep-dependent mechanism of learning and plasticity [cf. 63]. These PGO waves occur in a phase-locked manner with theta wave activity during REM sleep [88,89]. This is particularly interesting because experimental hippocampal stimulation at the peaks of theta waves facilitate LTP. The same stimulation applied at the troughs of the theta waves instead leads to long-term depression of synaptic responses [86,90]. These findings suggest that natural PGO activity during REM sleep may serve as an endogenous mediator of synaptic plasticity, based on its coincidence with theta wave oscillations, which, depending on its phase relationship, could either strengthen or weaken synaptic connections, both of which are necessary for efficient network plasticity.
Selective reactivation is seen not only from the human neuroimaging studies described above but also from more precise measurement of sleep-dependent network reactivation in the rat. Several groups have investigated the firing patterns of large networks of individual neurons across the wake-sleep cycle in a variety of cortical and subcortical regions of the rat brain. The signature firing patterns of these networks, expressed during waking performance of spatial tasks and novel experiences, are replayed during subsequent SWS and REM sleep, with replay during REM at speeds similar to those seen during waking but those in SWS being an order of magnitude faster in some, but not all, studies [84,91–94]. Dave and Margoliash [95,96] have shown that waking patterns of pre-motor activity observed during song-learning in the zebra finch are also replayed during sleep, with a temporal structure similar to that seen in wake.
Together, these data indicate that temporal patterns of network activity seen during waking experiences are consistently reacted during subsequent sleep across a broad spectrum of phylogeny. This replay of events is hypothesized to trigger distinct but complimentary processes within reactivated neuronal ensembles. Ribeiro et al. [94] have suggested that SWS reinstantiates the memory representation through network reverberation, while subsequent REM sleep then potentiates the memory for subsequent post-sleep recall, through gene-induction mediated synaptic plasticity.
Cellular studies
Recently, a form of sleep-dependent plasticity at the cellular level has been elegantly demonstrated during early post-natal development of the cat visual system [97,98]. Under normal circumstances, brief periods of monocular visual deprivation during critical periods of development lead to the remodeling of synaptic connectivity, with the deprived eye’s inputs to cortical neurons being first functionally weakened and then anatomically diminished [99]. Frank et al. [100] have now shown that when 6 hr of monocular deprivation are followed by 6 hr of sleep, the size of the monocularity shift doubles. In contrast, if the cats are kept awake for these same 6 hr (in the dark, without input to either eye), a non-significant reduction in the size of the shift occurs. Thus, sleep can contribute as much to developmental changes in synaptic connectivity as does visual experience, presumably by enhancing the initial changes occurring during a prior period of monocular deprivation. In contrast, sleep-deprivation results in a loss of previously formed, experience-dependent synaptic changes, a pattern seen as well in humans, albeit at the behavioral level [19,42].
Shaffery et al. [101] have reported similar findings of sleep-dependent plasticity in the rat visual cortex, suggesting that REM sleep, in conjunction with visual experience, modulates the initial course of visual cortex maturation. In rats under 30 days of age, electrical stimulation produces increased excitability (potentiation) in specific layers of the visual cortex, while stimulation after this early developmental stage fails to produce such potentiation. Depriving rats of REM sleep during this period extends this window of plasticity by as much as seven days, suggesting that events occurring during REM sleep modulate the duration of this period of experience-dependent plasticity.
Molecular studies
At the molecular level, Smith et al. [102] have shown that administration of protein synthesis inhibitors to rats during REM sleep windows thought to be critical for consolidation prevents behavioral improvement following the sleep period. Such protein synthesis could reflect the activation of genetic cascades which produce key molecules for synaptic remodeling. Our understanding of such gene inductions during sleep is only beginning. Although several of the known “immediate early genes” (IEGs) are specifically down-regulated during sleep [103–105], approximately 100 genes are specifically up-regulated during sleep [106], almost the same number that are up-regulated during wakefulness. Moreover, up-regulation of these genes during sleep was seen only in the brain tissue.
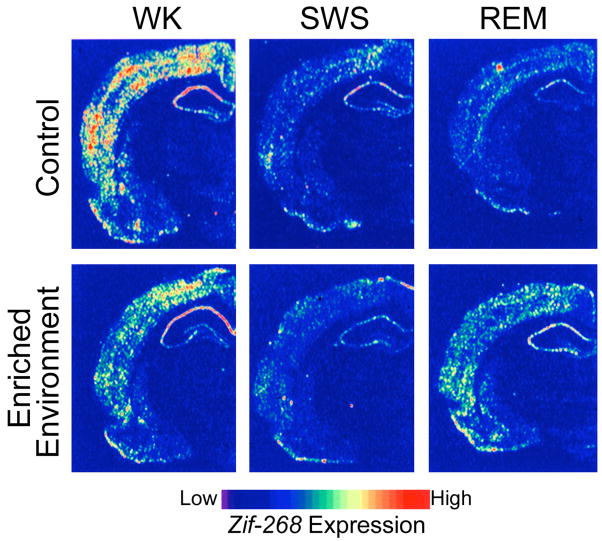
This extensive up-regulation of genes during sleep is seen even in the absence of any specific learning tasks being performed prior to sleep. If this up-regulation were specifically related to consolidation of recent learning and memory formation, one would expect that such gene inductions would be seen specifically after training on tasks that undergo sleep-dependent consolidation. Indeed, such learning-specific up-regulation has been observed. Ribeiro and colleagues found up-regulation in rats of zif-268, a plasticity associated IEG, during REM sleep following exposure to a rich sensorimotor environment, but found down-regulation during both SWS and REM sleep in the absence of such exposure [107]. This provides additional molecular evidence for the existence of windows for increased neuronal plasticity during REM sleep periods following enriched waking experience (Fig. 6), in agreement with both behavioral, physiological, and neuroimaging studies.
Figure 6.
Experience-dependent up-regulation of the synaptic plasticity related immediate early gene zif-268 during periods of wakefulness and REM sleep in the rat. Autoradiograms of frontal coronal brain sections in which gene-expression levels best represent the means for each group studied. (Top panels) In controls, zif-268 expression decreased from wake (WK) to SWS and REM. (Bottom panels) In enriched-environment animals, zif-268 levels decreased from WK to SWS, but then increased in REM. This effect was particularly noticeable in the cerebral cortex and the hippocampus. (Reproduced with permission from [107])
This rich environment effect can be mimicked by brief electrical stimulation of the medial perforant pathway [108], which normally carries signals from the cortex into the hippocampus. Unilateral stimulation results in a wave of zif-286 expression during subsequent REM sleep, with expression seen predominantly in the ipsilateral amygdala, entorhinal, and auditory cortices during the first REM sleep episodes after LTP induction, but extending into somatosensory and other cerebral cortices during subsequent REM periods [108]. These distinct phases of induction may correspond to the unique stages of consolidation previously reported from behavioral studies [14].
Conclusions
Learning and memory are dependent on processes of brain plasticity, and sleep-dependent learning and memory consolidation must be mediated by such processes. Many examples of such plasticity during sleep have now been reported, with several of them specifically induced by waking experiences. What remains to be demonstrated is that these specific components of brain plasticity, aside from the overall requirement for protein synthesis, specifically mediate sleep-dependent learning and memory consolidation. Such evidence would require elegant interventions in the cellular and molecular processes of brain plasticity during the normal course of sleep-dependent consolidation, studies which most likely are already in progress.
The opposing processes of synaptic stabilization and plasticity have recently been highlighted in a review by Abraham and Robins [109], who argue that functional stability requires molecular plasticity, so that the encoding of new information necessarily modifies the storage of older memories. The processes of memory consolidation and reconsolidation offer a series of opportunities for such plastic modification to occur. As such, they may be thought of as processes of memory organization, reorganization and refinement. While some of these events, such as initial stabilization, might reflect simple strengthening of the initial memory trace, sleep-dependent stages of consolidation and possibly reconsolidation are likely more complex, integrating memories within neural networks and memory systems.
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH48,832, MH65,292, MH67,754, and MH69,985).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Squire LR, Zola-Morgan S. Structure and function of declarative and non-declarative memory systems. Proc Natl Acad Sci USA. 1996;93:13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schacter D, Tulving E. What are the memory systems of 1994? In: Tulving E, editor. Memory systems 1994. viii. The MIT Press; 1994. pp. 1–38. [Google Scholar]
- 3.Misanin JR, et al. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 4.Schneider AM, Sherman W. Amnesia: a function of the temporal relation of footshock to electroconvulsive shock. Science. 1968;159:219–221. doi: 10.1126/science.159.3811.219. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 6.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 7.Rechtschaffen A, Kales A. A manual standardized terminology, techniques and scoring system for sleep stages of human subjects. U.S. Department of Health; 1968. [DOI] [PubMed] [Google Scholar]
- 8.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 9.McClelland JL, et al. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 10.Muller GE, Pilzecker A. Experimentelle Beitrage zur Lehre von Gedachtnis. Z Psychol. 1900;1:1–300. [Google Scholar]
- 11.Duncan CP. The retroactive effect of electroshock on learning. Journal of Comparative Physiology and Psychology. 1949;42:32–44. doi: 10.1037/h0058173. [DOI] [PubMed] [Google Scholar]
- 12.Agranoff BW, et al. Memory fixation in the goldfish. Proceedings of the National Academy of Science USA. 1965;54:788–793. doi: 10.1073/pnas.54.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brashers-Krug T, et al. Consolidation in human motor memory. Nature. 1996;382:252–255. doi: 10.1038/382252a0. [DOI] [PubMed] [Google Scholar]
- 14.Walker MP, et al. Sleep and the time course of motor skill learning. Learning and Memory. 2003;10:275–284. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caithness G, et al. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci. 2004;24:8662–8671. doi: 10.1523/JNEUROSCI.2214-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muellbacher W, et al. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- 17.Walker MP, et al. Practice with sleep makes perfect: sleep dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 18.Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005 doi: 10.1017/s0140525x05000026. In Press. [DOI] [PubMed] [Google Scholar]
- 19.Stickgold R, et al. Visual discrimination learning requires post-training sleep. Nature Neuroscience. 2000;2:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 20.Stickgold R. EMDR: a putative neurobiological mechanism of action. Journal of Clinical Psychology. 2002;58:61–75. doi: 10.1002/jclp.1129. [DOI] [PubMed] [Google Scholar]
- 21.Dumay N, Gaskell MG. Do words go to sleep? Exploring consolidation of spoken forms through direct and indirect measures. Behav Brain Sci. 2005;28 in press. [Google Scholar]
- 22.Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 23.Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 24.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 25.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 26.Nader K, et al. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krakauer JW, et al. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. Journal of Neuroscience. 2005;25:473–478. doi: 10.1523/JNEUROSCI.4218-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Przybyslawski J, et al. Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci. 1999;19:6623–6628. doi: 10.1523/JNEUROSCI.19-15-06623.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gruest N, et al. Memory consolidation and reconsolidation in the rat pup require protein synthesis. J Neurosci. 2004;24:10488–10492. doi: 10.1523/JNEUROSCI.2984-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debiec J, et al. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 32.Duvarci S, Nader K. Characterization of fear memory reconsolidation. J Neurosci. 2004;24:9269–9275. doi: 10.1523/JNEUROSCI.2971-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boccia MM, et al. Memory consolidation and reconsolidation of an inhibitory avoidance response in mice: effects of i.c.v. injections of hemicholinium-3. Neuroscience. 2004;124:735–741. doi: 10.1016/j.neuroscience.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Myers KM, Davis M. Systems-level reconsolidation: reengagement of the hippocampus with memory reactivation. Neuron. 2002;36:340–343. doi: 10.1016/s0896-6273(02)01017-6. [DOI] [PubMed] [Google Scholar]
- 36.Nader K, et al. Society for Neuroscience Program No 327.329. Society for Neuroscience; 2004. Double dissociation of the mechanisms mediating the induction of reconsolidation from those mediating the expression of a conditioned response. [Google Scholar]
- 37.Walker M, et al. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- 38.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Vertes RP. Memory consolidation in sleep; dream or reality. Neuron. 2004;44:135–148. doi: 10.1016/j.neuron.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005;28:51–104. doi: 10.1017/s0140525x05000026. [DOI] [PubMed] [Google Scholar]
- 41.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 42.Fischer S, et al. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002;99:11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuriyama K, et al. Sleep-dependent learning and motor skill complexity. Learn Mem. 2004;11:705–713. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robertson EM, et al. Awareness modifies the skill-learning benefits of sleep. Curr Biol. 2004;14:208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Huber R, et al. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 46.Karni A, et al. Dependence on REM sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 47.Gais S, et al. Early sleep triggers memory for early visual discrimination skills. Nat Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 48.Stickgold R, et al. Visual discrimination task improvement: A multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 49.Gaab N, et al. The influence of sleep on auditory learning - A behavioral study. Neuroreport. 2004;15:731–734. doi: 10.1097/00001756-200403220-00032. [DOI] [PubMed] [Google Scholar]
- 50.Atienza M, et al. The time course of neural changes underlying auditory perceptual learning. Learn Mem. 2002;9:138–150. doi: 10.1101/lm.46502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atienza M, et al. Posttraining sleep enhances automaticity in perceptual discrimination. J Cogn Neurosci. 2004;16:53–64. doi: 10.1162/089892904322755557. [DOI] [PubMed] [Google Scholar]
- 52.Fenn KM, et al. Consolidation during sleep of perceptual learning of spoken language. Nature. 2003;425:614–616. doi: 10.1038/nature01951. [DOI] [PubMed] [Google Scholar]
- 53.Fogel S, et al. Congress Physiological Basis for Sleep Medicine. Vol. 7. Actas de Fisiología; 2001. Increased sleep spindle activity following simple motor procedural learning in humans; p. 123. [Google Scholar]
- 54.Giuditta A, et al. The sequential hypothesis of the function of sleep. Behavioral Brain Research. 1995;69:157–166. doi: 10.1016/0166-4328(95)00012-i. [DOI] [PubMed] [Google Scholar]
- 55.Cohen DA, et al. Off-line learning of motor skill memory: A double dissociation of goal and movement. Proceedings of the National Academy of Science USA. 2005;102:18237–18241. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith C. Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med Rev. 2001;5:491–506. doi: 10.1053/smrv.2001.0164. [DOI] [PubMed] [Google Scholar]
- 57.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci U S A. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gais S, et al. Learning-dependent increases in sleep spindle density. J Neurosci. 2002;22:6830–6834. doi: 10.1523/JNEUROSCI.22-15-06830.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molle M, et al. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004;10:13963–13968. doi: 10.1073/pnas.0402820101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peigneux P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 61.Maquet P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 62.Peigneux P, et al. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20:125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- 63.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walker MP, et al. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;I133:911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Ohyama T, et al. What the cerebellum computes. Trends Neurosci. 2003;26:222–227. doi: 10.1016/S0166-2236(03)00054-7. [DOI] [PubMed] [Google Scholar]
- 66.Ungerleider LG, et al. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–564. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- 67.Schendan HE, et al. An FMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron. 2003;37:1013–1025. doi: 10.1016/s0896-6273(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 68.Poldrack RA, Rodriguez P. Sequence learning: what’s the hippocampus to do? Neuron. 2003;37:891–893. doi: 10.1016/s0896-6273(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 69.Koechlin E, et al. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koechlin E, et al. Medial prefrontal and subcortical mechanisms underlying the acquisition of motor and cognitive action sequences in humans. Neuron. 2002;35:371–381. doi: 10.1016/s0896-6273(02)00742-0. [DOI] [PubMed] [Google Scholar]
- 71.Toni I, et al. The time course of changes during motor sequence learning: a whole-brain fMRI study. Neuroimage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- 72.Seitz RJ, et al. Motor learning in man: a positron emission tomographic study. Neuroreport. 1990;1:57–60. doi: 10.1097/00001756-199009000-00016. [DOI] [PubMed] [Google Scholar]
- 73.Muller RA, et al. Functional MRI of motor sequence acquisition: effects of learning stage and performance. Brain Res Cogn Brain Res. 2002;14:277–293. doi: 10.1016/s0926-6410(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 74.Walker MP, et al. The functional anatomy of sleep-dependent visual skill learning. Cerebral Cortex. 2005;15:1666–1675. doi: 10.1093/cercor/bhi043. [DOI] [PubMed] [Google Scholar]
- 75.Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 76.Maquet P, et al. Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci. 2003;23:1432–1440. doi: 10.1523/JNEUROSCI.23-04-01432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Prog Neurobiol. 2003;69:71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- 78.Contreras D, et al. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J Neurophysiol. 1997;78:335–350. doi: 10.1152/jn.1997.78.1.335. [DOI] [PubMed] [Google Scholar]
- 79.Sejnowski TJ, Destexhe A. Why do we sleep? Brain Res. 2000;886:208–223. doi: 10.1016/s0006-8993(00)03007-9. [DOI] [PubMed] [Google Scholar]
- 80.Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cerebral Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- 81.Steriade M. Coherent oscillations and short-term plasticity in corticothalamic networks. Trends Neurosci. 1999;22:337–345. doi: 10.1016/s0166-2236(99)01407-1. [DOI] [PubMed] [Google Scholar]
- 82.Steriade M. The Intact and Sliced Brain. MIT Press; 2001. [Google Scholar]
- 83.Cantero JL, et al. Sleep-dependent theta oscillations in the human hippocampus and neocortex. J Neurosci. 2003;23:10897–10903. doi: 10.1523/JNEUROSCI.23-34-10897.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poe GR, et al. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 85.Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 86.Pavlides C, et al. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- 87.Sanford LD, et al. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139:169–183. [PubMed] [Google Scholar]
- 88.Karashima A, et al. Elicited ponto-geniculo-occipital waves by auditory stimuli are synchronized with hippocampal theta-waves. Psychiatry Clin Neurosci. 2002;56:343–344. doi: 10.1046/j.1440-1819.2002.01019.x. [DOI] [PubMed] [Google Scholar]
- 89.Karashima A, et al. Phase-locking of spontaneous and elicited ponto-geniculo-occipital waves is associated with acceleration of hippocampal theta waves during rapid eye movement sleep in cats. Brain Res. 2002;958:347–358. doi: 10.1016/s0006-8993(02)03673-9. [DOI] [PubMed] [Google Scholar]
- 90.Holscher C, et al. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 92.Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- 93.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 94.Ribeiro S, et al. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. PLoS Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–816. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- 96.Dave AS, et al. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- 97.Shaffery JP, et al. REM sleep deprivation in monocularly occluded kittens reduces the size of cells in LGN monocular segment. Sleep. 1998;21:837–845. doi: 10.1093/sleep/21.8.837. [DOI] [PubMed] [Google Scholar]
- 98.Shaffery JP, et al. Ponto-geniculo-occipital-wave suppression amplifies lateral geniculate nucleus cell-size changes in monocularly deprived kittens. Brain Res Dev Brain Res. 1999;114:109–119. doi: 10.1016/s0165-3806(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 99.Antonini A, Stryker MP. Rapid remodeling of axonal arbors in the visual cortex. Science. 1993;260:1819–1821. doi: 10.1126/science.8511592. [DOI] [PubMed] [Google Scholar]
- 100.Frank MG, et al. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 101.Shaffery JP, et al. Rapid eye movement sleep deprivation modifies expression of long-term potentiation in visual cortex of immature rats. Neuroscience. 2002;110:431–443. doi: 10.1016/s0306-4522(01)00589-9. [DOI] [PubMed] [Google Scholar]
- 102.Smith C, et al. Some biochemical and behavioural aspects of the paradoxical sleep window. Can J Psychol. 1991;45:115–124. doi: 10.1037/h0084279. [DOI] [PubMed] [Google Scholar]
- 103.Cirelli C, Tononi G. Differences in gene expression between sleep and waking as revealed by mRNA differential display. Brain Res Mol Brain Res. 1998;56:293–305. doi: 10.1016/s0169-328x(98)00057-6. [DOI] [PubMed] [Google Scholar]
- 104.Cirelli C, Tononi G. Gene expression in the brain across the sleep-waking cycle. Brain Res. 2000;885:303–321. doi: 10.1016/s0006-8993(00)03008-0. [DOI] [PubMed] [Google Scholar]
- 105.Cirelli C, Tononi G. On the functional significance of c-fos induction during the sleep-waking cycle. Sleep. 2000;23:453–469. [PubMed] [Google Scholar]
- 106.Cirelli C, et al. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. 2004;41:35–43. doi: 10.1016/s0896-6273(03)00814-6. [DOI] [PubMed] [Google Scholar]
- 107.Ribeiro S, et al. Brain gene expression during REM sleep depends on prior waking experience. Learn Mem. 1999;6:500–508. doi: 10.1101/lm.6.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ribeiro S, et al. Induction of hippocampal long-term potentiation during waking leads to increased extrahippocampal zif-268 expression during ensuing rapid-eye-movement sleep. J Neurosci. 2002;22:10914–10923. doi: 10.1523/JNEUROSCI.22-24-10914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abraham WC, Robins A. Memory retention - the synaptic stability versus plasticity dilemma. Trends in Neurosciences. 2005;28:73–78. doi: 10.1016/j.tins.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 110.Tulving E. How many memory systems are there? American Psychologist. 1985;40:385–398. [Google Scholar]
- 111.Walker MP, Stickgold R. Sleep, Memory, and Plasticity. Annual Review of Psychology. 2005;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]