Abstract
Background
Muir-Torre Syndrome (MTS) is a rare autosomal-dominant disorder characterized by the predisposition to both sebaceous neoplasm and internal malignancies. MTS-associated sebaceous neoplasms reveal mutations in DNA mismatch repair (MMR) genes and microsatellite instability. A significant part of MTS patients represents a phenotypic variant, the hereditary nonpolyposis colorectal cancer (HNPCC). A strong correlation between microsatellite instability and immunostaining has been demonstrated. The early recognition of sebaceous neoplasm as part of MTS, and their differentiation from sporadic sebaceous neoplasm may have an important application in a clinical setting. The absence of MLH-1 or MSH-2 expression by immunostaining identifies tumors with mismatch repair deficiency.
Objectives
Our aim is to determine whether an immunohistochemical approach, targeting DNA repair proteins MSH-2 and MLH-1 in MTS-related sebaceous neoplasm and their sporadic counterparts, can be used for their identification.
Methods
We examined 15 sebaceous neoplasms (including 6 internal malignancy- associated sebaceous neoplasms and 8 sporadic sebaceous neoplasms) from 11 patients for the expression of MSH-2 and MLH-1 by immunohistochemistry.
Results
Four of 5 internal malignancy-associated sebaceous neoplasms showed loss of expression of MSH-2 or MLH-1. Correlation of the immunostaining pattern of the sebaceous neoplasms and the patients’ positive history of colon carcinoma was 80%. Seven of 8 sporadic sebaceous neoplasms showed a positive expression of MSH-2 and MLH-1. The prevalence for loss of expression of MMR proteins in sebaceous neoplasms was 38.5%. MMR immunostaining had 87.5% specificity and 80% sensitivity.
Limitations
This study is limited by a small sample size, and by bias selection due to the use of non nationwide data-base as the resource of cases.
Conclusions
Our findings demonstrate that immunohistochemical testing for internal malignancy-associated sebaceous neoplasms is a practical approach to confirm a suspected inherited MMR gene defect, and an accurate method to distinguish between sporadic and MTS-associated sebaceous lesions.
Keywords: Immunohistochemistry, DNA mismatch repair, Sebaceous neoplasm, Muir-Torre syndrome, MSH-2
Muir-Torre Syndrome (MTS) is a rare autosomal-dominant disorder, described independently by Muir, Yates Bell, Barlow (1) and Torre (2) that is characterized by the predisposition to both sebaceous neoplasms and internal malignancies. The cutaneous neoplasms that are characteristic of the syndrome include sebaceous adenomas, sebaceous carcinomas, and sebaceomas, in addition to multiple keratoacanthomas (3–5). MTS is a clinical diagnosis that can be made in the setting of concurrent or sequential presence of a sebaceous neoplasm and one internal malignancy. This diagnosis can also be made in the presence of multiple keratoacanthomas, a visceral malignancy and a positive family history of MTS (5). The occurrence of any of these tumors, especially with isolated cystic type sebaceous tumors, even if solitary, mandates consideration of MTS (6–8). An evaluation of the patient and family members to rule out internal malignancies is mandatory.
MTS is caused by an inherited germ-line mutation in one allele of MMR genes. A somatic loss-of-function alteration of the remaining wild type allele may occur, leading to MMR deficiency (9–10). The MMR system repairs small errors that occur during replication in repeated sequences of DNA known as microsatellites. Consequently, MMR deficiency produces accumulation of mutations in microsatellites resulting in their instability. A strong correlation between microsatellite instability and immunostaining has been demonstrated (11–16). The absence of MLH-1 or MSH-2 expression by immunostaining identifies tumors with mismatch repair deficiency (11–18).
A spectrum of malignant visceral tumors can occur in MTS patients, but colorectal carcinomas occur more frequently. A significant part of MTS represents a phenotypic variant of the hereditary nonpolyposis colorectal cancer (HNPCC or Lynch Syndrome) (19–20). In HNPCC the proportion of MSH-2 mutations almost equals the proportion of MLH-1 mutations, whereas MTS is most frequently caused by germline mutations in MSH-2. Ponti, Losi, Pedroni, et al. (21) demonstrated that MLH-1 and MSH-2 gene mutations have an equivalent etiopathological role for both Lynch Syndrome and MTS, so a broadened clinical criterion for Lynch Syndrome has been proposed.
The objective of this study was to determine whether an immunohistochemical approach targeting DNA mismatch repaired proteins, MSH-2 and MLH-1, in MTS-related sebaceous neoplasms can be used for their identification when compared to their sporadic counterparts. The early recognition of sebaceous neoplasms as part of MTS, and their differentiation from sporadic sebaceous neoplasms, may have an important application in a clinical setting.
Materials and Methods
Patients and Tumor Samples
A total of 15 paraffin-embedded sebaceous neoplasms from 11 patients were randomly selected from a dermatopathology report database system: 5 sebaceous adenomas, 4 sebaceous carcinomas, and 6 sebaceomas. None of these patients had been previously analyzed for microsatellite instability. Six patients had an internal malignancy-associated sebaceous neoplasm, and 8 patients had sporadic sebaceous neoplasms (Table 1). In addition, one patient with an unknown past medical history was initially included to the immunohistochemical analysis for MLH-1 and MSH-2 expression.
Table 1.
Patient’s demographics, histopathologic diagnosis, and MMR proteins expression in sebaceous neoplasms
| Group | Case (n=15) |
Sex | Age (years old) |
History of internal malignancy |
Histology | MSH-2/ MLH-1 |
|---|---|---|---|---|---|---|
| A | 1A | m | 48 | yes | s. adenoma | −/+ |
| 2A | f | 43 | yes | s. adenoma | −/+ | |
| 3A | m | 67 | no | s. adenoma | −/+ | |
| 4A | m | 91 | no | s. adenoma | +/+ | |
| 5A | m | 78 | no | s. adenoma | +/+ | |
| B | 1B | m | 48 | yes | s. carcinoma | +/+ |
| 2B | m | 90 | no | s. carcinoma | +/+ | |
| 3B | f | 81 | no | s. carcinoma | +/+ | |
| 4B | f | 84 | no | s. carcinoma | +/+ | |
| C | 1C | m | 56 | yes | sebaceoma | −/+ |
| 2C | m | 58 | yes | sebaceoma | undiagnostic | |
| 3C | m | 58 | yes | sebaceoma | −/+ | |
| 4C | f | 82 | no | sebaceoma | +/+ | |
| 5C | m | 66 | no | sebaceoma | +/+ | |
| 6C | m | 77 | unknown | sebaceoma | undiagnostic |
A, sebaceous adenoma; B, sebaceous carcinoma; C, sebaceoma
* (−), loss of staining for MSH-2/MLH-1; (+), presence of nuclear immunoreactivity for MSH-2/ MLH-1
* cases 1a and 1b, 1c and 3c, 4b and 4c corresponded to same patients
The study was approved by the Institutional Review Board of the University of Puerto Rico Medical Sciences Campus.
Immunohistochemistry
Freshly cut paraffin sections were deparaffinized in xylene, and rehydrated in graded alcohols. Sections were subjected to heat-induced epitope retrieval, using a commercially available steamer, and Tris EDTA (Rockland Gilbertsville, PA, 10x TE Buffer, ph 7.5). The slides were steamed for 45 minutes, cooled at room temperature on Tris EDTA for 20 minutes, and then rinsed with Dako buffer (10x) at room temperature. Immunohistochemical staining was performed with an automated immunostainer (Dako). The mouse anti-human monoclonal antibodies against hMLH-1 and hMSH-2 were employed at 1:60 and 1:30 dilutions, respectively. Then, slides were subjected to a secondary antibody, Labelled polymer HRP anti-mouse, followed by DAB+ substrate buffer as the chromogen. The slides were counterstained with hematoxylin (Dakocytomation Automation Hematoxylin Histological Staining Reagent). After automated immunostaining, slides were washed with Dako buffer, rehydrated in graded alcohols and fixed with Xylol. Negative and positive controls were obtained with normal colon mucosa. Staining was scored as positive when nuclear immunoreactvity was present. Tumors were considered to demonstrate inactivation of hMSH-2 or MLH-1 when there was a complete absence of detectable nuclear staining of neoplastic cells. Positive nuclear staining of adjacent non-neoplastic epithelium, stromal cells, or lymphocytes served as an internal positive control, and was a requirement for each specimen evaluation. Two pathologists (J.S and C.G.K.) assessed all cases blinded of patients’ history of internal malignancy.
Results
The mean age of patients included in the analysis was 68 years old, with a male predominance. Results of the immunohistochemical staining are detailed in Table 1. Representative cases are shown in Figure 1 to Figure 3. Two specimens (cases 2C and 6C) were excluded from the analysis due to undiagnostic immunostaining results. Both excluded specimens were sebaceomas, one of them belonged to the group of sebaceous neoplasms in patients with a history of internal malignancy (Table 1). No appropriate internal control was achieved in these two cases.
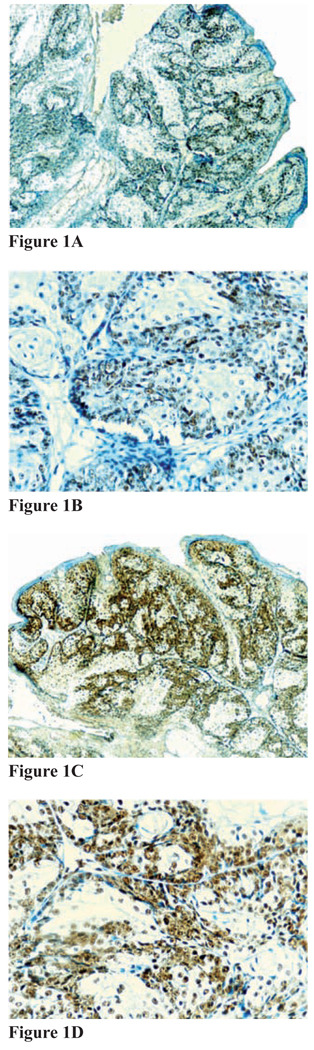
Figure 1. Figures 1A–1D.
Immunohistochemical staining for MMR proteins for sebaceus adenoma, subject 5A. A and B, Positive immunostaining for MSH-2 (A, magnification 20x; B, magnification 40x); C and D, positive immunostaining for MLH-1 (C, magnification 20x; D, magnification 40x)
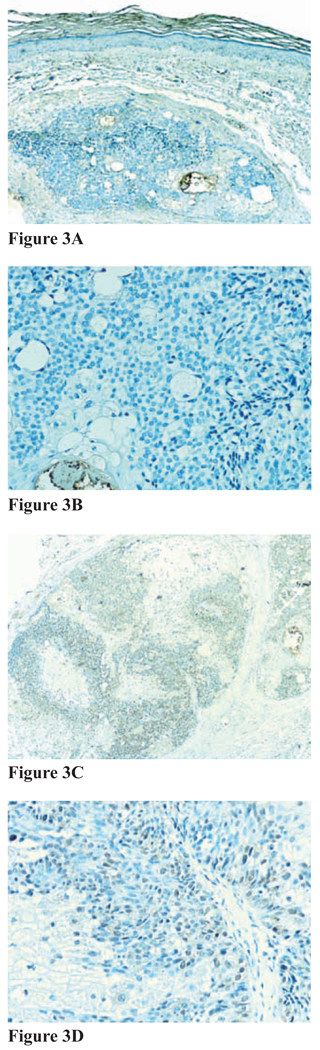
Figure 3. Figures 3A–3D.
Immunohistochemical staining for MMR proteins for sebaceoma, subject 1C. A and B, Negative immunostaining for MSH-2 (A, magnification 20x; B, magnification 40x); C and D, positive immunostaining for MLH-1 (C, magnification20x; D, magnification 40x)
In the group of patients with a history of colon cancer, 4 of 5 sebaceous neoplasms showed loss of expression of MSH-2 and MLH-1 (Table 2). Overall, the prevalence of MSH-2 and MLH-1 loss of expression was 80% among patients with a history of colon carcinoma.
Table 2.
Comparison of MSH-2/MLH-1 expression of sebaceous neoplasms in patients with a history of internal malignancy
| MMR protein status |
Sebaceous adenoma (n=2) |
Sebaceous carcinoma (n=1) |
Sebaceoma (n=2) | Combined (n=5) |
|---|---|---|---|---|
| MSH-2 loss or MLH-1 loss | 2 | 0 | 2 | 4 |
| No loss | 0 | 1 | 0 | 1 |
In the group of patients with a negative history for internal malignancy, 7 of 8 sebaceous neoplasms showed a positive expression of MSH-2 and MLH-1 (Table 2).
Overall, the prevalence of MSH-2 and MLH-1 loss of expression was 38.5% among patients with a negative history of colon carcinoma.
Generally, the specificity and sensitivity of MLH-1 and MSH-2 immunostaining of sebaceous neoplasms was 87.5% and 80%, respectively (Table 3). The positive predictive value of MLH-1/MSH-2 immunostaining for identification of MTS-associated sebaceous lesions was 80%.
Table 3.
Comparison of MSH-2/MLH-1 expression of sebaceous neoplasms in patients with a negative history of internal malignancy
| MMR protein status |
Sebaceous adenoma (n=3) |
Sebaceous carcinoma (n=3) |
Sebaceous (n=2) | combined (n=8) |
|---|---|---|---|---|
| MSH-2 loss or MLH-1 loss | 1 | 0 | 0 | 1 |
| No loss | 2 | 3 | 2 | 7 |
Discussion
Sebaceous neoplasms are rare skin tumors that may occur sporadically or in association to MTS. The diagnosis of a sebaceous neoplasm should give rise to the suspicion of an inherited MMR gene defect. The distinction between patients with MTS and sporadic sebaceous neoplasms is of paramount importance, particularly in those patients presenting sebaceous neoplasms as an initial manifestation of the syndrome.
MTS-sebaceous neoplasms originate from an inherited germ-line mutation in one allele of MMR genes, in combination with a somatic loss-of-function of the remaining wild type allele (12–13). The MMR deficiency leads to an inability to correct mutations, and eventually leads to microsatellites instability. Marcus, Madlensky, Gryfe, et al. (16) clearly demonstrated that immunostaining can accurately discriminate between microsatellite unstable and microsatellite stable neoplasms. Thus, immunohistochemistry can identify patients affected by an MMR deficiency.
Most studies on MMR defects published so far have concentrated on microsatellite instability analysis, and a 100% correlation between microsatellite instability analysis and immunostaining has been well documented (14–19).
In this study we analyzed MTS-associated sebaceous neoplasms and sporadic counterparts to determine the prevalence of loss of expression of MMR proteins, the specificity, and the sensitivity of the immunohistochemical analysis for sebaceous adenomas, sebaceous carcinomas and sebaceomas.
Considered as a whole, 5 cases (42%) were MMR deficient and 7 (58%) had an intact expression of MMR proteins. This is similar to the findings of Singh, Grayson, Redston, et al. (22) who found a 35% of the sebaceous neoplasms to be MMR deficient.
Similar to the findings of Popnikolov, Gatalica, Colome-Grimmer and Sánchez (23), we found a loss of MLH-1/MSH-2 protein in four of five (80%) internal malignancy-associated sebaceous neoplasms. One of the five (20%) internal malignancy-associated sebaceous neoplasms included in our study did not show loss of MLH-1/MSH-2 protein expression (case 1B- sebaceous carcinoma). There is a theoretical chance that the somatic mutation of the second allele of the MSH-2 gene in a patient with a germline mutation may lead to the synthesis of a defective, but still detectable protein (17–23). This may explain why discordance between immunostaining and clinical diagnosis of MTS may be seen. Otherwise, there may be a different mutated MMR gene of the MMR complex responsible for the development of this tumor. Further molecular analysis is required to clarify these types of discrepancies.
Loss of MLH-1/MSH-2 proteins was detected in one of eight sporadic sebaceous neoplasms (case 3A-sebaceous adenoma). This lesion may represent the first manifestation of MTS in this patient, and may indicate an increased risk for visceral malignancy.
In our study, the percentage of loss of expression of MMR proteins in internal malignancy associated sebaceous neoplasms was significantly higher (80% versus 36.3%, respectively) than the recently published findings of Cesinaro, Ubiali, Sighinolfi, et al. (24). In their study, only a minority of cases of sebaceous tumors associated with extracutaneous cancer displayed MMR abnormalities and/or microsatellite instability, arguing that these techniques are limited in their ability to identify patients with “clinically defined” MTS (24). These discrepancies could be related to differences in the definition of MTS cases. In our study, we strictly defined cases of MTS as those presenting at least one sebaceous neoplasm in addition to internal malignancy, all of them being carcinoma of colon. We did not include sebaceous hyperplasia, nor adenomatous polyps and/or hyperplastic polyps of the colon to identify our MTS cases.
No concurrent loss of both MLH-1 and MSH-2 was observed in our study, similar to previous findings (23). Overall, we found a strong positive correlation (85%) between the staining pattern and the clinical diagnosis of MTS or sporadic sebaceous neoplasms.
There was an observed difference between sebaceous adenomas, carcinomas, and sebaceomas in the rate of the overall loss of the expression of MMR proteins and a positive correlation to the patients’ history for internal malignancy. In our study, the sebaceoma presented the strongest positive correlation.
Previously, Marcus, Madlensky, et al. (16) reported that immunohistochemistry for MSH-2 and MLH-1 identifies MMR deficiency with 97% sensitivity and 100% specificity in microsatellite instability-analyzed neoplasms. In our study, MLH-1 and MSH-2 immunohistochemistry identifies MTS patients with 80% sensitivity and 87.5% specificity, supporting that immunohistochemistry can be used with a high degree of accuracy for the identification of those sebaceous neoplasms originated by mutations in MMR genes.
There are some inherent limitations in this study, including the possibility that some patients may have an undiagnosed internal malignancy, leading to an underestimation of MTS cases. Another limitation of this study stems from our inability to generalize our findings of prevalence as a nationwide prevalence, because the selected cases did not come from a nationwide data-base. This study includes a relatively small sample size.
This study supports earlier reports of alterations in mismatch repair gene expression for sebaceous neoplasms in patients with MTS (9, 14–23) and brings evidence in favor of immunohistochemical analysis as a practical first-line screening test. In those cases where internal malignancy-associated sebaceous neoplasm presents discordant immunostaining results, microsatellite analysis would be required to identify possible false-negative cases. Also, molecular analysis would be required for those tumors that may be originated from mutations in other genes of the mismatch repair complex (16).
In summary, our findings support immunohistochemical testing for internal malignancy-associated sebaceous neoplasms as a reliable and practical screening method with a high predictive value for the diagnosis of MMR deficient neoplams. It is an accurate way to distinguish between sporadic and MTS-associated sebaceous lesion.
Resumen
El síndrome de Muir-Torre (SMT) es un desorden autosómico dominante poco común, que se caracteriza por una predisposición a neoplasmas de origen sebáceo y a malignidades internas. Los neoplasmas sebáceos asociados al SMT revelan mutaciones en los genes de reparación de pareo incorrecto del ADN, además de una inestabilidad de microsatélite. Un grupo significativo de pacientes con SMT representan una variante fenotípica del síndrome hereditario de cáncer colorrectal sin poliposis. Se ha demostrado una correlación fuerte entre la inestabilidad de microsatélite y la inmunotinción. El reconocimiento temprano de los neoplasmas sebáceos como parte del SMT y su diferenciación de los neoplasmas sebáceos esporádicos, puede ser de gran importancia clínica. La ausencia de la expresión MLH-1 o MSH-2 identifica los tumores con deficiencia de las proteínas encargadas de la reparación del pareo incorrecto del ADN. Nuestro propósito es determinar si un análisis por inmunohistoquímica para las proteínas MSH-2 y MLH-1 en neoplasmas sebáceos asociados al SMT y sus contrapartes esporádicas, se puede utilizar para su distinción. Examinamos 15 neoplasmas sebáceos (6 neoplasmas asociados a malignidad interna y 8 neoplasmas sebáceos esporádicos) de 11 pacientes para la expresión por inmunohistoquímica de MSH-2 y de MLH-1. Cuatro (4) de 5 neoplasmas sebáceos asociados a malignidad interna mostraron la pérdida de expresión de MSH-2 o MLH-1. La correlación del patrón de inmunotinción y un historial positivo de los pacientes de carcinoma fue de un 80%. Siete (7) de 8 neoplasmas sebáceos esporádicos demostraron una expresión positiva de MSH-2 y MLH-1. La prevalencia de la pérdida de expresión de las proteínas del MMR en neoplasmas sebáceos fue de un 38.5%. La inmunotinción tuvo una especificidad de un 87.5% y una sensitividad de un 80%. Este estudio está limitado debido al tamaño pequeño de la muestra y por el uso de una base de datos no nacional como fuente de los casos. Nuestros resultados demuestran que las pruebas de inmunohistoquímica para los neoplasmas sebáceos asociados a malignidad interna son un análisis práctico. Es un método preciso para distinguir entre las lesiones esporádicas y las asociadas al SMT.
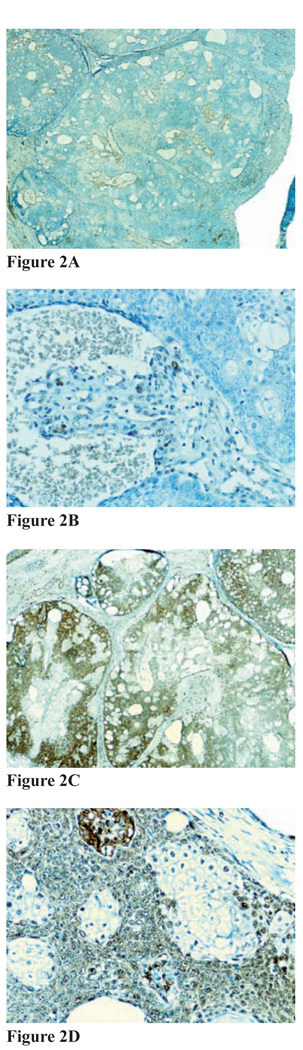
Figure 2. Figures 2A–2D.
Immunohistochemical staining for MMR proteins for sebaceus carcinoma, subject 4B. A and B, Positive immunostaining for MSH-2 (A, magnification 20x; B, magnification 40x); C and D, positive immunostaining for MLH-1 (C, magnification 20x; D, magnification 40x)
Acknowledgments
The authors thank Nelson Santiago, HTL for technical support; and Francisco Arroyo Vega for photographical assistance. This study was partially supported by grants P20RR11126 and R25RR17589 awarded by the National Centers for Research Resources, National Institutes of Health (NIH, Bethesda, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Muir EG, Yates Bell AJ, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with keratoacanthomata of the face. Br J Surg. 1967;54:191–195. doi: 10.1002/bjs.1800540309. [DOI] [PubMed] [Google Scholar]
- 2.Torre D. Multiple sebaceous neoplasms of the skin. Arch Dermatol. 1968;98:549–551. doi: 10.1001/archderm.98.5.549. [DOI] [PubMed] [Google Scholar]
- 3.Ackerman B, Nussen S. New Concept: Sebaceous “adenoma” is sebaceous carcinoma. Dermatopathol: Pract & Concep. 1998:4. [Google Scholar]
- 4.Ackerman B, Nussen S. The history: Respective contributions of Muir and Torre. Dermatopathol: Practl & Concept. 1999:5. [Google Scholar]
- 5.Schwartz R, Torre D. The Muir-Torre syndrome: A 25-year retrospect. J Am Acad Dermatol. 1995;33:90–103. doi: 10.1016/0190-9622(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 6.Kruse R, Rütten A, Malayeri HR, et al. A novel germline mutation in the hMLH-1 DNA mismatch repair gene in a patient with an isolated cystic sebaceous tumor. J Invest Dermatol. 1999;112:117–118. doi: 10.1046/j.1523-1747.1999.00469.x. [DOI] [PubMed] [Google Scholar]
- 7.Lynch HT, Fusaro RM. The Muir-Torre syndrome in kindreds with hereditary nonpolyposis colorectal cancer (Lynch syndrome): A classic obligation in preventive medicine. J Am Acad Dermatol. 1999;41:797–799. doi: 10.1016/s0190-9622(99)70017-4. [DOI] [PubMed] [Google Scholar]
- 8.Rothenberg J, Lambert WC, Vail JT, et al. The Muir-Torre syndrome: the significance of a solitary sebaceous tumor. J Am Acad Dermatol. 1990;23:638–640. doi: 10.1016/s0190-9622(08)81072-9. [DOI] [PubMed] [Google Scholar]
- 9.Kruse R, Rütten A, Hosseiry-Malayeri HR, et al. “Second hit” in sebaceous tumors from Muir-Torre patients with germ-line mutations in MSH-2: allele loss is not the preferred mode of inactivation. J Invest Dermatol. 2001;116:463–465. doi: 10.1046/j.1523-1747.2001.01265.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Lindroos A, Ollila S, et al. Gene conversion is a frequent mechanism of inactivation of the Wild-Type Allele in cancers from MLH1/ MSH2 deletion carriers. Cancer Res. 2006;66:659–664. doi: 10.1158/0008-5472.CAN-05-4043. [DOI] [PubMed] [Google Scholar]
- 11.Entius ME, Keller JJ, Drillenburg P, et al. Microsatellite Instability and Expression of hMLH-1 and hMSH-2 in sebaceous gland carcinomas as markers for Muir-Torre syndrome. Clin Cancer Res. 2000;6:1784–1789. [PubMed] [Google Scholar]
- 12.Machin P, Catasus L, Pons C, et al. Microsatellite instability and immunostaining for MSH-2 and MLH-1 in cutaneous and internal tumors for patient of Muir-Torre syndrome. J Cutan Pathol. 2002;29:415–420. doi: 10.1034/j.1600-0560.2002.290705.x. [DOI] [PubMed] [Google Scholar]
- 13.Marcus VA, Madlensky L, Gryfe R, et al. Immunohistochemistry for hMLH1 and hMSH2: A Practical Test for DNA Mismatch Repair-Deficient Tumors. Am J Surg Pathol. 1999;23:1248–1255. doi: 10.1097/00000478-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Mathiak M, Rütten A, Mangold E, et al. Loss of DNA Mismatch Repair Proteins in Skin Tumors From Patients with Muir-Torre Syndrome and MSH2 or MLH1 Germline Mutations: Establishment of Immunohistochemical Analysis as a Screening Test. Am J Surg Pathol. 2002;26:338–343. doi: 10.1097/00000478-200203000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Ponti G, Ponz de Leon M, Losi L, et al. Different Phenotypes in Muir-Torre Syndrome: clinical and biomolecular characterization in two Italian families. Br J Dermatol. 2005;152:1335–1338. doi: 10.1111/j.1365-2133.2005.06506.x. [DOI] [PubMed] [Google Scholar]
- 16.Southey M, Young MA, Whitty J, et al. Molecular pathologic analysis enhances the diagnosis and management of Muir-Torre Syndrome and gives insight its underlying molecular pathogenesis. Am J Surg Pathol. 2001;25:936–941. doi: 10.1097/00000478-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Kruse R, Rütten A, Schweiger N, et al. Frequency of microsatellite instability in unselected sebaceous gland neoplasias and hyperplasias. J Invest Dermatol. 2003;120:858–864. doi: 10.1046/j.1523-1747.2003.12125.x. [DOI] [PubMed] [Google Scholar]
- 18.Marazza G, Masouyé I, Taylor S, et al. An Illustrative Case of Muir-Torre Syndrome. Arch Dermatol. 2006;142:1039–1042. doi: 10.1001/archderm.142.8.1039. [DOI] [PubMed] [Google Scholar]
- 19.Kruse R, Rütten A, Lamberti C, et al. Muir Torre Phenotype has a frequency of DNA mismatch-repair-gene mutations similar to that in hereditary nonpolyposis colorectal cancer families defined by the Amsterdam criteria. Am J Hum Genet. 1998;63:63–70. doi: 10.1086/301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mangold E, Pagenstrecher C, Leister M, et al. A genotype-phenotype correlation in HNPCC: strong predominance of MSH-2 mutations in 41 patients with Muir-Torre Syndrome. J Med Genet. 2004;41:567–572. doi: 10.1136/jmg.2003.012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponti G, Losi L, Pedroni M, et al. Value of MLH1 and MSH2 Mutations in the appearance of Muir-Torre syndrome phenotype in HNPCC patients presenting sebaceous gland tumors or keratoacanthomas. J Invest Dermatol. 2006;126:2302–2307. doi: 10.1038/sj.jid.5700475. [DOI] [PubMed] [Google Scholar]
- 22.Singh R, Grayson W, Redston M, et al. Site and tumor type predicts DNA mismatch repair status in cutaneous sebaceous neoplasia. Am J Surg Pathol. 2008;32:936–942. doi: 10.1097/pas.0b013e31815b0cc2. [DOI] [PubMed] [Google Scholar]
- 23.Popnikolov NK, Gatalica Z, Colome-Grimmer MI, Sánchez RL. Loss of mismatch repair proteins in sebaceous gland tumors. J Cutan Pathol. 2003;30:178–184. doi: 10.1034/j.1600-0560.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 24.Cesinaro AM, Ubiali A, Sighinolfi P, et al. Mismatch repair proteins expression and microsatellite instability in skin lesions with sebaceous differentiation: a study in different clinical subgroups with and without extracutaneous cancer. Am J Dermatopathol. 2007;29:351–358. doi: 10.1097/DAD.0b013e318057713c. [DOI] [PubMed] [Google Scholar]