Abstract
Background
Xenogeneic thymus transplantation is an effective approach to achieving T cell tolerance across highly disparate xenogeneic species barriers. We have previously demonstrated that phenotypically normal, specifically tolerant human T cells are generated in porcine thymic grafts. In this study, we assessed the diversity of the human T cell repertoire generated in porcine thymic xenografts. We also examined the ability of porcine thymus grafts to coexist with human thymus grafts.
Methods
Fetal swine (SW) or human (HU) thymus with human fetal liver (FL) fragments were transplanted under the kidney capsule of 3Gy irradiated NOD/SCID mouse recipients. Thymus tissue was harvested approximately 16 weeks post-transplant for analysis of mixed lymphocyte reactions and spectratyping of human CD4 and CD8 single positive (SP) thymocytes.
Results
TCR β genes of human CD4 and CD8 SP cells developing in HU and SW thymus grafts showed similar, normal CDR3 length distributions. Human T cells developing in SW thymus grafts showed specific unresponsiveness to the MHC of the donor swine, in MLR assays. In 2 of 3 animals receiving SW and HU thymus grafts under opposite kidney capsules, both grafts functioned. In animals with surviving SW grafts, thymocytes from the SW but not the HU grafts showed specific unresponsiveness to the SW donor.
Conclusion
SW thymus grafts support generation of human T cells with a diverse TCR repertoire. Human thymocytes in human thymus grafts are not tolerized by the presence of an additional porcine thymus, but tolerance might be achieved by post-thymic encounter with porcine antigens.
Keywords: Xenotransplantation, thymus, thymopoiesis, T cell repertoire, Spectratyping
Introduction
The inadequate supply of allogeneic donors presents a major obstacle to clinical organ and tissue transplantation, and xenogeneic transplantation has the potential to overcome this limitation (1). Because of similarities in size and physiology, the pig is considered to be one of the most attractive candidate xenograft source animals for transplantation to humans. While natural antibodies against α1,3Gal (Gal) have presented a major barrier to successful pig to primate xenotransplantation, the recent development of Gal transferase (GalT) knockout pigs (2) has provided a solution to this problem (3). Nevertheless, organ grafts from GalT knockout pigs have undergone rejection despite the use of substantial immunosuppession (4,5), making it likely that T cell tolerance will be necessary for safe and effective pig to human xenotransplantation.
We have developed a strategy for inducing T cell tolerance across the most disparate species barriers, involving xenogeneic thymic transplantation. Thymectomized mice that receive fetal or neonatal porcine thymus grafts demonstrate recipient T cell repopulation within the xenogeneic thymus graft (6–8). Normal mouse T cells maturing within the thymus graft repopulate the peripheral immune system, conferring immunocompetence (9) and, importantly, donor-specific tolerance (8).
This approach was subsequently extended to the pig-to-human species combination, in which normal human thymopoiesis occurred in porcine thymic tissue grafted with human FL tissue into immunodeficient mice. Importantly, human T cells developing in porcine thymus grafts demonstrated specific tolerance to the MHC of the porcine thymus donor with normal reactivity to allogeneic pig and human antigens (10). The thymic transplantation approach has further been extended to a large animal allogeneic transplantation model, in which T cells from swine receiving SLA mismatched donor thymus grafts developed donor-specific unresponsiveness (11). More recently, this strategy has been extended to a pig-to baboon model (12–14), providing the first demonstration that pig to primate renal transplants can avoid all rejection processes when GalT knockout pigs were used as source animals (3).
In normal animals, T cell progenitors from the bone marrow undergo thymopoiesis, which involves generation of T cell receptor (TCR) heterodimers composed of somatically rearranged α and β chains. These heterodimers are first expressed at the CD4/CD8 “double-positive” (DP) stage of T cell ontogeny in the thymus. TCR diversity is generated by the random rearrangement of variable, diversity and joining gene segments as well as α and β chain pairing (15,16). DP thymocytes undergo positive and negative selection in the thymus and mature into CD4 or CD8 “single positive” (SP) cells if they survive these selective processes. Consequently, the repertoire of TCRs expressed by thymic and consequently peripheral SP T cells differs markedly from that of DP thymocytes.
Evidence for polyclonality of the human SP T cell populations developing in porcine xenogenic swine thymus grafts was obtained by demonstrating the use of multiple TCR-Vβ chains (10). While the demonstration of a normal Vβ usage pattern by human SP T cells developing in porcine thymus grafts suggests that the repertoire is polyclonal, a more definitive approach to addressing this question involves the analysis of CDR3 lengths, which, based on the variable number of N insertions that may occur during the TCR rearrangement process, should follow a normal distribution for TCR using a given Vβ in a polyclonal repertoire (17,18). In contrast, skewing of CDR3 length distributions within some or all of the Vβ families would suggest that the T cell repertoire might lack normal diversity (19,20). Thus, we have now performed spectratyping analysis to compare CDR3 length distributions among human SP thymocytes developing in porcine versus human thymus grafts in immunodeficient mice. In addition, we have begun to address the requirements for and mechanisms of donor-specific tolerance achieved among human T cells developing in porcine thymic grafts by examining the effect of a human thymus graft on survival of porcine thymus grafts. Our results have implications for the clinical application of thymic transplantation as an approach to xenogeneic tolerance induction.
Materials and Methods
Animals and tissues
NOD/SCID mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Fetal human tissue was obtained from Advanced Bioscience Resource (Alameda, CA) with approval from the Massachusetts General Hospital Institutional Review Board. Fetal porcine tissue was harvested from pregnant sows, which were obtained from our MHC-defined miniature swine colony maintained at Tufts University School of Veterinary Medicine (Grafton, MA). SLAdd sows were used as thymic donors. PBL were prepared from the blood of MHC-matched SLAdd and fully MHC-mismatched SLAcc pigs as described (21,22).
Transplantation
NOD/SCID mouse recipients were maintained in microisolator cages and received 3-Gy total body irradiation on day 0. Human (HU) fetal thymic (THY) and liver (FL) fragments (gestational week 17) and second trimester (gestational day 50–72) miniature swine (SW) fetal THY fragments (approximately 0.5 × 0.5 × 1 mm) were transplanted under the kidney capsule via a midline laparotomy incision on the same day. All surgical procedures, including harvest of porcine tissue, were performed under general anesthesia using sterile conditions as described (7). In accordance with the recent demonstration that human peripheral T cell reconstitution is enhanced in HU THY/FL NOD/SCID mice (HU/HU mice) by the i.v. co-administration of CD34+ cells collected from the donor FL (23), we also injected donor CD34+ cells i.v. to recipients of SW or HU THY plus HU FL fragments (SW/HU and HU/HU mice, respectively). CD34+ cells were collected from cell suspensions prepared from the fetal liver of the fetal human thymus and liver donor using anti-human CD34 microbeads (Miltenyi Biotec, Auburn, CA,) and magnetic cell sorting (MACS) according to the manufacturer’s instructions. The purity of CD34+ cells (>95%) was confirmed by flow cytometry. 2–5×105 CD34 positive cells were injected i.v. on the day of thymus transplantation.
Histology
Formalin-fixed and paraffin-embedded and processed THY/FL grafted tissue was sectioned and then stained with hematoxylin and eosin (H&E) and examined microscopically using standard methods. Serial sections were performed to examine most of the tissue.
CD4 and CD8 SP thymocytes isolation
Thymocytes suspensions were prepared as described (10) from human HU/HU and SW/HU grafts at 16 to 20 weeks following implantation under the kidney capsules of NOD/SCID mice. The cells were magnetically labeled with anti-human CD4 microbeads (Miltenyi Biotec) or anti-human CD8 microbeads (Miltenyi Biotec), and negatively selected CD4 or CD8 single positive cells were collected with MACS for spectratyping. These selected cell populations contained >80% CD4 or CD8 SP target populations.
Spectratyping
Total RNA was extracted directly from 1~2×106 CD4 or CD8 single positive thymocytes, and single-strand cDNA synthesis was performed using SuperScript III (Invitrogen, CA, USA) according to the manufacturer’s instructions. Amplification reactions were performed using a TCR β chain constant region primer and individual variable region (Vβ1-24) primers as described (24). Products were then used for run-off reactions with Jβ-specific FAM-labeled primer (Integrated DNA Technologies, Coralville, IA) (25). The labeled products were then used for TCR Vβ length analysis. The size and area of the peaks corresponding to the DNA products were determined using the ABI 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA) and analyzed using Applied Biosystems Genotyper 3.7 NT at Harvard Partners Center for Genetics and Genomics High Throughput Genotyping Facility (Brigham and Women’s Hospital Boston, MA).
Flow cytometric analysis
Blood samples from recipient NOD/SCID mice were collected from the tail veins at 9 and 12 weeks after transplantation. Human and porcine PBL were prepared by centrifugation over a Ficoll layer. Cells were washed with PBS twice, and then used for flow cytometry. The following mAbs were used for staining: FITC-RPA-T4 (anti-human CD4), PE-RPA-T8 (anti-human CD8), FITC-HIT3a (anti-human CD3) (all anti-human mAbs were purchased from PharMingen, San Diego, CA), FITC-MSA-4 (anti-porcine CD2; all anti-porcine mAbs were prepared in our laboratory), PE-30-F11 (anti-mouse CD45) (PharMingen) FITC-HOPC-1 (PharMingen), and PE-Rat IgG2a (PharMingen) (as negative controls). After 30 min of incubation, cells were washed twice. All data were collected using a FACSCalibur (Becton Dickinson, Mountain View, CA) and analyzed with Flowjow v6.2 (Tree Star, Inc., San Carlos, CA) software. The anti-human, anti-mouse, and anti-porcine mAbs were used at dilutions previously shown to be optimal for staining, without significant interspecies cross-reactivity. Non-viable cells were excluded using the vital nucleic stain proidium iodide.
Mixed Lymphocyte Reactions
Thymocyte suspensions were prepared from HU/HU and SW/HU THY/FL grafts and washed in AIM-V medium (Life Technologies, Gaithersburg, MD) supplemented with 10% (v/v) human serum and 1% HEPES buffer. Triplicate wells containing 4 × 105 responders with 4 × 105 allogeneic human PBL stimulators (30 Gy irradiated), or 4 × 105 xenogeneic SLAcc or SLAdd PBL stimulators (30 Gy irradiated) in a total volume of 0.2 ml of medium were incubated at 37°C for 4 days in 5% CO2. Cultures were pulsed with 1 μCi of 3H-TdR on the fifth day, harvested on the sixth day with a Tomtec automated harvester, and assayed in a Pharmacia LKB liquid scintillation counter. Results are expressed as the stimulation index (SI), which equals: mean c.p.m. for test. sample/mean c.p.m. of unstimulated control sample.
Statistical analysis
The results were analyzed by the Mann Whitney test. A P value less than 0.05 was considered to be statistically significant.
Results
Improved peripheral T cell reconstitution by i.v. injection of donor CD34+ cells in recipients of SW THY grafts
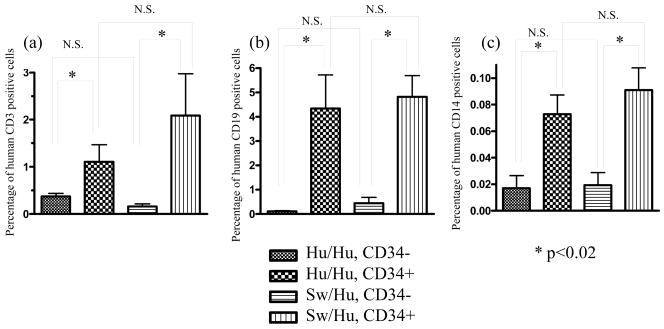
Previous studies have shown that human immune reconstitution, including that of T cells, is enhanced in HU/HU mice by the i.v. injection of CD34+ cells from the same human donor (23). Therefore, to evaluate the effect of adding i.v. injection of human donor CD34 cells to the previous regimen for achieving human T cell reconstitution in porcine thymic grafts (10), 2–5×105 CD34+ cells isolated from the human donor fetal liver (FL) were administrated i.v. on the same day as swine or human fetal thymus and human FL transplantation (SW/HU or HU/HU mice, respectively) under the kidney capsule. Reconstitution of human cell populations in PBL was evaluated by flow cytometry. Compared to HU/HU or SW/HU mice not receiving i.v. co-administration of CD34 cells, human T and B cells tended to appear in the periphery much earlier in both HU/HU and SW/HU mice receiving CD34 cells i.v.. At 9 weeks after transplantation, average percentages of CD3 positive cells were 0.28±0.13 (±SEM) (n=11) and 1.03±0.65 (n=23) in HU/HU and SW/HU mice receiving CD34+ cells i.v., compared to 0.17±0.06 (n=10) and 0.18±0.04 (n=14) in HU/HU and SW/HU mice not receiving CD34+ cells i.v.. CD19 positive cells represented 0.99±0.28 and 1.4±0.25 percent, respectively, of PBL in HU/HU and SW/HU mice that received CD34+ cells at 9 weeks after transplantation, compared to 0.25±0.10 and 0.36±0.17 in HU/HU and SW/HU mice not receiving CD34+ cells i.v. (p<0.05). While the increased T cell reconstitution in recipients of i.v. CD34 cells did not achieve significance at 9 weeks, significantly increased repopulation of human T cells, B cells and monocytes was detected in PBL of both SW/HU and HU/HU mice receiving human CD34 cells i.v. at 12 weeks after transplantation (Figure 1). The similar levels of human T cell reconstitution in the periphery of SW/HU and HU/HU mice receiving human CD34+ cells i.v. has been confirmed in several repeat experiments (T. Onoe and M. Sykes, unpublished data).
Figure 1. Human leukocyte reconstitution in immunodeficient mice transplanted with human or porcine fetal thymus with human fetal liver.
NOD/SCID mice received 3Gy irradiation and human or swine thymus tissue with human fetal liver under the kidney capsule on the same day. 2 to 5 × 105 human CD34 positive cells isolated from the same human fetal liver were injected i.v. on the same day. Reconstitution of PBL by human CD3+ (a), human CD19+ (b), and human CD14+ (c) cells at 12 weeks is shown. Cells were analyzed in a lymphocyte forward-side scatter gate in (a) and (b). Forward-side scatter gates for analyses in (c) included monocytes and granulocytes. Number of recipient mice were n=10, n=11, n=14, and n=23 for HU/HU without CD34 injection, HU/HU with CD34 injection, SW/HU without CD34 injection, SW/HU with CD34 injection, respectively. N.S., not significantly different.
Diverse repertoire of TCR of human CD4 and CD8 single positive (SP) T cells maturing in porcine thymic xenografts
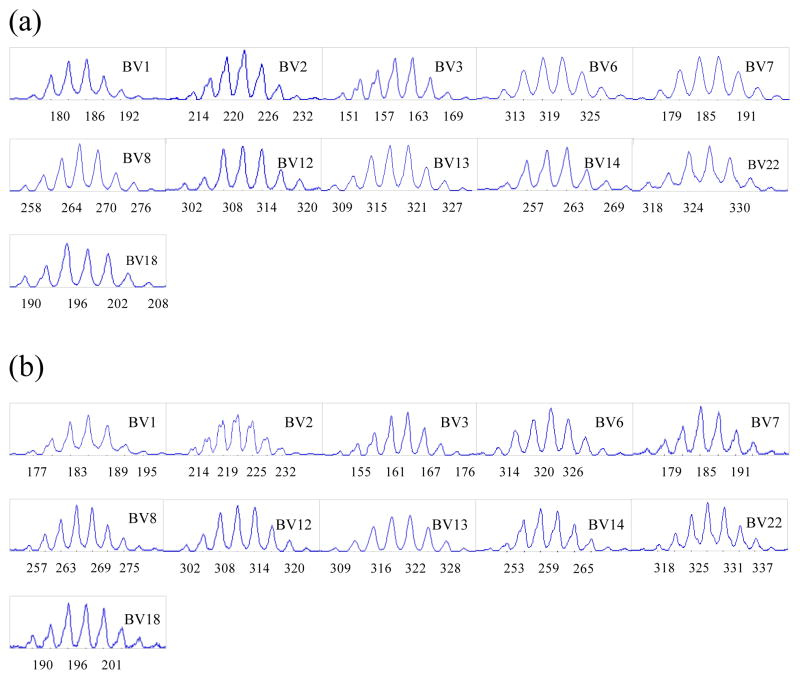
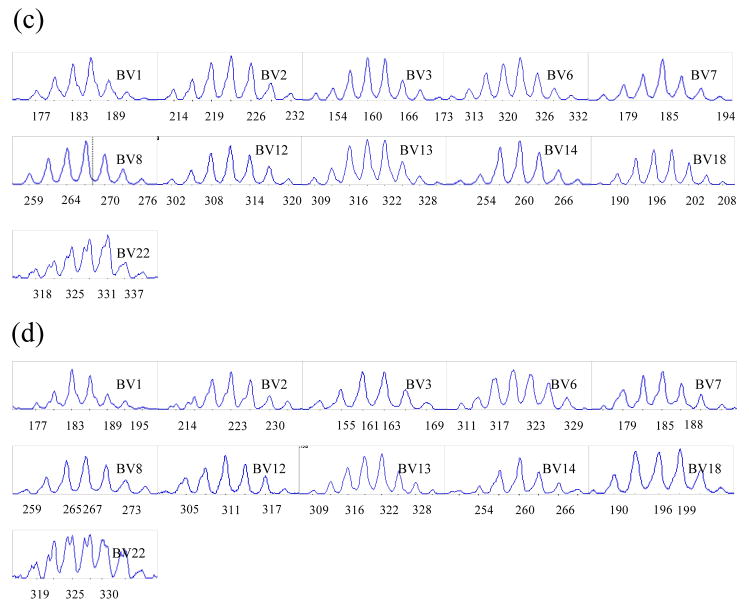
Spectratyping analysis was performed on SP thymocytes from 3 SW/HU and 2 HU/HU grafts harvested 16–20 weeks following implantation in NOD/SCID mice and compared to that of normal human peripheral CD4+ and CD8+ T cells. Similar Vβ were successfully amplified from normal peripheral human T cells and from CD4 and CD8 single positive thymocytes of both HU/HU and SW/HU grafts. One exception was Vβ10, which could not be amplified in either CD4 or CD8 SP thymocytes from SW thymus, but was clearly amplified in SP thymocytes from HU thymus grafts, Spectratypes from the amplified Vβ of CD4 (Figure 2a) and CD8 (Figure 2c) SP cells from human thymic grafts followed a normal distribution. Human TCR of CD4 and CD8 SP cells maturing in SW thymic xenografts also showed multiple peaks with a similar, normal distribution to those seen for normal human T cells (Figure 2b and 2d, respectively). These results indicate that TCR of human CD4 and CD8 SP cells show normal polyclonality, regardless of whether they develop in human or porcine thymic grafts.
Figure 2. Spectratyping of human CD4 and CD8 single positive T cells developing from the same human fetal liver/CD34 cell donor in human versus porcine thymic grafts.
Human CD4 or CD8 single positive (SP) thymocytes were collected from THY/FL grafts between 16 to 20 weeks after transplantation of human or porcine thymus tissue with human fetal liver under the kidney capsule and i.v. injection of CD34 cells from the same donor. Purity of >80% CD4 SP cells was confirmed by flow cytometry for each sample analyzed. Purity of >80% CD8 SP cells was confirmed by flow cytometry for each sample analyzed. RT-PCR was performed using human Vβ1 to 24 primers, and spectratyping was performed after run-off reaction with Jβ-specific FAM-labeled primer. Compared to CD4 and CD8 SP T cells developing in human thymic grafts (a and c, repectively), CD4 and CD8 SP T cells developing in porcine thymic grafts (b and d, respectively) showed similar, normal CDR3 length distributions. CDR3 analysis was performed on each Vβ, from Vβ1 to Vβ24 with similar results, except that Vβ10 could not be amplified from cells in porcine grafts. Spectratypes from one representative animal of three SW/HU and two HU/HU grafts are shown.
Coexistence of simultaneously-grafted porcine and human thymus grafts
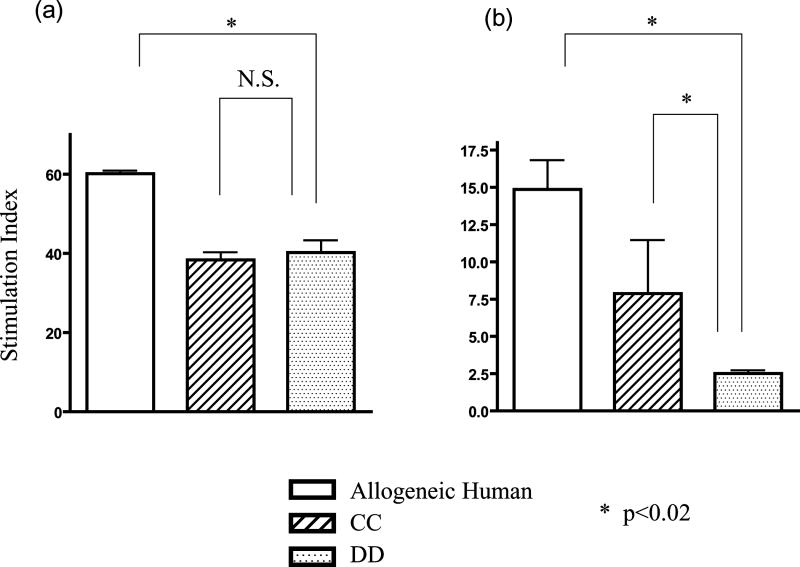
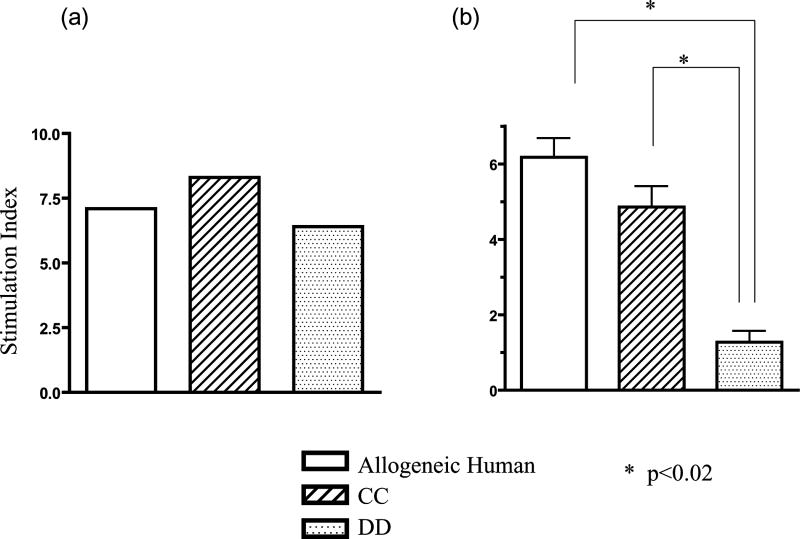
To analyze tolerance of human T cells developing in xenogeneic porcine thymic grafts, MLR assays were performed on graft thymocytes from HU/HU (Figure 3a) and SW/HU (SLAdd pig) (Figure 3b) mice. Consistent with previous results (10), human T cells that developed in porcine grafts showed specific tolerance to the MHC of the porcine donor in MLR, with responsiveness both to human alloantigens and to non-donor, SLA-mismatched porcine xenoantigens (p<0.02 comparing anti-SLAdd to SLA-mismatched SLAcc porcine stimulators). In contrast, human T cells developing in HU grafts showed similar responses to SLAdd and SLAcc. These data confirm that human T cells developing in porcine thymic xenografts were specifically tolerant to the porcine donor MHC antigens.
Figure 3. Human T cells developing in porcine thymus grafts are specifically tolerant toward porcine donor MHC antigens.
Either human (a) or swine (b) thymic grafts were transplanted under the kidney capsule of recipient NOD/SCID mice together with donor human fetal liver tissue, and isolated human CD34 positive cells from the same fetal liver were injected i.v.. Twenty weeks after transplantation, thymocytes were harvested and used for the assay. MLR was performed using third party human stimulators and stimulators expressing third party or donor SLA. Human T cells developing in porcine thymus grafts (SLAdd) showed markedly reduced MLR responses against SLAdd (DD) stimulators (b) compared to SLAcc (CC) or allogeneic human (Allogeneic Human) stimulators. One representative result from each of 2 SW/HU and 2 HU/HU individual mice is shown.
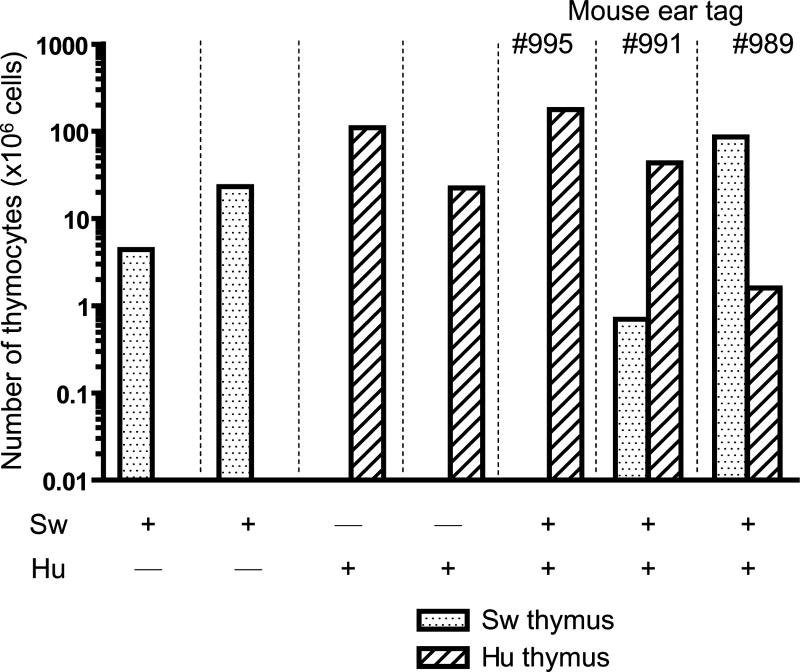
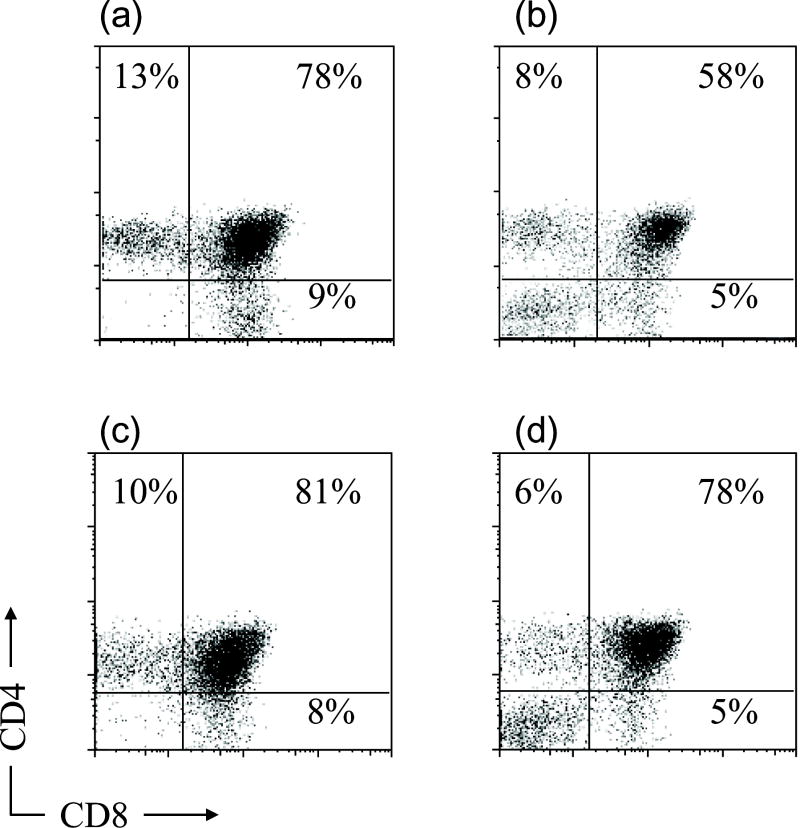
Next, we performed human and porcine dual grafting to analyze whether both xenogeneic thymic grafts could be accepted in the same donor. Human and porcine fetal thymus grafts were transplanted under the opposite kidney capsules along with a human fetal liver fragment and i.v. CD34+ cells from the same human donor (HU+SW mice). Twenty weeks after grafting, thymic grafts were harvested and human thymocytes and CD4 and CD8 profiles were analyzed (Figure 4). As shown in Figure 5a, both grafts yielded measurable numbers of thymocytes in 2 of 3 dual graft recipients. In the third animal (#995), only the human thymus graft could be found. The total thymocyte numbers in the porcine and human thymus grafts of dual graft recipients were similar to the numbers detected in grafts of single graft recipients (Figure 4A). As shown in Figure 4B, CD4 versus CD8 staining profiles were generally similar for recipients of HU/HU, SW/HU and HU+SW double grafts.
Figure 4. Thymocytes in swine or human thymus grafts implanted as single or double grafts.
A) Total thymocyte numbers. Swine or human thymus grafts, transplanted with human fetal liver and human CD34 positive cells injected i.v., were harvested and thymocytes were counted 20 weeks after transplantation. Each bar represents one individual animal. B) Human thymocyte subsets in thymic grafts 20 weeks after double graft implantation. Human (a) and porcine (b) thymic grafts were harvested from HU/HU and SW/HU mice, respectively. Human CD4 and CD8 cell populations were analyzed using flow cytometry. Similar thymocyte populations were detected in human (c) and porcine (d) thymic grafts of HU+SW dual-grafted mice. One representative result from 2 individual mice of each type is shown.
Figure 5. Human T cells developing in porcine but not human thymus grafts of dual graft recipients are specifically tolerant to porcine donor MHC antigens.
Both human or swine thymic grafts were transplanted under opposite kidney capsules of recipient NOD/SCID mice together with human fetal liver, and isolated human CD34 positive cells from the same fetal donor were given i.v.. Twenty weeks later, thymocytes were harvested from each human (a) and swine (b) thymic graft and used for the MLR assay. MLR was performed using allogeneic human, third party SLA, and donor SLA stimulators. Human T cells developing in swine (b) but not human (a) thymus grafts showed markedly and specifically reduced MLR responses against donor-type SLAdd stimulators. One representative result from 2 individual HU+SW recipients is shown.
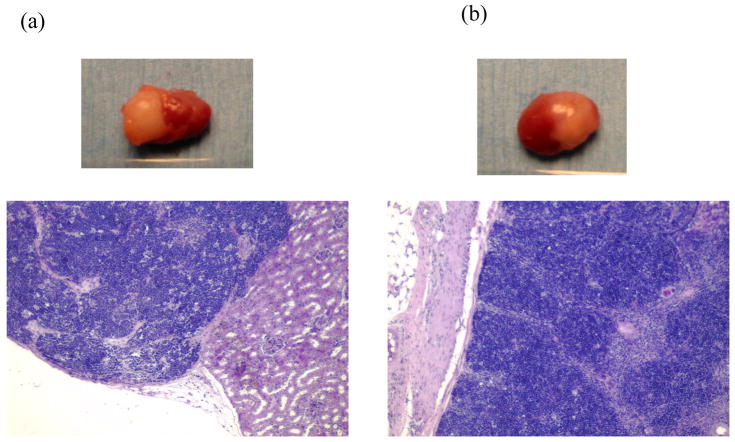
MLR responses were examined for thymocytes collected from swine and human grafts in HU+SW dual-grafted mice. Human T cells in xenogeneic SLAdd thymic grafts showed markedly and significantly reduced proliferative responses toward porcine donor SLAdd compared to the third party SLAcc or allogeneic human stimulators (Figure 5b), demonstrating xenogeneic donor-specific tolerance. In contrast, the human thymocytes in human thymus grafts of all 3 doubly grafted recipients showed similar responses to the porcine donor SLAdd as to third party porcine antigens and human alloantigens (Figure 5a). Histologically, both human and porcine thymus grafts in dual graft recipients showed normal thymic architecture with normal cortical and medullary structure (Figure 6).
Figure 6.
Histology and gross appearance of thymic grafts obtained at 20 weeks post-implantation from a NOD/SCID mouse transplanted with both human and swine thymus grafts under opposite kidney capsules. Both human (a) and swine (b) thymic grafts showed normal thymic architecture with readily visible cortical and medullary structure (H-E staining).
Discussion
We have previously demonstrated in thymectomized and T-cell-depleted mice that porcine thymus grafts can replace the host thymus to support normal mouse thymopoiesis and repopulate the peripheral T cell repertoire (7,8). Importantly, these murine T cells developing in porcine thymus grafts show specific tolerance to the donor, even by the stringent test of skin grafting (7,8). This approach has recently been extended to allogeneic and xenogeneic (pig to baboon) large animal models (3), suggesting that it has considerable potential to promote the success of xenotransplantation.
Positive selection in porcine thymic xenografts in immunocompetent mice is mediated entirely by the porcine MHC of the thymic epithelium, but both porcine donor antigens and recipient MHC antigens contribute to negative selection (26–29). Nevertheless, the murine T cells that repopulate the periphery can recognize antigens presented by host MHC molecules and can clear opportunistic infections (9). These data suggest that recipient mouse-restricted immune reactivity reflects cross-reactivity on murine MHC of a broad repertoire of T cells selected in the porcine thymus grafts.
More recently, we extended these findings to the pig/human combination using immunodeficient mice and demonstrated that normal human thymopoiesis could take place in xenogeneic porcine thymus grafts (10). These results were replicated here, with normal proportions of all human thymocyte subsets in the swine thymus graft. The slight reduction in percentages of DP and SP thymocytes and increase in “double negative” cells in the xenogeneic grafts (Figure 4B) probably reflects the presence of porcine thymocytes that often persist long-term in the porcine grafts, as we have previously reported (10). We also confirmed here that the human T cells developing in porcine thymus grafts are tolerant to the xenogeneic source animal in MLR assays. Remarkably, this unresponsiveness is specific for the MHC (the SLA) of the xenograft donor, as these human T cells showed normal responses to SLA-mismatched porcine stimulators, as well as to allogeneic human stimulators. In our previous study, the failure to overcome tolerance to the donor porcine SLA with exogenous IL-2 suggested that the tolerance was achieved by intrathymic deletion rather than by an anergy mechanism (10). Similar TCR Vβ usage was observed in human thymocytes developing in porcine versus human thymic grafts, suggesting that a polyclonal repertoire was generated in the xenogeneic thymus graft.
We have now addressed the polyclonality of the human T cell repertoire developing in a xenogeneic thymus in a more direct fashion, using spectratype analysis. Our studies reveal similar, polyclonal, normal TCR repertoires among single positive human thymocytes generated in xenogeneic porcine and autologous human thymus grafts. Given that all positive selection of murine T cells in porcine thymus grafts in immunocompetent mice is mediated by the porcine donor MHC, whereas negative selection is mediated by both mouse and pig MHC (26–29), it is reasonable to assume that this diverse human TCR repertoire results from positive selection by porcine thymic epithelium and negative selection on human, porcine and murine elements in the grafts, which we have previously shown to be present (10). The ability of a porcine thymic epithelium to positively select human TCR may reflect in part the extensive homology between SLA and HLA. SLA class Ia (30) and class Ib genes (31) show homology to human class Ia and Ib molecules, respectively. Class II genes also showed strong homology between SLA and HLA (32,33). However, we favor another explanation, given that murine T cells positively selected by porcine thymic epithelium also show evidence for polyclonality (see above) (29), despite the lower species homology of pig and mouse MHC molecules. TCR structure seems to have evolved to have reactivity to MHC (34–36) and this evolution likely occurred before mammalian speciation, resulting in conservation of inherent TCR MHC reactivity between species. This reactivity may ensure the selection of a polyclonal repertoire in the xenogeneic thymic environment.
While the failure to amplify Vβ10 from single positive cells in porcine thymus grafts might suggest that this family of TCR could be deleted by a porcine endogenous superantigen, we have previously succeeded in detecting Vβ10+ human thymocytes in porcine thymus grafts (10), arguing against this possibility. Nevertheless, these previous studies involved whole thymocyte cell suspensions rather than single positive thymocytes, and it is possible that deletion due to a porcine endogenous superantigen occurs at the late double positive and/or single positive stage.
The double grafting experiments performed here may be relevant to the question of whether or not recipient thymectomy will be needed to achieve xenogeneic tolerance through the thymus transplantation approach. In immunocompetent, T cell-depleted mice, recipient thymectomy was found to be essential for the successful engraftment and function of porcine thymus xenografts (37). Recipient thymectomy was also found to be essential for xenogeneic thymus engraftment in a pig to baboon model (13). In a separate ongoing study, we have observed that untreated humanized mice are capable of rejecting porcine thymic tissue grafted after human immune reconstitution has occurred (K. Habiro and Y. Yang, personal communication). However, the data presented here show that two of three animals receiving porcine thymus grafts simultaneously with human thymus tissue and human HSCs accepted the pig thymus long-term, despite the lack of xenogeneic MLR tolerance observed among thymocytes in the human graft. These studies raise the possibility that post-thymic mechanisms, with or without a role for presentation of soluble porcine antigen in the human thymus, may lead to tolerance of human thymocytes after they leave the human thymus graft. Not all self-reactive thymocytes are normally deleted (38–40), and regulatory T cells play an important role in suppressing autoreactivity of these cells (41). Likewise, regulatory cells developing in porcine thymus grafts may suppress the ability of non-tolerant human T cells developing in human thymus grafts to reject porcine thymic grafts. Indeed, thymic epithelium has been shown to be capable of inducing dominant tolerance to its antigens in an allogeneic model (42). It is also possible that the thymus is an immunoprotected site and that non-tolerant T cells generated in the human thymus grafts simply “ignored” the porcine thymus grafts, which were healed in by the time human T cells had developed in the human grafts. However, previous thymic grafting studies in allogeneic mouse models showed that a second thymus graft could tolerize T cells developing in a first thymus graft to minor antigens but not to MHC alloantigens (43). Since we did not analyze tolerance of peripheral T cells in these mice, further studies will be needed to address their tolerance.
The studies presented here demonstrate that the addition of human CD34 cells i.v. can enhance human T cell reconstitution from a porcine graft. Improved human immune reconstitution, including T cells, B cells, dendritic cells, the ability to reject xenografts and the ability to mount specific class-switched antibody responses have recently been demonstrated in HU/HU mice receiving i.v. injections of fetal CD34+ cells from the same human donor (23,44–46). The presence of human APC in the periphery seems to enhance the survival and function of T cells generated in a human thymus graft, consistent with studies showing a need for self MHC/peptide in the periphery (47). Our demonstration of a similar phenomenon in mice receiving porcine thymic xenografts with human FL and CD34 cells, in which the thymic epithelium and human APC in the periphery are completely MHC-mismatched, is somewhat surprising. We hypothesize that because human T cells interact more efficiently with human than mouse APCs in the periphery and because the human TCR repertoire developing in a porcine thymus is diverse, that cross-reactivity with human MHC of TCR selected on porcine MHC allows human APC to provide MHC/peptide signals that support the survival and function of the human T cells. Our observations suggest an excellent model for dissecting the impact of disparity between thymic epithelium (pig) and APC in the periphery (human) on human T cell function. For example, several studies in rodents suggest that self-tolerance may be incomplete, with an increased tendency to develop autoimmunity, when T cells develop in a xenogeneic thymus graft (28,48–50) The improved understanding of immune function obtained from such studies in humanized mice should help to bring the xenogeneic thymic transplantation approach closer to clinical application for the induction of tolerance.
Acknowledgments
We thank Drs. Thomas Fehr and Hideki Ohdan for helpful review of the manuscript and Ms. Kelly Walsh for expert assistance with its preparation.
ABBREVIATIONS
- HU
human
- SW
swine
- TCR
T cell receptor
- FT
fetal thymus
- FL
fetal liver
- DP
double positive
- SP
single positive
- THY
thymus
Footnotes
This work was supported by NIH Grant # PO1 HL 18646 and PO1 AI 045897
Reference List
- 1.Sachs DH, Sykes M, Greenstein JL, Cosimi AB. Tolerance and xenograft survival. Nat Med. 1995;1:969. doi: 10.1038/nm0995-969. [DOI] [PubMed] [Google Scholar]
- 2.Dor FJ, Tseng YL, Cheng J, et al. alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation. 2004;78:15–20. doi: 10.1097/01.tp.0000130487.68051.eb. [DOI] [PubMed] [Google Scholar]
- 3.Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11:32–34. doi: 10.1038/nm1172. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Qian H, Starzl T, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11:1295–1298. doi: 10.1038/nm1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan A, Sergio JJ, Zhao Y, Pearson DA, Sachs DH, Sykes M. Discordant xenogeneic neonatal thymic transplantation can induce donor-specific tolerance. Transplantation. 1997;63:124–131. doi: 10.1097/00007890-199701150-00023. [DOI] [PubMed] [Google Scholar]
- 7.Lee LA, Gritsch HA, Sergio JJ, et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc Natl Acad Sci U S A. 1994;91:10864–10867. doi: 10.1073/pnas.91.23.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y, Swenson K, Sergio JJ, Arn JS, Sachs DH, Sykes M. Skin graft tolerance across a discordant xenogeneic barrier. Nat Med. 1996;2:1211–1216. doi: 10.1038/nm1196-1211. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Fishman JA, Sergio JJ, et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J Immunol. 1997;158:1641–1649. [PubMed] [Google Scholar]
- 10.Nikolic B, Gardner JP, Scadden DT, Arn JS, Sachs DH, Sykes M. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J Immunol. 1999;162:3402–3407. [PubMed] [Google Scholar]
- 11.Kamano C, Vagefi PA, Kumagai N, et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc Natl Acad Sci U S A. 2004;101:3827–3832. doi: 10.1073/pnas.0306666101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barth RN, Yamamoto S, LaMattina JC, et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation. 2003;75:1615–1624. doi: 10.1097/01.TP.0000064335.50622.20. [DOI] [PubMed] [Google Scholar]
- 13.Wu A, Yamada K, Neville DM, et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation. 2003;75:282–291. doi: 10.1097/01.TP.0000044137.97841.99. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto S, Lavelle JM, Vagefi PA, et al. Vascularized thymic lobe transplantation in a pig-to-baboon model: a novel strategy for xenogeneic tolerance induction and T-cell reconstitution. Transplantation. 2005;80:1783–1790. doi: 10.1097/01.tp.0000184445.70285.4b. [DOI] [PubMed] [Google Scholar]
- 15.Toyonaga B, Yoshikai Y, Vadasz V, Chin B, Mak TW. Organization and sequences of the diversity, joining, and constant region genes of the human T-cell receptor beta chain. Proc Natl Acad Sci U S A. 1985;82:8624–8628. doi: 10.1073/pnas.82.24.8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia KC, Degano M, Stanfield RL, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–219. doi: 10.1126/science.274.5285.209. [DOI] [PubMed] [Google Scholar]
- 17.Genevee C, Diu A, Nierat J, et al. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 18.Gorski J, Yassai M, Zhu X, et al. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994;152:5109–5119. [PubMed] [Google Scholar]
- 19.Dietrich PY, Caignard A, Lim A, et al. In vivo T-cell clonal amplification at time of acute graft-versus-host disease. Blood. 1994;84:2815–2820. [PubMed] [Google Scholar]
- 20.Douillard P, Josien R, Pannetier C, Bonneville M, Soulillou JP, Cuturi MC. Selection of T cell clones with restricted TCR-CDR3 lengths during in vitro and in vivo alloresponses. Int Immunol. 1998;10:71–83. doi: 10.1093/intimm/10.1.71. [DOI] [PubMed] [Google Scholar]
- 21.Leight GS, Sachs DH, Rosenberg SA. Transplantation in miniature swine. II. In vitro parameters of histocompatibility in MSLA homozygous minipigs. Transplantation. 1977;23:271–276. [PubMed] [Google Scholar]
- 22.Haller GW, Esnaola N, Yamada K, et al. Thymic transplantation across an MHC class I barrier in swine. J Immunol. 1999;163:3785–3792. [PubMed] [Google Scholar]
- 23.Lan P, Wang L, Diouf B, et al. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- 24.Genevee C, Diu A, Nierat J, et al. An experimentally validated panel of subfamily-specific oligonucleotide primers (V alpha 1-w29/V beta 1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–1269. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 25.Gea-Banacloche JC, Weiskopf EE, Hallahan C, et al. Progression of human immunodeficiency virus disease is associated with increasing disruptions within the CD4+ T cell receptor repertoire. J Infect Dis. 1998;177:579–585. doi: 10.1086/514233. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Sergio JJ, Swenson K, Arn JS, Sachs DH, Sykes M. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J Immunol. 1997;159:2100–2107. [PubMed] [Google Scholar]
- 27.Zhao Y, Rodriguez-Barbosa JI, Zhao G, Shaffer J, Arn JS, Sykes M. Maturation and function of mouse T-cells with a transgenic TCR positively selected by highly disparate xenogeneic porcine MHC. Cell Mol Biol (Noisy -le-grand) 2001;47:217–228. [PubMed] [Google Scholar]
- 28.Zhao Y, Rodriguez-Barbosa JI, Shimizu A, Sachs DH, Sykes M. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation. 2003;75:1832–1840. doi: 10.1097/01.TP.0000065292.20062.F0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Y, Swenson K, Sergio JJ, Sykes M. Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. J Immunol. 1998;161:1320–1326. [PubMed] [Google Scholar]
- 30.Renard C, Vaiman M, Chiannilkulchai N, Cattolico L, Robert C, Chardon P. Sequence of the pig major histocompatibility region containing the classical class I genes. Immunogenetics. 2001;53:490–500. doi: 10.1007/s002510100348. [DOI] [PubMed] [Google Scholar]
- 31.Chardon P, Rogel-Gaillard C, Cattolico L, Duprat S, Vaiman M, Renard C. Sequence of the swine major histocompatibility complex region containing all non-classical class I genes. Tissue Antigens. 2001;57:55–65. doi: 10.1034/j.1399-0039.2001.057001055.x. [DOI] [PubMed] [Google Scholar]
- 32.Gustafsson K, Germana S, Hirsch F, Pratt K, LeGuern C, Sachs DH. Structure of miniature swine class II DRB genes: conservation of hypervariable amino acid residues between distantly related mammalian species. Proc Natl Acad Sci U S A. 1990;87:9798–9802. doi: 10.1073/pnas.87.24.9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirsch F, Sachs DH, Gustafsson K, Pratt K, Germana S, LeGuern C. Class II genes of miniature swine. III. Characterization of an expressed pig class II gene homologous to HLA-DQA. Immunogenetics. 1990;31:52–56. doi: 10.1007/BF00702489. [DOI] [PubMed] [Google Scholar]
- 34.Huseby ES, Crawford F, White J, Marrack P, Kappler JW. Interface-disrupting amino acids establish specificity between T cell receptors and complexes of major histocompatibility complex and peptide. Nat Immunol. 2006;7:1191–1199. doi: 10.1038/ni1401. [DOI] [PubMed] [Google Scholar]
- 35.Huseby E, Kappler J, Marrack P. TCR-MHC/peptide interactions: kissing-cousins or a shotgun wedding? Eur J Immunol. 2004;34:1243–1250. doi: 10.1002/eji.200425000. [DOI] [PubMed] [Google Scholar]
- 36.Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. doi: 10.1016/s0092-8674(00)81905-4. [DOI] [PubMed] [Google Scholar]
- 37.Yamada K, Shimizu A, Utsugi R, et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J Immunol. 2000;164:3079–3086. doi: 10.4049/jimmunol.164.6.3079. [DOI] [PubMed] [Google Scholar]
- 38.Dai Z, Li Q, Wang Y, et al. CD4+CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–822. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 40.Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080–1086. doi: 10.4049/jimmunol.168.3.1080. [DOI] [PubMed] [Google Scholar]
- 41.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 42.Modigliani Y, Thomas-Vaslin V, Bandeira A, et al. Lymphocytes selected in allogeneic thymic epithelium mediate dominant tolerance toward tissue grafts of the thymic epithelium haplotype. Proc Natl Acad Sci U S A. 1995;92:7555–7559. doi: 10.1073/pnas.92.16.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamoyska R, Waldmann H, Matzinger P. Peripheral tolerance mechanisms prevent the development of autoreactive T cells in chimeras grafted with two minor incompatible thymuses. Eur J Immunol. 1989;19:111–117. doi: 10.1002/eji.1830190118. [DOI] [PubMed] [Google Scholar]
- 44.Yang YG, Chen AM, Garrett LJ, et al. Development and analysis of transgenic mice expressing porcine hematopoietic cytokines: a model for achieving durable porcine hematopoietic chimerism across an extensive xenogeneic barrier. Xenotransplantation. 2000;7:58–64. doi: 10.1034/j.1399-3089.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 45.Lan P, Tonomura N, Shimizu A, Wang S, Yang YG. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- 46.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen specific human T cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jung S. Good, bad and beautiful--the role of dendritic cells in autoimmunity. Autoimmun Rev. 2004;3:54–60. doi: 10.1016/S1568-9972(03)00066-1. [DOI] [PubMed] [Google Scholar]
- 48.Taguchi O, Takahashi T, Seto M, Namikawa R, Matsuyama M, Nishizuka Y. Development of multiple organ-localized autoimmune diseases in nude mice after reconstitution of T cell function by rat fetal thymus graft. J Exp Med. 1986;164:60–71. doi: 10.1084/jem.164.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan Y, Devos T, Yu L, et al. Pathogenesis of autoimmunity after xenogeneic thymus transplantation. J Immunol. 2003;170:5936–5946. doi: 10.4049/jimmunol.170.12.5936. [DOI] [PubMed] [Google Scholar]
- 50.Devos T, Sprangers B, Lin Y, et al. Occurrence of autoimmunity after xenothymus transplantation in T-cell-deficient mice depends on the thymus transplant technique. Transplantation. 2008;85:640–644. doi: 10.1097/TP.0b013e3181613f0c. [DOI] [PubMed] [Google Scholar]