SUMMARY
The Drosophila embryonic salivary gland is a migrating tissue that undergoes a stereotypic pattern of migration into the embryo. We demonstrate that the migratory path of the salivary gland requires the PDGF/VEGF pathway. The PDGF/VEGF receptor, Pvr, is strongly expressed in the salivary glands, and Pvr mutations cause abnormal ventral curving of the glands, suggesting that Pvr is involved in gland migration. Although the Pvr ligands, Pvf1 and Pvf2, have distinct expression patterns in the Drosophila embryo, mutations for either one of the ligands result in salivary gland migration defects similar to those seen in embryos that lack Pvr. Rescue experiments indicate that the PDGF/VEGF pathway functions autonomously in the salivary gland. The results of this study demonstrate that the Drosophila PDGF/VEGF pathway is essential for proper positioning of the salivary glands.
Keywords: Pvr, Pvf1, Pvf2, Pvf3, VEGF, PDGF, salivary gland, migration
INTRODUCTION
Cell migration is an essential part of the development and function of many cell types in all multicellular organisms. Guidance by external spatial cues directs a migrating cell or tissue to maintain an appropriate migratory path within an organism and ultimately reach the correct target. There are many examples of this, including immune cells that receive chemical gradient cues throughout development, as well as during their lifetime as pathogen fighting cells, neurons that receive cues promoting axon guidance, the multistep migration of the primordial germ cells and migration of the border cells in the Drosophila ovary (Bonasio et al., 2006; Luster et al., 2005; Montell, 2006; Tessier-Lavigne and Goodman, 1996).
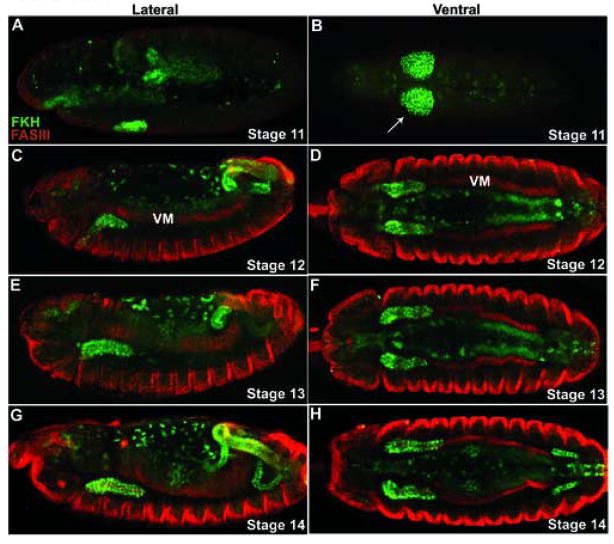
The embryonic development of the Drosophila salivary glands provides a good system to study guided cell migration (Andrew et al., 2000; Bradley et al., 2001; Kerman et al., 2006). The salivary glands consist of two cell types: gland cells and duct cells, which are specified on the ventral surface of parasegment 2. During stage 11, the circular salivary placodes form and are visible as two groups of cells on either side of the ventral midline (Fig. 1A–B). The placodes are separated ventrally by cells that will give rise to the salivary ducts. After specification, the salivary placodes begin to invaginate into the embryo (Fig. 1C–D). When the salivary glands reach the visceral mesoderm, the glands turn and begin posterior migration. The glands are completely internalized by stage 13 and lie parallel to the anteroposterior axis of the embryo (Fig. 1E–H). This posterior migration is a heavily regulated process involving attractive and repulsive cues and complex tissue-tissue interactions that are just beginning to be understood. Recent work on these cues has revealed startling similarities between salivary gland migration and axonal development (Harris and Beckendorf, 2007; Kolesnikov and Beckendorf, 2005; Vining et al., 2005).
Figure 1. Morphogenesis of the salivary glands.
(A–H) Salivary gland cells (arrows) are stained for FKH in green and the circular visceral mesoderm is stained for FASIII in red. (A, C, E G) Lateral and (B, D, F, H) corresponding ventral views of embryos stages 11 through 14. (A, B) Salivary glands begin as a pair of single-layered epithelial disks, the salivary placodes that invaginate by apical constriction to form slender tubes. (C, D) As they leave the surface these tubes extend dorsally and posteriorly at a 45 degree angle until they reach the visceral mesoderm. (E, F) Then they change paths and migrate actively along the mesoderm until they lie horizontally within the embryo (G, H) By stage 15 the glands reach their final position within the embryo.
Here we characterize the role in salivary gland development of Pvr, the gene coding for the single Drosophila homolog of the mammalian PDGF/VEGF receptors. Previous studies have shown that Pvr is needed for border cell migration, hemocyte migration and survival, thorax closure during metamorphosis, and the rotation and dorsal closure of the male terminalia (Bruckner et al., 2004; Duchek et al., 2001; Ishimaru et al., 2004; Macias et al., 2004). These processes involve concerted morphogenetic cell movements which are disrupted in Pvr mutants. In this paper we report that Pvr is expressed in Drosophila embryonic salivary glands and that mutations in Pvr disrupt the concerted migration of the salivary glands. Furthermore, we show that at least two of the Pvf ligands, Pvf1 and Pvf2 are required for this migration.
RESULTS AND DISCUSSION
Pvr is expressed in the developing salivary glands
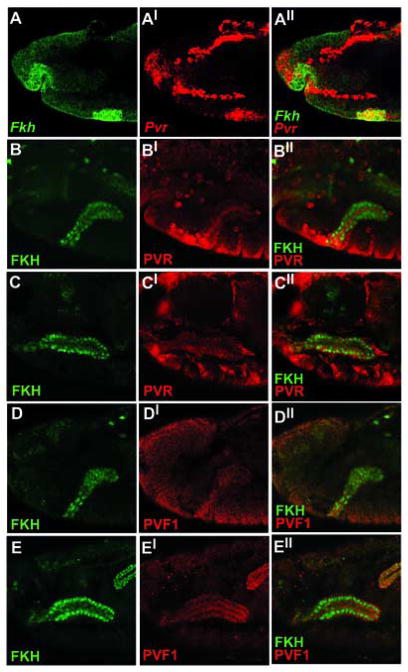
In Drosophila embryos the Pvr receptor is expressed in the hemocytes where it is necessary for cell survival and for migration of the hemocytes throughout the embryo (Bruckner et al., 2004; Cho et al., 2002; Wood et al., 2006). Another site of embryonic Pvr expression is the developing salivary gland. Salivary expression of Pvr mRNA is strongest at stage 11 of embryonic development, when salivary gland cells are still situated on the surface of the embryo as circular placodes (Fig. 2A). Transcript levels steadily decrease through stage 12, during which time the placode cells invaginate. At stage 13 Pvr transcripts are practically undetectable (data not shown). PVR protein is detected in the gland beginning at stage 12 and is localized to the cell membrane (Fig 2B–C).
Figure 2. Pvr and Pvf1 are expressed in the developing salivary glands.
(A) w1118 embryos were hybridized in situ with Fkh and Pvr probes. Lateral view of a stage 11 embryo. (A-A″) The Pvr receptor is expressed in the salivary placode. (B–C) Lateral view of wild-type embryos stained for FKH and PVR proteins. (B-B″) PVR is first detected as the salivary gland invaginates into the embryo (stage 12) and is enriched at the apical membrane. (C-C″) PVR expression reaches peak levels by stage 15. (D–E) Lateral view of wild-type embryos stained for FKH and PVF1 proteins. (D-D″) PVF1 expression begins in the salivary glands at stage 12. (E-E″) PVF1 expression increases during salivary gland development and reaches peak levels by stage 15.
The three Pvr ligands have distinct expression patterns within the Drosophila embryo
Three genes in the Drosophila genome code for Pvr ligands: Pvf1, Pvf2, and Pvf3. Both Pvf2 and Pvf3 are expressed in the ventral midline, where they are thought to act in a partially redundant manner as attractive cues for hemocytes migrating out of the head (Cho et al., 2002; Wood et al., 2006). Previous studies have shown that Pvf2 and Pvf3 share more than just a similar expression pattern. These genes are located only 16 kb apart and may have been generated by recent gene duplication. Sequence similarities indicate that they are likely to be functionally similar to each other as well (Cho et al., 2002). In contrast, Pvf1 contains unique, cysteine-rich CXCXC motifs not found in the other two ligands, and it has a distinct expression pattern. The developing salivary gland is the strongest site of Pvf1 expression, beginning at stage 12 and persisting through stage 17 (Fig. 2D–E). Interestingly, Pvf1 protein is expressed at highest levels in the cells near the tip of the gland that are the most actively involved in migration (Bradley et al., 2003). Pvf1 is not expressed in the ventral midline and has does not have a significant effect on embryonic hemocyte migration (Cho et al., 2002; Wood et al., 2006).
Pvr and the Pvf ligands are necessary for salivary gland development
The importance of Pvr and the Pvf ligands for salivary gland development was confirmed when Pvr-mutant embryos were found to have salivary gland migration defects. Pvr null mutant embryos have salivary glands that curve abnormally toward the ventral surface of the embryo, instead of lying parallel to the A-P axis of the embryo as in wild type embryos (Fig. 3B, F). In contrast to hemocytes, salivary gland survival, as tested by TUNEL staining, was not affected by Pvr mutations (data not shown).
Figure 3. Pvr and the Pvf ligands are required for proper salivary gland positioning.
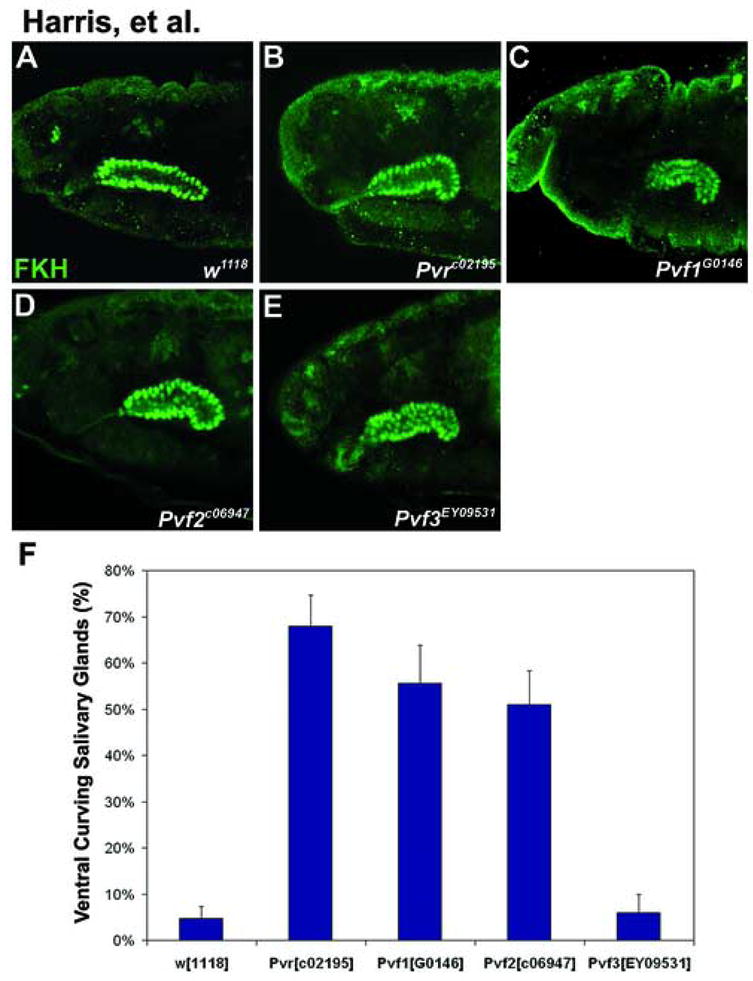
(A–E) Lateral views of stage 15 embryos stained with FKH. (B) In Pvr null embryos the salivary glands curve ventrally. (C–D) Embryos lacking Pvf1 or Pvf2 have salivary glands that bend ventrally, similar to Pvr mutants. Two Pvf1 alleles (Pvf1G0146, Pvf1EP1624) and two Pvf2 alleles (Pvf2C06947, Pvf2d02444) were tested. All caused similar phenotypes at 40–60% penetrance. (E) Pvf3 mutant embryos have an impenetrant phenotype that resembles Pvr mutants. (F) Graphical representation of phenotypic penetrance in embryos scored for salivary gland migration defects at stages 14–16. At least 30 embryos were scored for each genotype represented on the graph.
Mutations in the Pvf1 or Pvf2 ligands caused ventral curving similar to that in Pvr mutant embryos (Fig. 3C–D, F). In contrast, Pvf3EY09531, a P-element insertion located in the first intron of the Pvf3 gene, very infrequently affects embryonic gland positioning (Fig. 3E, F). This impenetrant phenotype may be due to the timing of Pvf3 expression, which occurs prior to salivary gland invagination and decreases during the time that the salivary gland migrates posteriorly. Alternatively, the EY09531 P-element insertion may be a weak, hypomorphic mutation that does not eliminate Pvf3 function.
Several factors required for salivary gland guidance and migration have already been identified. After the salivary gland contacts the VM and begins to move posteriorly within the embryo, the attractant Netrin and two repellents, Slit and Wnt4, guide the salivary glands (Harris and Beckendorf, 2007; Kolesnikov and Beckendorf, 2005). Netrin, which is expressed in the CNS and the visceral mesoderm, works as an attractant to maintain salivary gland positioning on the visceral mesoderm. At the same time, CNS expression of Slit and Wnt4 keeps the salivary glands away from the CNS and parallel to it. The early timing of the Pvr phenotype and its similarity to the slit and Wnt4 phenotypes suggest that PDGF/VEGF signaling is required at the same time as Netrin, Slit and Wnt4 signaling and might be required for the same process, salivary gland guidance. Near the end of salivary migration, a different signal-receptor pair, Wnt5 and Derailed, is required at the distal tip of the glands to mediate attachment to the longitudinal visceral mesoderm (Harris and Beckendorf, 2007).
The salivary gland defect caused by Pvr mutation is salivary gland autonomous
In addition to its role in the salivary gland, Pvr is essential for hemocyte migration throughout the embryo (Cho et al., 2002; Wood et al., 2006). During their migration hemocytes lay down components of the extracellular matrix needed by other cells for movement (Nelson & Fessler, 1994). For example, the extracellular matrix is indispensable for the process of ventral nerve cord condensation. In Pvr mutants, this condensation fails due to defects in hemocyte migration and extracellular matrix deposition (Olofsson and Page, 2005). This extracellular matrix might also be important for salivary gland migration. Therefore, we tested whether the salivary gland defects caused by mutations in Pvr and its ligands were autonomous to the salivary gland. When expression of a dominant negative allele of Pvr is driven in the salivary glands by fkh-GAL4, the glands curve ventrally as they do in Pvr-mutants (Fig 4B, F). In contrast, expression with hemocyte specific driver pxn-GAL4 results in near background levels of ventral curving, despite the inhibition of hemocyte migration (Fig 4C, F and data not shown). Thus, Pvr activity is autonomously required in the salivary glands. Neither proper hemocyte migration nor the extracellular matrix that hemocytes deposit is required for salivary gland migration. Furthermore, the gland migration defects in Pvr mutants are rescued in embryos carrying fkh-GAL4 and either UAS-Pvr or the constitutively active UAS-λPvr construct (Fig. 4E–G).
Figure 4. Pvr function is autonomous to the salivary gland.
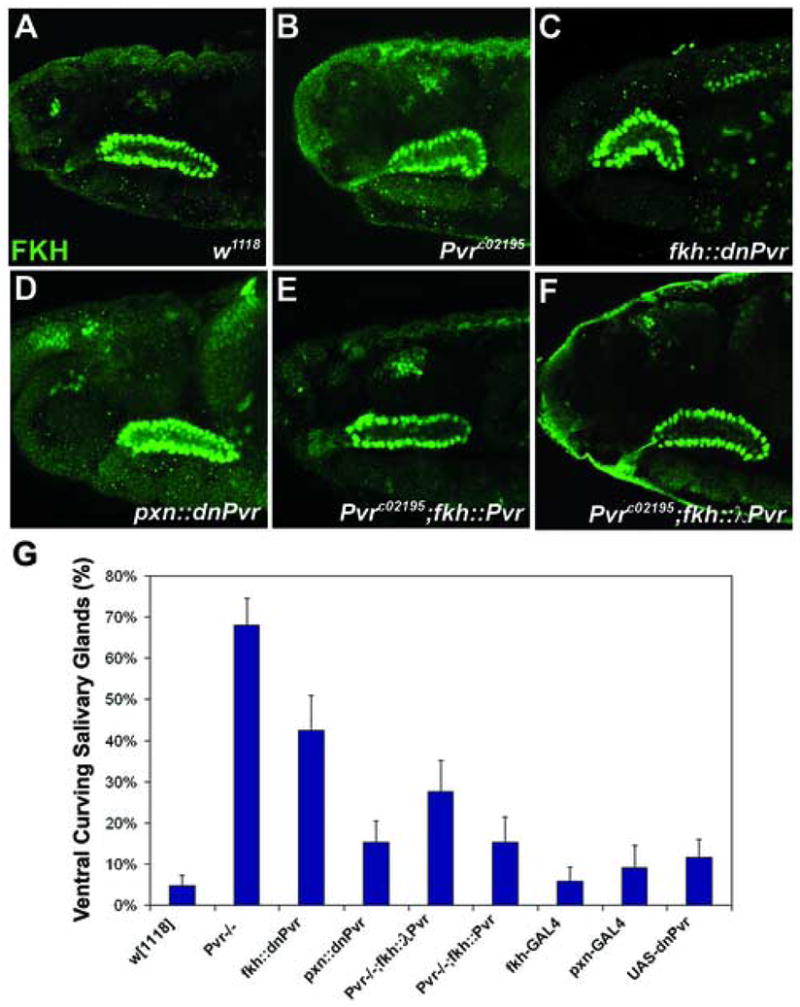
(A–F) Lateral views of stage 15 embryos stained with FKH. (B) In Pvr mutant embryos the salivary glands curve ventrally. (C) Expression of a dominant negative transgene for Pvr in the gland phenocopies the Pvr mutant phenotype (compare B and C). (D,G) Expression of dominant negative Pvr in the hemocytes has little effect on salivary gland positioning. (E,G) The Pvr mutant phenotype can be rescued by UAS-Pvr using a salivary gland specific GAL4 driver, fkh-GAL4. (F,G) A constitutively active Pvr construct is also able to rescue the Pvr mutant phenotype when driven in the salivary gland. (G) Graphical representation of phenotypic penetrance in embryos scored for salivary gland migration defects at stages 14–16. At least 30 embryos were scored for each genotype represented on the graph.
Ectopic Expression of the Pvf ligands affects salivary gland position
Previous studies in Drosophila have shown that the Pvf ligands act as attractants. In the border cells, ectopic expression of PVF1 is sufficient to redirect border cells towards the site of expression (McDonald et al., 2003). Similarly, PVF2 and PVF3 in the ventral midline act as attractants for migrating hemocytes. Ectopic expression of PVF2 using breathless-GAL4 has been shown to induce a chemotactic response to the new source of PVF expression from the embryonic hemocytes (Cho et al., 2002). The ventral midline expression of Pvf2, along with its ventral curving phenotype, suggests that Pvf2 might be acting as a repellent for the salivary gland, despite the fact that there is no previous indication of Pvf ligands acting as repellents. However, ectopic expression of PVF2 in the visceral mesoderm was not sufficient to redirect the salivary glands away from the visceral mesoderm (data not shown).
We find that overexpression of Pvf1 in the salivary glands results in ventrally curved glands similar to those seen in Pvr mutants (Fig. 5B). Similarly, misrouted glands result from salivary-gland expression of Pvf2 and Pvf3 (Fig 5C–D). These results suggest that all three ligands may have the same or similar effects on the Pvr receptor, despite their differing expression patterns and slightly different sequences. Interestingly, expression of the constitutively active receptor, λPvr in otherwise wild-type embryos results in salivary glands that are ventrally curved, similar to overexpression of the individual ligands in the gland (Fig 5E). One possible explanation for these results is that the Pvf ligands might be required for proper salivary gland positioning by regulating adhesion or another migratory event autonomous to the salivary gland.
Figure 5. Ectopic expression of the Pvf ligands results in salivary gland positioning defects.
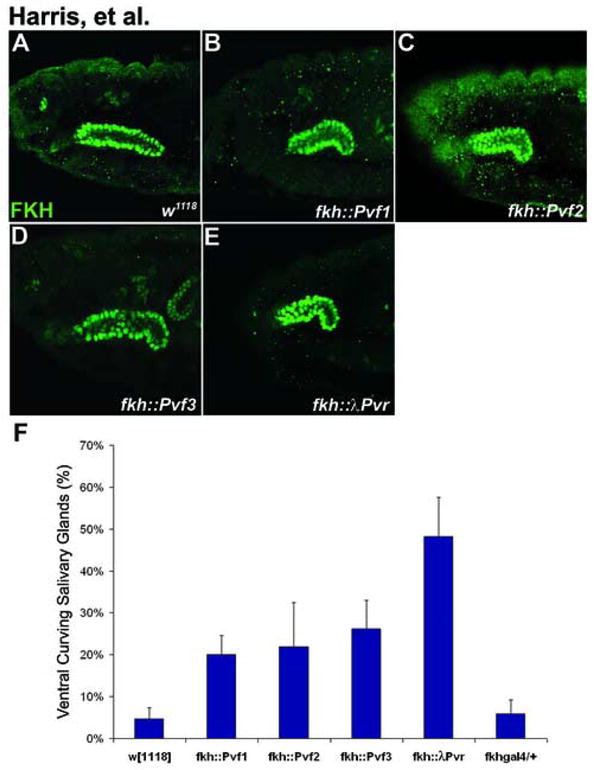
(A–D) Lateral views of stage 15 embryos stained with FKH. (B) Overexpression of Pvf1 using the salivary gland specific GAL4 driver, fkh-GAL4, results in ventrally curved salivary glands. (C–D) Misexpression of either Pvf2 (C) or Pvf3 (D) in the salivary gland also results in ventrally curved glands. (E) Similar results, though at a higher frequency, are seen when the constitutively active receptor, λPvr, is expressed in the salivary glands. (F) Graphical representation of phenotypic penetrance in embryos scored for salivary gland migration defects at stages 14–16. At least 30 embryos were scored for each genotype represented on the graph.
Alternatively, both the salivary gland expressed Pvf1 and ventral midline expressed Pvf2 may be required for a directional response to be received, perhaps forming a heterodimer to activate the Pvr receptor. This possibility is attractive since vertebrate studies have shown that heterodimers are formed between PDGF-A and PDGF-B that have a unique binding affinity for PDGF receptor subtypes that differs from the affinity of either homodimer (Heidaran et al., 1991). Furthermore, the PDGF-AB heterodimer is capable of activating different signal transduction pathways thus eliciting a different response on proliferation as well as gene expression compared to the PDGF-AA or PDGF-BB monomers (Kondo et al., 1993; Lepisto et al., 1995). It seems plausible that a similar situation may be occurring in the salivary glands, where both PVF1 and PVF2 are needed in order to activate PVR and direct the salivary glands.
EXPERIMENTAL PROCEDURES
Fly strains
The following alleles were used: PvrC02195, Pvf2C06947 (provided by M. Krasnow)(Cho et al., 2002), Pvf3EY09531 (Baylor stock center), Pvf1G0146, Pvf1EP1624, Pvf2d02444 and w1118 (Bloomington stock center). w1118 was used as the standard strain for comparison.
The following GAL4 and UAS constructs were used: UAS-Pvr, UAS-λPvr, UAS-dnPvr (Duchek et al., 2001), fkh-GAL4 (Zhou, 1995), pxn-GAL4 (Fessler et al., 1994), sca-GAL4 (provided by M. Foss), UAS-Pvf1 (provided by D. Montell)(McDonald et al., 2003), UAS-Pvf2 (provided by M. Krasnow)(Cho et al., 2002), UAS-Pvf3 (provided by B. Shilo)(Rosin et al., 2004).
Immunocytochemistry and in situ hybridization
Embryo fixation and staining were performed as described (Chandrasekaran and Beckendorf, 2003). The salivary gland nuclear-specific antibody used was rabbit anti-FKH (1:1000). Rabbit anti-β-galactosidase antibody (Roche) was used at 1:1000. The mouse anti-FASIII (7G10) antibody was obtained from the Hybridoma Bank and used at 1:10. Rat anti-PVR was used at 1:200 (provided by B. Shilo)(Rosin et al., 2004). Alexa Fluor-546- and 488- (Molecular Probes) secondary antibodies were used at 1:500. Fluorescent in situ hybridization was performed as described (Tautz and Pfeifle, 1989) with modifications (Harland, 1991) using antisense digoxigenin-labeled probes. Mouse anti-DIG (Roche) was used at 1:100 with the Alexa Fluor-546 (Molecular Probes) secondary antibody at 1:250. After washing, embryos were cleared with 50% glycerol, then 70% glycerol and visualized on a Zeiss 510 confocal microscope.
Acknowledgments
We thank D. Montell, M. Krasnow, P. Rørth, M. Foss and the Bloomington Stock Center for providing fly stocks and B. Shilo for providing fly stocks and antibodies. We also thank T. Kolesnikov for helpful discussion and critical reading of the manuscript. This work was supported by an NIH grant (DE012519) to SKB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrew DJ, Henderson KD, Seshaiah P. Salivary gland development in Drosophila melanogaster. Mech Dev. 2000;92:5–17. doi: 10.1016/s0925-4773(99)00321-4. [DOI] [PubMed] [Google Scholar]
- Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- Bradley PL, Haberman AS, Andrew DJ. Organ formation in Drosophila: specification and morphogenesis of the salivary gland. Bioessays. 2001;23:901–11. doi: 10.1002/bies.1131. [DOI] [PubMed] [Google Scholar]
- Bradley PL, Myat MM, Comeaux CA, Andrew DJ. Posterior migration of the salivary gland requires an intact visceral mesoderm and integrin function. Dev Biol. 2003;257:249–62. doi: 10.1016/s0012-1606(03)00103-9. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran V, Beckendorf SK. senseless is necessary for the survival of embryonic salivary glands in Drosophila. Development. 2003;130:4719–28. doi: 10.1242/dev.00677. [DOI] [PubMed] [Google Scholar]
- Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–76. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Duchek P, Somogyi K, Jekely G, Beccari S, Rorth P. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell. 2001;107:17–26. doi: 10.1016/s0092-8674(01)00502-5. [DOI] [PubMed] [Google Scholar]
- Fessler LI, Nelson RE, Fessler JH. Drosophila extracellular matrix. Methods Enzymol. 1994;245:271–94. doi: 10.1016/0076-6879(94)45016-1. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods in cell biology. 1991;36:685–95. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harris KE, Beckendorf SK. Different Wnt signals act through the Frizzled and RYK receptors during Drosophila salivary gland migration. 2007 doi: 10.1242/dev.001164. [DOI] [PubMed] [Google Scholar]
- Heidaran MA, Pierce JH, Yu JC, Lombardi D, Artrip JE, Fleming TP, Thomason A, Aaronson SA. Role of alpha beta receptor heterodimer formation in beta platelet-derived growth factor (PDGF) receptor activation by PDGF-AB. The Journal of biological chemistry. 1991;266:20232–7. [PubMed] [Google Scholar]
- Ishimaru S, Ueda R, Hinohara Y, Ohtani M, Hanafusa H. PVR plays a critical role via JNK activation in thorax closure during Drosophila metamorphosis. Embo J. 2004;23:3984–94. doi: 10.1038/sj.emboj.7600417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Andrew DJ. From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis. Differentiation; research in biological diversity. 2006;74:326–48. doi: 10.1111/j.1432-0436.2006.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. NETRIN and SLIT guide salivary gland migration. Dev Biol. 2005;284:102–11. doi: 10.1016/j.ydbio.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Kondo T, Konishi F, Inui H, Inagami T. Differing signal transductions elicited by three isoforms of platelet-derived growth factor in vascular smooth muscle cells. The Journal of biological chemistry. 1993;268:4458–64. [PubMed] [Google Scholar]
- Lepisto J, Peltonen J, Vaha-Kreula M, Niinikoski J, Laato M. Platelet-derived growth factor isoforms PDGF-AA, -AB and -BB exert specific effects on collagen gene expression and mitotic activity of cultured human wound fibroblasts. Biochemical and biophysical research communications. 1995;209:393–9. doi: 10.1006/bbrc.1995.1516. [DOI] [PubMed] [Google Scholar]
- Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–90. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- Macias A, Romero NM, Martin F, Suarez L, Rosa AL, Morata G. PVF1/PVR signaling and apoptosis promotes the rotation and dorsal closure of the Drosophila male terminalia. Int J Dev Biol. 2004;48:1087–94. doi: 10.1387/ijdb.041859am. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Pinheiro EM, Montell DJ. PVF1, a PDGF/VEGF homolog, is sufficient to guide border cells and interacts genetically with Taiman. Development. 2003;130:3469–78. doi: 10.1242/dev.00574. [DOI] [PubMed] [Google Scholar]
- Montell DJ. The social lives of migrating cells in Drosophila. Current opinion in genetics & development. 2006;16:374–83. doi: 10.1016/j.gde.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Olofsson B, Page DT. Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol. 2005;279:233–43. doi: 10.1016/j.ydbio.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Rosin D, Schejter E, Volk T, Shilo BZ. Apical accumulation of the Drosophila PDGF/VEGF receptor ligands provides a mechanism for triggering localized actin polymerization. Development. 2004;131:1939–48. doi: 10.1242/dev.01101. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–5. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–33. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Vining MS, Bradley PL, Comeaux CA, Andrew DJ. Organ positioning in Drosophila requires complex tissue-tissue interactions. Dev Biol. 2005;287:19–34. doi: 10.1016/j.ydbio.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–16. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B. Salivary determination and transcriptional control of fork head in Drosophila melanogaster. University of California; Berkeley: 1995. p. 220. [Google Scholar]