Abstract
A mucocutaneous reaction in mice associated with Doxil® treatment was identified as Auricular Erythema (AE). Given that the immuno-targeting of Doxil® to tumors was found to influence also its systemic biodistribution pattern, the attempt was made to exploit a specific targeting of Doxil® to reduce the manifestation of this adverse reaction. This problem is of general significance, since cutaneous reactions often lead to alterations of Doxil® dosing regimen in patients and might subsequently compromise the therapeutic outcome of cancer treatment. Tumor-bearing mice were used to study the biodistribution and skin-tissue accumulation effects of the tumor-targeted Doxil® (the clinically used anti-cancer formulation) coupled with the anti-cancer monoclonal 2C5 antibody (mAb 2C5) as well as AE caused by Doxil® application. The modification of Doxil® with mAb 2C5 resulted in a significant decrease in the normal skin accumulation of doxorubicin compared to original Doxil® and substantially reduced AE. The frequency of AE was decreased by 3-4-fold with the mAb 2C5-modified doxorubicin-loaded long-circulating liposomes. Thus, targeting of Doxil® with the anticancer mAb 2C5 not only can increase the tumor-specific accumulation of the drug, but also diminishes the cutaneous side-effect of the original Doxil® therapy.
Keywords: Doxorubicin, Long-circulating liposomes, Doxil®, Tumor targeting, Tumor-specific antibody, Anti-nucleosome antibody, Auricular Erythema
INTRODUCTION
The enhanced tumoral delivery of anti-cancer drugs in long-circulating pharmaceutical nanocarriers is based on the passive accumulation of such carriers via the enhanced permeability and retention (EPR) effect responsible for the extravasation of large molecules and nanoparticles into the tumor mass in the cancerous areas with “leaky” vasculature (Iyer, et al. 2006). Doxil® [doxorubicin-loaded polyethylene glycol(PEG)-coated liposomes] embodies this principle of passive targeting. It was shown earlier, that PEG-liposomes demonstrate prolonged circulation in the blood (Klibanov, et al. 1990), and encapsulating various drugs including doxorubicin within these sterically-stabilized PEG-liposomes favorably alters their pharmacokinetics and biodistribution (Allen, et al. 1991, Papahadjopoulos, et al. 1991). This has resulted in the significant decrease in doxorubicin-associated dose-limiting toxicities: cardiomyopathy and myelosuppression (Berry, et al. 1998, Safra, et al. 2000).
However, due to this alteration of the pharmacokinetic profile of free doxorubicin, the dose-limiting toxicities of the long-circulating liposomal doxorubicin have been shifted towards mucocutaneous reactions such as palmar-plantar erythrodysesthesia (PPE) and mucositis/stomatitis (Hamilton, et al. 2002, Lotem, et al. 2000, Uziely, et al. 1995). The current hypothesis for the development of PPE is that the small size and long circulation time of Doxil® allow for the liposome accumulation in the skin where the basal layers of the skin are damaged with prolonged exposure to doxorubicin as the liposomes slowly release this drug with known vesicant properties. Moreover, liposomes with long circulation times were found to accumulate in the skin of experimental animals to a greater extent than liposomes with shorter circulation times (Allen and Hansen 1991, Papahadjopoulos, et al. 1991). This is believed to be a result of the pressure-dependent extravasation of such liposomes into cutaneous tissues (Allen and Hansen 1991, Hamilton, et al. 2002, Hensley, et al. 2001), which is currently supported by experimental and clinical data indicating that the accumulation of the long-circulating liposomes in fact repeats the anatomical distribution of PPE lesions in regions of skin that are subjected to the pressure or irritation, like the flexure creases of the hands, soles of the feet, or belt lines (Gordon, et al. 2000, Hensley, et al. 2001, Lotem, et al. 2000). As indicated earlier, the incidence and severity of PPE associated with common Doxil® therapeutic regimens, by extrapolation of murine PK data to humans, can be controlled by increasing dose intervals, allowing time for the drug to clear from skin and for the ulcers to heal. Nevertheless, the benefit of dose delay would be offset by reduced therapeutic efficacy (Charrois and Allen 2003, Ranson, et al. 1997, Uziely, et al. 1995).
The modification of drug-loaded pharmaceutical carriers with tumor-specific ligands, such as anti-tumor monoclonal antibodies, is used to achieve higher local drug concentration at the target zone and enhanced anticancer effect of the chemotherapeutic agent (Noble, et al. 2004, Park, et al. 2004). The idea of adding monoclonal antibodies or their fragments to passively targeted long-circulating PEGylated drug carriers, including liposomes, may not only render the whole formulation more tumor-specific, hence demonstrating a superior therapeutic effectiveness (Torchilin 2005), but could also result in decreasing drug presence in non-targeted areas, thus reducing possible adverse effects of drugs in long-circulating carriers onto normal areas of the body.
Earlier, we have described the monoclonal antinuclear antibody 2C5 (mAb 2C5) with the nucleosome-restricted specificity and unique ability to selectively bind to the surface of a broad variety of lymphoid and non-lymphoid tumor cells of murine and human origin (but not to the surface of normal cells) via the tumor cell surface-bound nucleosomes released from the apoptotically dying neighboring tumor cells (Iakoubov, et al. 1995, Iakoubov and Torchilin 1997). We have also used this antibody as a targeting moiety to actively target various drug-loaded long-circulating pharmaceutical nanocarriers, including doxorubicin-loaded PEGylated liposomes (Doxil®) to different tumors (Elbayoumi, et al. 2007, Elbayoumi and Torchilin 2006, Elbayoumi and Torchilin 2007, Gupta, et al. 2005, Lukyanov, et al. 2004).
Here, we report our findings from the experiments with animals receiving different Doxil® formulations, plain and immuno-modified with the mAb 2C5. The animals subjected to original Doxil® treatment exhibited a blistering skin reaction, limited to the auricular region of the ears, hence named “Auricular Erythema” (AE). The manifestations of this AE cutaneous reaction closely resemble the PPE noticed earlier known for doxorubicin in PEG-liposomes. The occurrence and severity of the AE can be correlated with the reported drug release from the liposomes and its skin accumulation profiles. The data also suggest that the modification of Doxil® with the tumor-specific mAb 2C5 significantly reduces the manifestation of AE. Therefore, mAb 2C5-modification of Doxil® can potentially help alleviate the therapy-associated mucocutaneous adverse effects known for the original Doxil®.
MATERIALS AND METHODS
Materials
Doxil® was purchased from Pharmaceutics Inc. (West Roxbury, MA). Cholesterol (Chol), fully hydrogenated soy phosphatidylcholine (HSPC), N-(carbonyl-methoxy poly (ethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (MPEG2000-DSPE), and phosphatidylethanolamine (PE) were from Avanti Polar Lipids, Inc. (Alabaster, AL) and used without further purification. Doxorubicin was purchased from Sigma Chem., Inc. (St. Louis, MO). Triethylamine (TEA), octyl glycoside (OG) and diethylene triamine pentaacetic acide anhydride (DTPA) were also products of Sigma. Poly-oxyethylene3400-bis(p-nitrophenyl carbonate) [PEG(pNP)2] was purchased from SunBio (Orinda, CA). Cell culture media, DMEM, EMEM and RPMI 1640, fetal bovine serum (FBS) and concentrated solutions of Na-pyruvate, non-essential amino acids, L-glutamine and penicillin/streptomycin stock solutions were purchased from CellGro (Kansas City, MO). Female BALB/C and C57/BL mice were purchased from Charles River Laboratories (Cambridge, MA). All solvents and other chemicals were analytical grade preparations.
Monoclonal 2C5 antibody was produced in ascites via the I.P injection of 1.5×106 cells of the hybridoma cell line (obtained in our laboratory) into pristine primed BALB/C 4-week old male mice. The production and the purification of the mAb 2C5 were carried out by Harlan Bioproducts (Indianapolis, IL).
Methods
Antibody modification
To prepare mAb 2C5 conjugates with PEG3400-PE, a 40 molar excess of p-nitrophenylcarbonyl(pNP)-PEG-PE prepared as in (Torchilin, et al. 2001), dispersed in 10 mg/ml solution of OG in 5 mM Na-citrate, 150 mM NaCl, pH 5.0, was added to an equal volume of 16.7 μM of the antibody in Tris-buffered saline (TBS), pH 8.5. The mixture was incubated for 24 hr at pH 8.5 at 4°C (Lukyanov, et al. 2004).
Preparation of antibody-modified liposomes
To modify doxorubicin-loaded PEG-liposomes, the mAb 2C5-PEG3400-PE conjugate obtained as described above was co-incubated with Doxil® preparation. For this purpose, the reaction mixture form the conjugation reaction (see the previous paragraph) was mixed in equal volumes with Doxil® and incubated for 6-to-8 h at 4°C. Following the incubation, the remaining impurities and non-incorporated mAb 2C5 were removed by dialysis using cellulose ester dialysis tubes with a cutoff size of 250,000 Da. The complete removal of the free antibody was confirmed by the presence of only one peak of liposomes on the gel-chromatogram of the final sample (with no peaks corresponding to free mAb 2C5-PEG-PE or mAb 2C5-PEG-PE micelles) (Elbayoumi and Torchilin 2007, Lukyanov, et al. 2004).
Preparation of control liposomes
Control PEGylated liposomes mimicking Doxil® composition but containing no doxorubicin were prepared using the same lipid components and in the same concentrations as in Doxil®. A lipid film was obtained from MPEG2000-DSPE (3.19 mg/ml), HSPC (9.58 mg/ml), and Chol (3.19 mg/ml). The lipid film was suspended in HEPES-buffered saline (HBS), pH 7.4, and sonicated with a probe-type sonicator at 10 Watts power for 15 min, followed by several passages through the mini-extruder with 100 nm pore size polycarbonate filter, until approximately 100 nm liposomes with narrow size distribution were obtained (Elbayoumi and Torchilin 2007, Lukyanov, et al. 2004).
In vitro release of doxorubicin from liposomes
The in vitro release of doxorubicin from different Doxil® formulations was conducted in DMEM cell culture medium containing 10% fetal bovine serum (FBS). Liposomes at a concentration of 0.5 mg/ml of doxorubicin, diluted in the media, were sealed into dialysis tubes with the cutoff size of 12,000–14,000 Da. The liposome-loaded dialysis tubes were incubated in 50 ml of the media for 48 h at 37°C, with continuous stirring. At various time points, aliquots were withdrawn, and replaced with the equal volume of the media. Doxorubicin concentrations were measured at 485 nm using a Hitachi U-1500 spectrophotometer, Hitachi Instruments (Schaumburg, IL) (Ishida, et al. 2001, Xiong, et al. 2005).
111In radiolabeling of liposomes
Doxil®-mimicking liposomes containing the amphiphilic chelate DTPA-PE (HSPC:Chol:MPEG200-DSPE:DTPA-PE in 3:2:0.3:0.3 molar ratio) were prepared along with the mAb 2C5-immunoanalogues. The loading of the liposome-incorporated DTPA-PE with 111In was performed via the transchelation mechanism from a weak citrate complex. DTPA-PE-containing liposomes were supplemented with 0.1 M citrate buffer and incubated for 1 h with 111In (as 111In chloride in citrate buffer) at RT, and then dialyzed overnight against HBS at 4°C to remove the free label (Elbayoumi and Torchilin 2006, Torchilin, et al. 2001).
Growth of tumors in mice
Murine breast carcinoma (4T1) and colon carcinoma (C26) tumors were implanted in 8 week-old BALB/C mice by the subcutaneous injection of 105 cancer cells into the fat pads in the lower abdominal region. Similarly, murine Lewis lung carcinoma (LLC) cells were S.C. injected in 8 week-old C57/BL mice (5×104 cells/mouse). The time for the appearance of the palpable tumor (varies from one cell line to another and usually is 7–12 days. Mice were regularly monitored, with free access to food and water (following animal care protocol no. R01210 approved by Northeastern University Institutional Animal Care and Use Committee, in accordance with the “Principles of laboratory animal care”, NIH publication no. 85–23, revised in 1985), and tumor volumes were calculated by measuring the length and width of the tumor at regular intervals (Chakilam 2004, Elbayoumi and Torchilin 2006).
Single-dose pharmacokinetics and biodistribution of 111In-labeled liposomes
In vivo biodistributon studies of 111In-radiolabeled Doxil®-mimicking liposomal formulation along with mAb 2C5-modified analogue were performed in 8 week-old female BALB/C mice, both healthy and 4T1 tumor-bearing (tumor volume range: 200–300 mm3), in two separate experiments. In each experiment, BALB/C mice were injected with 0.1 ml of 1 mg/ml 111In-radiolabeled Doxil®-mimicking liposomes via the tail vein. At 15, 30, 120, 360, 720, 1440 minutes post injection, blood was collected using a Pasteur pipette from the retro-orbital plexus of the eye, and the mice were euthanized by CO2 followed by collection of different organ tissues. The amount of radioactivity was quantified as CPM using a Beckman 5500B gamma-counter, and the amount of the accumulated radioactivity per gram of tissue was calculated followed by calculation of the temporal biodistribution and tissue accumulation parameters (Chakilam 2004, Elbayoumi and Torchilin 2006, Pastorino, et al. 2003).
Assessment of auricular erythema as Drug-induced side effect
Starting one week after the initiation of the drug administration (given in a sub-therapeutic dose regimen, as 2.2 mg/kg/dose every 5 days, for four consecutive doses) into tumor-bearing mice, location, count and severity of any developed auricular erythma (AE) lesions were monitored and documented for the rest of the treatment, in BALB/C mice bearing C26 tumors, and C57/BL bearing LLC tumors. Finally, AE percentage (AE%) was estimated for each group.
Statistics
Differences in the apparent AE occurrence percentage, and percentage of ears exhibiting AE manifestations during the treatment were compared using logistic regression, and comparing the odds ratio (OR) between the treatment groups [(AE events vs. no event) of mAb-2C5-modified Doxil® intervention/(AE events vs. no event) of original Doxil® intervention]. The significance level for the OR proportion of probability (ORp) was set at 0.1. (Batist, et al. 2006, Greenhalgh 1997, Potamianou, et al. 2005).
RESULTS & DISCUSSION
Doxorubicin leakage from antibody-modified liposomes
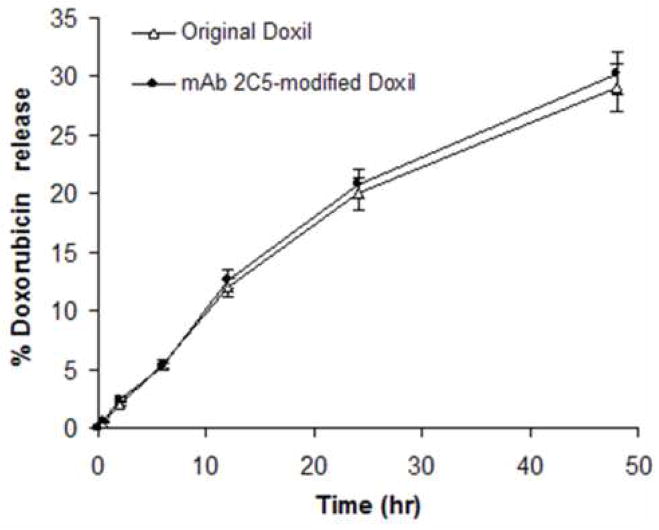
Similar to earlier data (Elbayoumi and Torchilin 2006, Elbayoumi and Torchilin 2007, Lukyanov, et al. 2004), the post-insertional technique for immuno-modification of Doxil® with the mAb 2C5-PEG-PE conjugate did not markedly alter the physicochemical properties of the original Doxil® formulation and resulted in the final preparation of 112±7 nm liposomes with 73±3 antibody molecules attached per single liposome. The complete release profiles (Fig. 1, panel A) of the original Doxil® and mAb 2C5-Doxil® also did not show any significant difference between these formulations. These in vitro release profiles have also demonstrated a slow first order release of doxorubicin from its carrier with just about 30% drug release after two days, which extrapolates to ca. 80 % of the encapsulated drug to be released after approximately 5 days. This corresponds well to the estimated in vitro doxorubicin release half-time of 85 hrs for similar HSPC liposomes (Allen, et al. 2005). This noteworthy prolonged release profile of the drug from the liposomal carrier, extending for up to 5 or even more days, evidently plays an important part of sustaining an effective local tissue concentration at the tumor site, combined with an extended doxorubicin tumor half-life reaching up to 70 hrs, as demonstrated earlier by other groups (Charrois and Allen 2003, Charrois and Allen 2004, Papahadjopoulos, et al. 1991). On the other hand, it may play a causative role in the necrotic manifestations at normal mucocutaneous tissues, especially on the multiple dosing treatment regimen of Doxil®. Especially when considering the reported protracted skin tissue half-life (approx. 80–110 hr) of the released drug (Charrois and Allen 2003, Charrois and Allen 2004).
Figure 1.
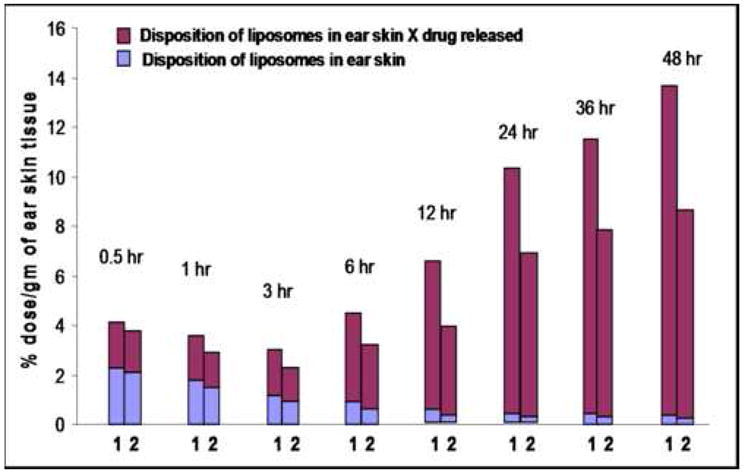
Figure 1A. In vitro doxorubicin release from various Doxil® preparations in cell culture media containing 10% BSA
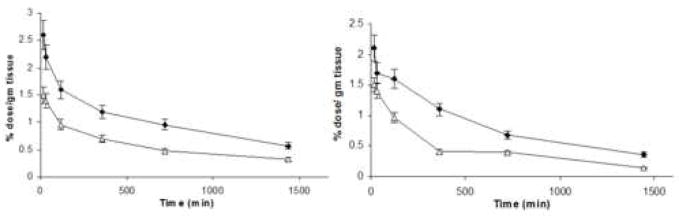
Figure 1B. Biodistribution data of 111In-labeled Doxil®-mimicking liposomal preparations in skin (auricular) tissue of the ears, in healthy BALB/C mice (left panel); and 4T1-tumor-bearing mice (right panel). (◆) Doxil®-mimicking liposomes; and (Δ) mAb 2C5-modified Doxil®-mimicking liposomes. (n = 4, results indicated ± SD)
Figure 1C. Hybrid representation of the different Doxil®-mimicking liposome accumulation and doxorubicin release profiles; 1-original Doxil®; 2- mAb 2C5- Doxil®, determined for 24 hrs and extrapolated up to 4 days.
In vivo biodistribution and skin accumulation of 111In-labeled liposomes
Using liposomes mimicking the lipid composition of Doxil® and radiolabeled with 111In, the tissue biodistribution of different liposomal formulations was determined. Blood clearance data of analogous formulations in tumor-bearing BALB/C mice reported earlier (Elbayoumi and Torchilin 2006), indicated that mAb 2C5-Doxil®-mimicking liposomes cleared faster from the body compared to plain liposomes (MRT ≈ 12 hrs for mAb 2C5-liposomes, compared to approx. 17 hrs for plain liposomes, as derived from AUC data using a non-compartmental analysis model). This somewhat accelerated clearance of the antibody-modified liposomes from the body can be attributed to the presence of antibody molecules on the liposomal surface, which made liposomes more prone to the uptake by RES cells, and this is true for the cases when both tumor-specific and non-specific immunoglobulins are present on the PEG-liposome surface (Chakilam 2004, Xiong, et al. 2005). However, mAb 2C5-immunoliposomes still demonstrated a sufficiently prolonged circulation, which corresponds well to the results reported with similar formulations in healthy mice (Elbayoumi and Torchilin 2006, Pastorino, et al. 2003). Furthermore, one can also expect that the higher dose of liposomes ends in the tumor in the case of mAb 2C5-Doxil® (Elbayoumi and Torchilin 2006).
As for the accumulation in the normal skin tissue of the ears (Fig. 1, Panel B), it is lower for mAb 2C5-immunoliposomes compared to original Doxil®, most probably due to their shorter blood residence time. Further more, one can suggest that in case of tumor-bearing mice, the tumor-targeted nature of immuno-liposomes could be partially involved, when the tumor attracts and accumulates circulating targeted liposomes in a shorter time period (revealed as a shorter plasma half-life), similar to what was described in earlier reports (Charrois and Allen 2003, Elbayoumi and Torchilin 2006).
Therefore, combining the data collected from both the liposomal drug release profiles with the skin accumulation profiles clearly reveals an important fact. Despite the minor differences in skin accumulation between different liposomal preparations, especially at longer post-injection times, a significant amount of doxorubicin estimated to be released in the same time period would result in a marked amplification of these differences of the liposomal skin accumulation. Figure 1, panel C, illustrates the differential doxorubicin accumulation in the ear area, produced by factoring the liposomal accumulation by doxorubicin release. The graph clearly demonstrates that even after 48 hr post-injection (extrapolated), according to the drug release profile, the amount of doxorubicin accumulated in the ear tissue areas in case of mAb 2C5-Doxil® is still significantly lower than for the original Doxil®.
Assessment of drug-associated auricular erythema as toxicity-indicative side-effect
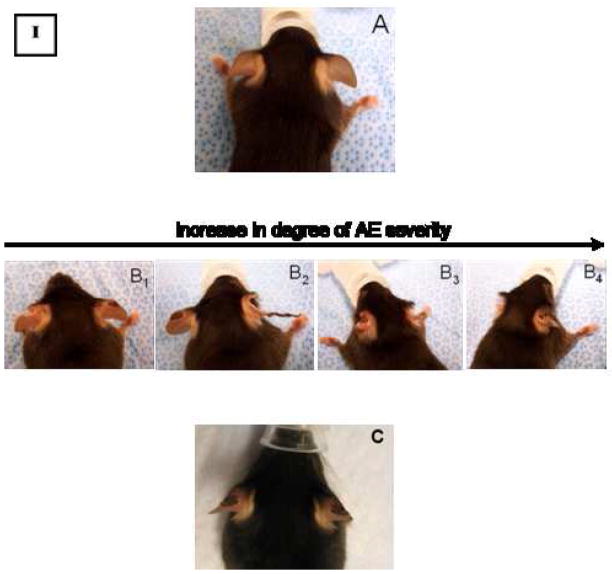
In accordance with the theory of pressure-dependent extravasation of PEG-liposomes and PPE (Charrois and Allen 2003, Charrois and Allen 2003, Lotem, et al. 2000), we observed that therapy-related symptoms appeared in mice treated with the different Doxil® formulations to a variable extent (but not in the PBS control groups). Observed skin damages are similar to the PPE lesions, but more confined to the auricles of the ears and tend to cause serious blistering and desquamation of the auricular tissue (Fig. 2, Panels A and B), hence termed “auricular erythema (AE)”. It was first observed in C57/BL mice starting after the administration of the second Doxil® dose (2.2 mg/kg/q 5 days), and in the BALB/C mice after the administration of the third Doxil® dose. Tables I and II show the data collected after recording the incidence of this AE side effect during the entire period of therapy.
Figure 2.
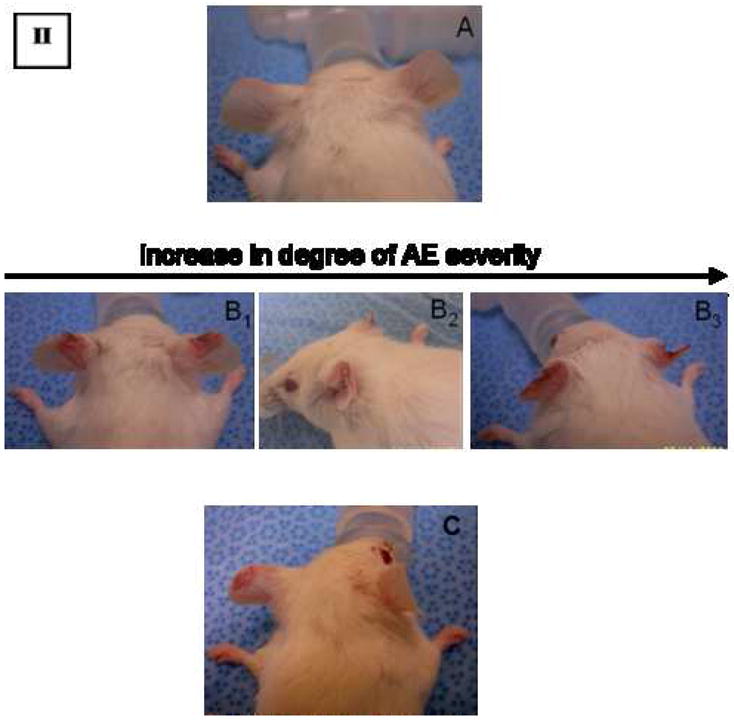
Auricular Erythema manifestation in C57/BL mice implanted with LLC tumors (I); and BALB/C mice implanted with C26 tumors (II), as a result of drug administration, showing untreated mouse (A); compared to Doxil®-treated mice (B1–4), exhibiting the progression of AE manifestation during chemotherapy; and to mAb 2C5-Doxil®-treated mice (C, showing AE manifestation developed only after the last administered dose).
Table I.
Occurrence of Auricular Erythema in LLC-bearing C57/BL mice, corresponding to each treatment.
| Treatment | % AE (No. C57/BL mice with AE/gp) | % of ears with AE (No. ears with AE/gp) |
|---|---|---|
| PBS | 0% (0/10) | 0% (0/20) |
| Doxil® | 80% (8/10)* | 80% (16/20)† |
| mAb-2C5-modified Doxil® | 30% (3/10)* | 20% (4/20)† |
(*† P≤0.1)
Table II.
Occurrence of Auricular Erythema in C26-bearing BALB/C mice, corresponding to each treatment.
| Treatment | % AE (No. BALB/C mice with AE/gp) | % of ears with AE(No. ears with AE/gp) |
|---|---|---|
| PBS | 0% (0/10) | 0% (0/20) |
| Doxil® | 56% (5/9) | 56% (10/18)† |
| mAb-2C5-modified Doxil® | 20% (2/10) | 10% (2/20)† |
P≤0.1
A plausible explanation for the appearance of AE lesions relates to the fact that the external auricles in mice ears have a relatively thin cutaneous tissue (acting as a cooling system to allow for the efficient dissipation of the body heat). Hence, the slow release of doxorubicin from the PEG-liposomes accumulated in the skin of the ear after several doses, causes severe necrosis in surrounding tissue. We have used this AE side effect as a means of estimating the toxicity related to the treatments with different Doxil® preparations.
It is clearly seen that the number of C57/BL mice exhibiting this AE side-effect is noticeably lower in the case of mAb 2C5-Doxil® (ORp ≤ 0.1) than in the case of the original Doxil® group. A similar pattern was also noted in the case of BALB/C mice. There is a clear significant difference in AE percentage between the original Doxil® treatment group and the mAb 2C5-Doxil® group. This low incidence of AE in case of animals treated with mAb 2C5-Doxil® compared to that of the original Doxil® formulation can be related to both the shorter blood-residence time of this immuno-formulation and to its tumor-specific accumulation. It seems that tumor-specific ligands, such as mAb 2C5, might play a considerable role in lowering side effects of Doxil®, by actively limiting its presence to the tumor tissue, hence avoiding non-targeted tissues and related side-effects. Nevertheless, further toxicological and pharmacokinetic studies concerning the AE and PPE phenomena, using larger number of animals/treatment group are still needed, in order to establish a rigorous correlation between the incidence profiles of AE in the animals and the dose intensities of different Doxil® formulations.
CONCLUSIONS
Here, we report a new cutaneous side effect of long-circulating liposomal carriers encapsulating the anti-cancer drug doxorubicin observed in mice. This syndrome appearing in the ear skin areas and termed “auricular erythema”, is associated with Doxil® treatment, hence becomes a candidate to be added to the family of the mucocutaneous adverse reactions caused by the long-circulating liposomal doxorubicin.
Our data suggest also that the immuno-modification of Doxil® with the anti-tumor antibody, mAb 2C5 in this particular case, can create a formulation that possesses the ability to specifically recognize and accumulate in various tumors, and at the same time lowers its presence in normal tissues. Therefore, the active targeting of the long-circulating liposomal preparations of doxorubicin, for example, with the mAb 2C5, presents an attractive opportunity to further control the in vivo behavior of the original Doxil® and improve its tolerability.
Acknowledgments
This work was supported by the NIH grant R01 HL55519 to V. P. Torchilin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TM, Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068:133–141. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- Allen TM, Hansen C, Martin F, Redemann C, Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- Allen TM, Mumbengegwi DR, Charrois GJ. Anti-CD19-targeted liposomal doxorubicin improves the therapeutic efficacy in murine B-cell lymphoma and ameliorates the toxicity of liposomes with varying drug release rates. Clin Cancer Res. 2005;11:3567–3573. doi: 10.1158/1078-0432.CCR-04-2517. [DOI] [PubMed] [Google Scholar]
- Batist G, Harris L, Azarnia N, Lee LW, Daza-Ramirez P. Improved anti-tumor response rate with decreased cardiotoxicity of non-pegylated liposomal doxorubicin compared with conventional doxorubicin in first-line treatment of metastatic breast cancer in patients who had received prior adjuvant doxorubicin: results of a retrospective analysis. Anticancer Drugs. 2006;17:587–595. doi: 10.1097/00001813-200606000-00014. [DOI] [PubMed] [Google Scholar]
- Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, Patek A, Martin FJ. The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol. 1998;9:711–716. doi: 10.1023/a:1008216430806. [DOI] [PubMed] [Google Scholar]
- Chakilam AR, Pabba S, Mongayt D, Iakoubov LZ, Torchilin VP. A single monoclonal antinuclear autoantibody with nucleosome-restricted specificity inhibits the growth of diverse human tumors in nude mice. Cancer Therapy. 2004;2:353–364. [Google Scholar]
- Charrois GJ, Allen TM. Multiple injections of pegylated liposomal Doxorubicin: pharmacokinetics and therapeutic activity. J Pharmacol Exp Ther. 2003;306:1058–1067. doi: 10.1124/jpet.103.053413. [DOI] [PubMed] [Google Scholar]
- Charrois GJ, Allen TM. Rate of biodistribution of STEALTH liposomes to tumor and skin: influence of liposome diameter and implications for toxicity and therapeutic activity. Biochim Biophys Acta. 2003;1609:102–108. doi: 10.1016/s0005-2736(02)00661-2. [DOI] [PubMed] [Google Scholar]
- Charrois GJ, Allen TM. Drug release rate influences the pharmacokinetics, biodistribution, therapeutic activity, and toxicity of pegylated liposomal doxorubicin formulations in murine breast cancer. Biochim Biophys Acta. 2004;1663:167–177. doi: 10.1016/j.bbamem.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Elbayoumi TA, Pabba S, Roby A, Torchilin VP. Antinucleosome antibody-modified liposomes and lipid-core micelles for tumor-targeted delivery of therapeutic and diagnostic agents. J Liposome Res. 2007;17:1–14. doi: 10.1080/08982100601186474. [DOI] [PubMed] [Google Scholar]
- Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. Eur J Nucl Med Mol Imaging. 2006;33:1196–1205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- Elbayoumi TA, Torchilin VP. Enhanced cytotoxicity of monoclonal anticancer antibody 2C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. Eur J Pharm Sci. 2007;32:159–168. doi: 10.1016/j.ejps.2007.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AN, Granai CO, Rose PG, Hainsworth J, Lopez A, Weissman C, Rosales R, Sharpington T. Phase II study of liposomal doxorubicin in platinum- and paclitaxel-refractory epithelial ovarian cancer. J Clin Oncol. 2000;18:3093–3100. doi: 10.1200/JCO.2000.18.17.3093. [DOI] [PubMed] [Google Scholar]
- Greenhalgh T. How to read a paper. Statistics for the non-statistician. II: “Significant” relations and their pitfalls. BMJ. 1997;315:422–425. doi: 10.1136/bmj.315.7105.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta B, Levchenko TS, Mongayt DA, Torchilin VP. Monoclonal antibody 2C5-mediated binding of liposomes to brain tumor cells in vitro and in subcutaneous tumor model in vivo. J Drug Target. 2005;13:337–343. doi: 10.1080/10611860500286239. [DOI] [PubMed] [Google Scholar]
- Hamilton A, Biganzoli L, Coleman R, Mauriac L, Hennebert P, Awada A, Nooij M, Beex L, Piccart M, Van Hoorebeeck I, Bruning P, de Valeriola D. EORTC 10968: a phase I clinical and pharmacokinetic study of polyethylene glycol liposomal doxorubicin (Caelyx, Doxil) at a 6-week interval in patients with metastatic breast cancer. European Organization for Research and Treatment of Cancer. Ann Oncol. 2002;13:910–918. doi: 10.1093/annonc/mdf157. [DOI] [PubMed] [Google Scholar]
- Hensley ML, Hoppe B, Leon L, Sabbatini P, Aghajanian C, Chi D, Spriggs DR. The costs and efficacy of liposomal doxorubicin in platinum-refractory ovarian cancer in heavily pretreated patients. Gynecol Oncol. 2001;82:464–469. doi: 10.1006/gyno.2001.6299. [DOI] [PubMed] [Google Scholar]
- Iakoubov LZ, Rokhlin O, Torchilin VP. Anti-nuclear autoantibodies of the aged reactive against the surface of tumor but not normal cells. Immunol Lett. 1995;47:147–149. doi: 10.1016/0165-2478(95)00066-e. [DOI] [PubMed] [Google Scholar]
- Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol Res. 1997;9:439–446. [PubMed] [Google Scholar]
- Ishida T, Kirchmeier MJ, Moase EH, Zalipsky S, Allen TM. Targeted delivery and triggered release of liposomal doxorubicin enhances cytotoxicity against human B lymphoma cells. Biochim Biophys Acta. 2001;1515:144–158. doi: 10.1016/s0005-2736(01)00409-6. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–818. doi: 10.1016/j.drudis.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- Lotem M, Hubert A, Lyass O, Goldenhersh MA, Ingber A, Peretz T, Gabizon A. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475–1480. doi: 10.1001/archderm.136.12.1475. [DOI] [PubMed] [Google Scholar]
- Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Noble CO, Kirpotin DB, Hayes ME, Mamot C, Hong K, Park JW, Benz CC, Marks JD, Drummond DC. Development of ligand-targeted liposomes for cancer therapy. Expert Opin Ther Targets. 2004;8:335–353. doi: 10.1517/14728222.8.4.335. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, Martin FJ. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Benz CC, Martin FJ. Future directions of liposome- and immunoliposome-based cancer therapeutics. Semin Oncol. 2004;31:196–205. doi: 10.1053/j.seminoncol.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Pastorino F, Brignole C, Marimpietri D, Sapra P, Moase EH, Allen TM, Ponzoni M. Doxorubicin-loaded Fab′ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003;63:86–92. [PubMed] [Google Scholar]
- Potamianou A, Androulakis N, Papakotoulas P, Toufexi H, Latoufis C, Kouroussis C, Christofilakis C, Xenidis N, Georgoulias V, Polyzos A. Sequential combination of paclitaxel-carboplatin and paclitaxel-liposomal doxorubicin as a first-line treatment in patients with ovarian cancer. A multicenter phase II trial. Oncology. 2005;69:348–353. doi: 10.1159/000089767. [DOI] [PubMed] [Google Scholar]
- Ranson MR, Carmichael J, O’Byrne K, Stewart S, Smith D, Howell A. Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol. 1997;15:3185–3191. doi: 10.1200/JCO.1997.15.10.3185. [DOI] [PubMed] [Google Scholar]
- Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m2. Ann Oncol. 2000;11:1029–1033. doi: 10.1023/a:1008365716693. [DOI] [PubMed] [Google Scholar]
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- Uziely B, Jeffers S, Isacson R, Kutsch K, Wei-Tsao D, Yehoshua Z, Libson E, Muggia FM, Gabizon A. Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies. J Clin Oncol. 1995;13:1777–1785. doi: 10.1200/JCO.1995.13.7.1777. [DOI] [PubMed] [Google Scholar]
- Xiong XB, Huang Y, Lu WL, Zhang H, Zhang X, Zhang Q. Enhanced intracellular uptake of sterically stabilized liposomal Doxorubicin in vitro resulting in improved antitumor activity in vivo. Pharm Res. 2005;22:933–939. doi: 10.1007/s11095-005-4588-x. [DOI] [PubMed] [Google Scholar]