Abstract
Coarse and fine particulate matter (PM2.5–10 and PM2.5, respectively) are regulated ambient air pollutants thought to have major adverse health effects in exposed humans. The role of endotoxin and other bioaerosol components in the toxicity of PM from ambient air is controversial. This study evaluated the inflammatory lung response in mice instilled intratracheally with PM2.5–10 and PM2.5 emitted from a working dairy barn, a source presumed to have elevated concentrations of endotoxin. PM2.5–10 was more pro-inflammatory on an equal weight basis than was PM2.5; both fractions elicited a predominantly neutrophilic response. The inflammatory response was reversible, with a peak response to PM2.5–10 observed at 24 hours after instillation, and a return to control values by 72 hours after instillation. The major active pro-inflammatory component in whole PM2.5–10, but not in whole PM2.5, is heat labile, consistent with it being endotoxin. A heat treatment protocol for the gradual inactivation of biological materials in the PM fractions over a measurable time course was developed and optimized in this study using pure lipopolysaccharide (LPS) as a model system. The time course of heat inactivation of pure LPS and of endotoxin activity in PM2.5–10 as measured by Limulus bioassay is identical. The active material in both PM2.5–10 and PM2.5 remained in the insoluble fraction when the whole PM samples were extracted with physiological saline solution. Histological analysis of lung sections from mice instilled with PM2.5–10 or PM2.5 showed evidence of inflammation consistent with the cellular responses observed in lung lavage fluid. The major pro-inflammatory components present in endotoxin-rich PM were found in the insoluble fraction of PM2.5–10; however, in contrast with PM2.5–10 isolated from ambient air in the Central Valley of California, the active components in the insoluble fraction were heat labile.
Keywords: Coarse PM (PM2.5–10), Fine PM (PM2.5), Bronchoalveolar Lavage, Endotoxin, Air pollution
Introduction
Particulate Matter (PM) is a mixture of respirable particles regulated as a National Ambient Air Quality Standard (NAAQS) pollutant by the U. S. Environmental Protection Agency (EPA) under the Clean Air Act. PM standards are currently based on total mass and size, which can range from a few nanometers to tens of micrometers in mass median aerodynamic diameter. Coarse particles (PM2.5–10; 2.5μm–10μm diameter) are the largest particles that can penetrate into the lung and potentially elicit health effects. Experiments evaluating lung response to ultrafine, fine, and coarse PM have suggested that coarse PM (2.5 – 10 μm) shows the highest potency at equivalent masses (Monn et al., 1999; Soukup et al., 2001; Hetland et al., 2005). Epidemiological studies have shown an association between increases in morbidity and respiratory diseases with increased concentrations of either coarse or fine particulate matter in ambient air, especially among susceptible populations (Dockery et al., 1993; Schwartz, 1994; Pope et al., 1995; Schwartz et al., 2000; Brook and Rajagopalan, 2007). Current air quality standards regulate both coarse and fine PM concentrations.
It remains unclear which constituents in the particulate mixture in ambient air are responsible for respiratory effects (Dreher, 2000; Schwarze et al., 2006) and what mechanisms are involved. PM in ambient air is a mixture of primary emissions and secondary air pollutants, but it has yet to be determined which specific component, or combinations of PM components, is responsible for the observed inflammatory and toxic effects in the respiratory system. Particulate matter collected in spatially and temporarily variable environments shows different inflammatory and toxic responses in the lung (Cassee et al., 2003; Peng et al., 2005); concentrations and properties of PM also vary with time, season, and climate. Steerenberg and colleagues (2004) showed that both the coarse and fine PM fractions from various geographical sites differ in adjuvant activity after administration; winter PM was found to be more active than PM collected in spring and summer. Current research suggests that possible toxic and pro-inflammatory effects of coarse PM may be contributed predominantly by its constituents, which include biological agents (Becker et al., 1996; Dong et al., 1996), metals (Pope et al., 1989; Costa et al., 1997; Ghio et al., 1999; Donaldson et al., 2005), organic compounds (Li et al., 2002), or ultrafine particles adhered to coarse particles (Oberdorster et al., 1994), each of which make up a minor, but potentially toxicologically active, proportion of the total PM.
A role for biological constituents of PM, specifically β-glucans, mold spores, and endotoxin (Schwarze et al, 2006), in PM toxicity has been suggested by numerous studies. Endotoxin, a common constituent of particulate matter in the relevant size range for the PM2.5–10 fraction, is a component of the outer membrane of gram-negative bacteria. The purified soluble fraction of endotoxin is known as lipopolysaccharide (LPS). Endotoxin can elicit inflammatory responses in the lung and is found predominantly in the coarse PM2.5–10 fraction (Monn et al., 1999; Soukup et al., 2001; Huang et al., 2002; Schins et al., 2004; Steerenberg et al., 2004). Endotoxin, like other constituents of particulate matter, can be spatially (Spaan et al., 2008), temporarily, and seasonally variable (Steerenberg et al., 2004). For example, endotoxin is present at higher concentrations in a dairy barn (584±2.7 EU/mg dust) than in a coal and biomass power plant (19.4±4.0 EU/mg dust) (Spaan et al., 2008). Since endotoxin and other bioaerosol components originate from bacteria and other microorganisms, moist conditions that favor microbial growth tend to be associated with higher endotoxin concentrations in the PM2.5–10 fraction. The role, if any, of high humidity during PM sample collection as a factor contributing to microbial growth (and, therefore, endotoxin contamination of ambient PM samples) on sample filters has not, to our knowledge, been systematically explored.
In a previous study (Wegesser and Last, 2008), we demonstrated that the insoluble fraction of coarse particulate matter collected during the dry summer months from the Central Valley of California was responsible for pro-inflammatory responses in the mouse. We also suggested based on the heat stability of the active components of this PM2.5–10 fraction that the acute lung inflammation was not caused by endotoxin in the PM (Wegesser and Last, 2008). A significant limitation of the previous study was the lack of a simultaneous test of actual particles from an environmental source known to contain endotoxin. Therefore, in the current experiments we evaluated responses of the mouse lung to PM collected using the same procedures as the earlier study from a source that contained endotoxin. To do this we collected PM2.5–10 and PM2.5 from a working dairy barn in Northern California during the summer months, a source of PM that we presumed would be rich in endotoxin, and measured the pro-inflammatory responses in mice intratracheally instilled with this material. We hypothesized that in this case the lung inflammation would be caused primarily by endotoxin in the PM, and asked whether the relatively simple bioassay we had described in our previous study (Wegesser and Last, 2008) could distinguish between endotoxin-induced lung inflammation and lung inflammation caused by components of the PM other than bioaerosol components. We anticipated that the major inflammatory components in an endotoxin-rich PM would also be found in the insoluble fraction of PM2.5–10, but in contrast to what we previously found with coarse PM from ambient air, the active components would be heat labile. Thus, we hypothesized that a simple comparison of whole PM and heat-treated PM in a mouse bioassay could be used to quantify the relative contributions of endotoxin and of other non-bioaerosol components to the lung inflammatory response caused by PM preparations arising from different sources.
Materials and Methods
Animals
Specific Pathogen-Free BALB/c mice (male, 8–10 weeks old, 25–30g) were purchased from Charles River Breeding Laboratories (Wilmington, MA). Animals were housed, 4 animals per cage, in filtered Bio-Clean facilities in the Animal Resources Center (UC Davis, CA). Mice received water and standard feed (Purina Rat Chow) ad lib and were allowed to acclimate for a week prior to any experimental procedures. The animals were kept on a 12-h light/dark cycle at room temperature (68–70°F) and 30–70% relative humidity. All procedures were performed under an Institutional Animal Care and Use Committee (IACUC) approved protocol.
Source of PM
Particulate matter used in this study was collected during the summer months with a high-volume air sampler (Model GS2310, Andersen Instruments Inc.; Smyrna, Georgia) equipped with a 4-stage cascade impactor (Series 230, Andersen Instruments Inc.; Smyrna, Georgia) from a dairy barn in Northern California. Slotted aluminum substrates (Tisch Environmental, Cleves, OH) were used for particulate matter collection. The nominal flow rate used for collection was 20 ft3/minute (CFM), with particle size cut-offs of 10.2, 4.2, 2.1, and 1.3μm (unit mass density at 25°C and 760mm Hg) at the various stages of the impactor. When discussing the results of this manuscript we will refer to “coarse” PM (or PM2.5–10) as those particles that fall within the range of 10.2 – 2.1 μm and “fine” PM (or PM2.5) as those particles that fall within the range of 2.1 – 1.3 μm mass median aerodynamic diameter. After collection, substrates from each stage were weighed and the particles were removed and stored in separate vials. We collected the following particulate mass yields of the various size fractions after an approximate sampling period of 48hrs. Stage 1 contained particles of 10.2 um and larger (33.6 mg). Stages 2 (4.2–10.2um; 12.3 mg) and 3 (2.1–4.2um; 11.4 mg) were combined to make up the coarse fraction. Stage 4 (1.3–2.1um; 2.6 mg) collected the fine fraction used in this study.
Particles were suspended in phosphate-buffered saline (PBS), pH 7.6 (Mediatech, Inc., Herndon, VA; the resulting PM suspension had a pH of >7.), aliquoted into separate vials, and stored at 80°C until 30 minutes prior the experiments. Particles were viewed under light and polarized light microscopy with an Olympus BH2 microscope connected to an OLY-750 Color Camera using a polarized filter (Vivitar Polarizing 49mm, Japan) attached to a neutral density filter.
Experimental Design
Mice were instilled, immediately after vigorous vortex mixing of the PM, with 50ul of a PM suspension at a concentration of 1mg/ml of PM (unless otherwise noted). Coarse PM exposures included: 1) whole particulate fraction, 2) heated whole fraction, 3) insoluble fraction, and 4) heated insoluble particulate fraction. Fine PM exposures included: 1) whole particulate fraction, 2) heated whole fraction, 3) insoluble fraction, and 4) heated insoluble particulate fraction. Each treatment group included 5–6 animals per group, and all experiments were replicated at least twice. The data presented are average values from two or more experiments. Bronchoalveolar lavage (BAL) and tissue were collected 24 hours after PM instillation (unless otherwise noted). BAL and tissues were also collected in a time course experiment and processed at 3, 24, 48, or 72 hours after IT instillation. Matched control animals were instilled with 50ul of PBS in each experiment.
Intratracheal Instillations
Prior to instillation, mice were anesthetized with Attane (isoflurane) via inhalation in an enclosed box chamber. Mice were positioned in a supine position and the jaw and tongue were drawn away from the esophageal region using forceps while inserting a blunt 22-gauge needle attached to a glass syringe (Model #1705, Hamilton, Reno, NV) into the trachea and instilling 50ul of the PM suspension. Tracheal insertion was confirmed by palpating the tracheal rings with the tip of the syringe. After instillation, the mouse was allowed to visibly recover before placement back into the cage for a predetermined period after exposure.
Bronchoalveolar Lavage
Mice were euthanized with an overdose of pentobarbital/dilantin via intraperitoneal injection (usually) 24 hours after PM instillation and exsanguinated by cardiac puncture. The tracheas were cannulated and the lungs were lavaged two times each with 1ml instillations (2 ml total) of PBS, pH 7.6 (Mediatech, Inc, Herndon, VA). More than 80% of the initially instilled PBS volume was recovered in the lavage fluid. The total lavage fluid from each mouse lung was combined and centrifuged at 1800–2000 rpm for 10 minutes using a bench top unit (Centrifuge #5415C, Eppendorf, New York, NY). The supernatant was removed and the resulting pellet was resuspended in ACK Lysis Buffer (0.15 M NH4Cl, 1.0 M KHCO3, 0.1 mM Na2EDTA, H2O) and centrifuged for another 10 minutes to remove lysed red blood cells. The supernatant was discarded and the pellet was suspended in 1ml of PBS. The trypan blue exclusion method (Mishell and Shiigi, 1980) was used to count the total number of viable cells in each BAL sample using a hemocytometer. Additional cell cytospin preparations were performed with 100ul of BAL fluid using a cytocentrifuge, (StatSpin Cytofuge 2, Iris, and Westwood, MA). Prepared cytospin slides were stained with DiffQuick® (International Reagent Corp, Kobe, Japan). Cell differentials were counted as described previously (Kenyon et al. 2006). Briefly, cells were counted and classified by counting 10 fields (400x) from each stained cytospin slide using a light microscope. Differential cell counts were expressed as percentages of total cell counts.
Lung Isolation, Fixation, and Immunohistochemistry
Lavaged lungs were inflated and fixed at 30 cm pressure with 1% paraformaldehyde in PBS for 1 hour for histopathological assessment. Fixed lungs were separated into right and left lobes, and processed for analysis via light microscopy in paraffin-embedded histological sections (5μm). Lung sections (5μm thick) were stained with Harris’ hematoxylin and eosin (H&E) stain and then viewed with an Olympus BH2 microscope connected to an OLY-750 Color Camera.
Soluble and Insoluble Particulate Matter Fractions
PM suspensions in PBS were separated into soluble and insoluble fractions (Ghio et al., 1999, Wegesser and Last, 2008). PM preparations were thoroughly mixed and centrifuged at 12,850 × g for 30 minutes. The supernatant (soluble fraction) was transferred to a vial, while the pellet (insoluble fraction) was either resuspended in a volume of PBS equal to the volume of supernatant removed or further treated to inactivate biological material by heating the contents (dry heat) in an oven at 120–130°C for 24 hours (Alexis et al., 2006, Wegesser and Last, 2008).
Heat Inactivation of LPS and Endotoxin
To determine the heat inactivation curve for authentic LPS, individual vials of lipopolysaccharide (Escherichia coli; 0111:B4, Lonza, MD), resuspended in endotoxin-free H2O, were heated at 130°C for different time intervals (0–4 hours). Vials were removed at each time point and stored on ice until all of the samples were collected, then simultaneously tested for endotoxin using a Limulus Amoebocyte Lysate (LAL) assay (QCL-1000 Chromogenic, Lonza, MD). The same protocol was conducted for PM2.5–10. After heating, the PM2.5–10 preparations were diluted in endotoxin-free water (Lonza, MD) to a concentration within the range of the assay, and to ensure a constant amount of salt in the vials during the assay.
Statistical Analysis of Data
Prism 4.0 or 5.0 (GraphPad Software, San Diego, CA) was used for the analysis of data. All values are expressed as mean values ± SE (standard error) unless otherwise noted. Parametric analysis of data was conducted using ANOVA with Tukey’s or Dunnet’s post-test for multiple comparisons, or with Student’s t-test. Welch’s correction was applied if variances were unequal.
Linear regression was performed using a 95% confidence interval. Data expressed in the figures were annotated as significant if the p value was <0.05.
Results
Particle Characteristics
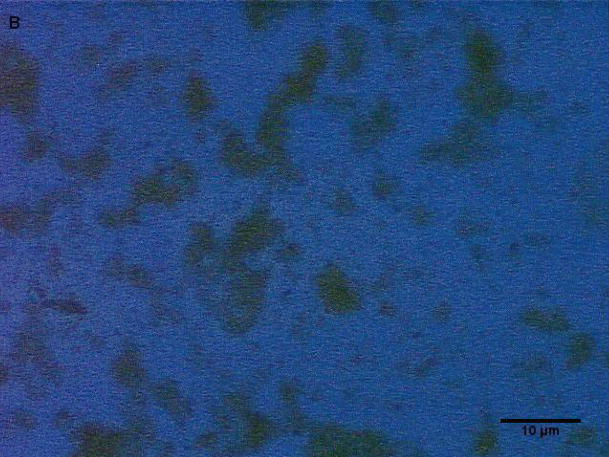
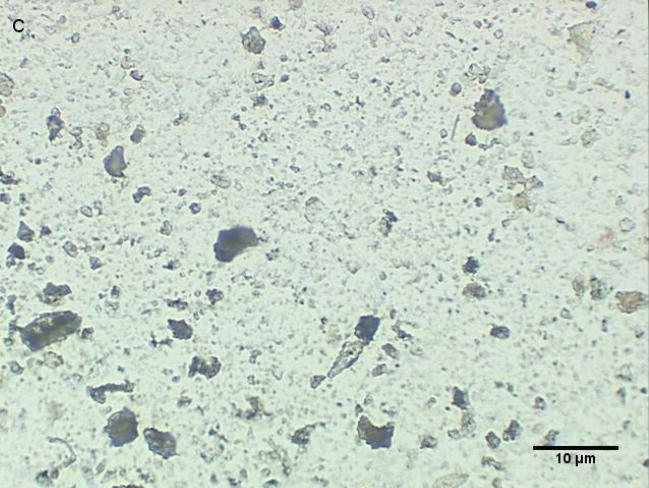
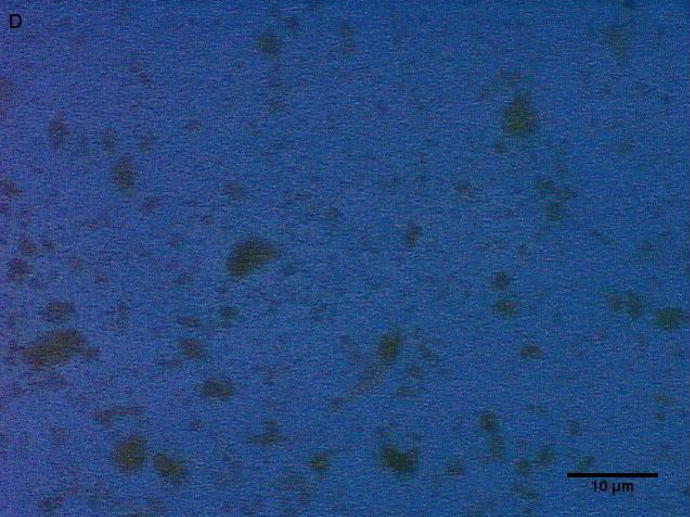
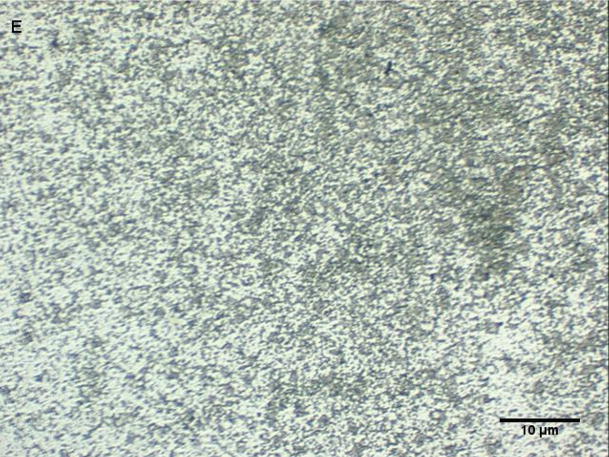
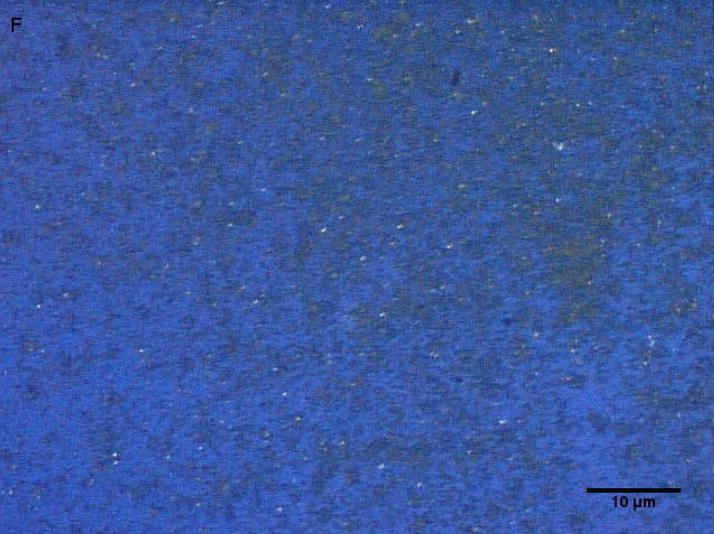
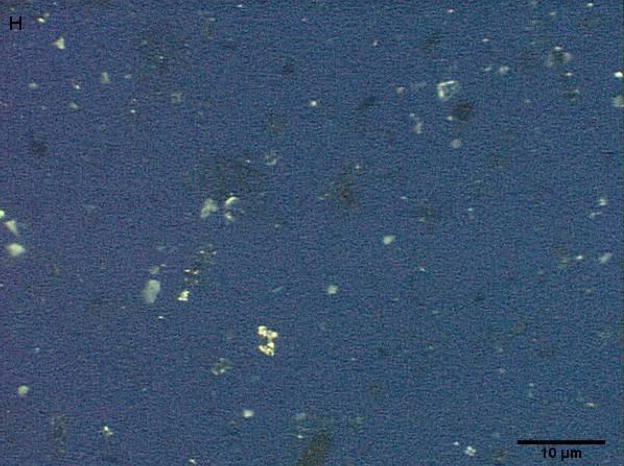
Coarse and fine particles from the dairy barn were compared with PM2.5–10 from a non-dairy source (ambient air collected from two different communities in the Central Valley of California) by microscopy under normal or polarized light (Fig. 1a–h). Under normal light, both the coarse and fine particles collected from the dairy barn displayed a very heterogeneous pattern with an array of different shapes and sizes. The same fields viewed under polarized light display a lack of birefringent particles, shown by an overall dark field (Fig. 1b, d). By comparison, particles (coarse and fine) collected from ambient air (a non-dairy source) display a more homogenous pattern, with comparable sizes and shapes. Unlike the dairy barn particles, these ambient coarse particles display birefringent, anisotropic or doubly refractive particles (shown as bright white speckles in the dark-field view) when particles are viewed under polarized light (Fig. 1f, h).
Figure 1a-h.
Representative normal light and dark-field (crossed polar filters) images of isotropic and anisotropic particles from: a, b) Dairy Barn PM2.5–10, c, d) Dairy Barn PM2.5, e, f) Coalinga, CA ambient air PM2.5–10, g, h) Parlier, CA ambient air PM2.5–10. Bar = 10μm.
Lung Lavage Response to PM2.5–10 or PM2.5 Instillation
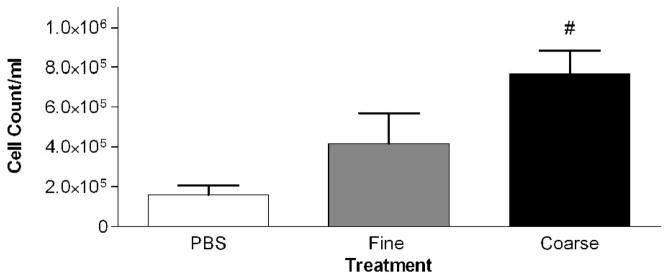
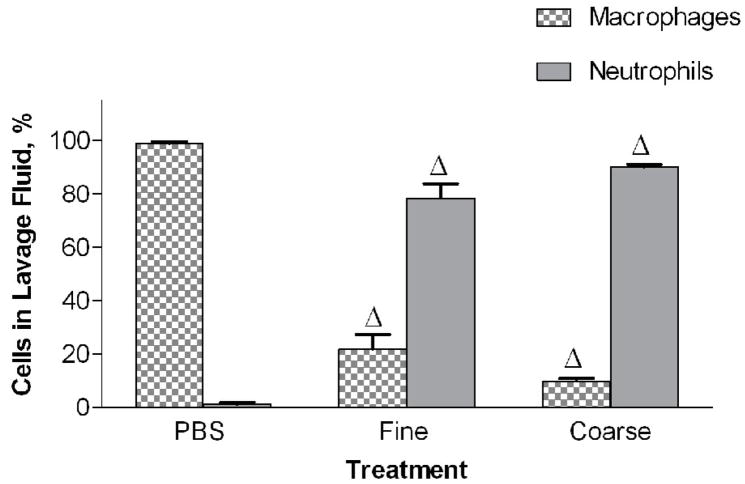
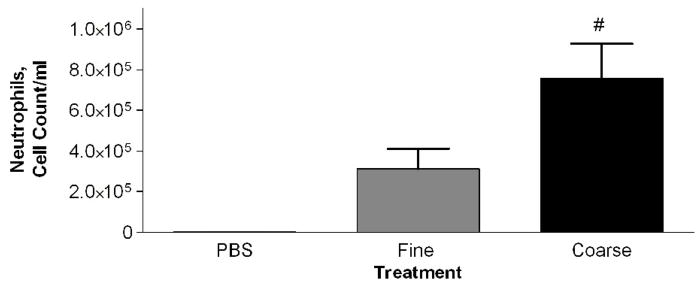
Mice were intratracheally instilled with either PM2.5 or PM2.5–10 (50 ug/per mouse). The PM2.5–10 treatment group showed a significant increase in total cells in the lavage fluid 24 hours after administration when compared to control (PBS instilled) mice (Fig. 2a). On the other hand, the PM2.5 treated group did not show a significant increase in total cells recovered from bronchoalveolar lavage (BAL) fluid, despite the apparent trend in Fig. 2a towards increased total cells in the lavage fluid. The cellular differential was analyzed to determine the total numbers of macrophages and neutrophils recovered from the BAL fluid. The relative percentages of macrophages and neutrophils recovered were calculated, and are presented in Figure 2b and in subsequent figures (Wegesser and Last, 2008). We found a significant decrease in the percentage of macrophages (compared to the PBS controls), and a concomitant significant increase in the percentage of neutrophils, for both treatment groups, PM2.5 and PM2.5–10 (Fig. 2b). The total number of recovered macrophages did not show any significant changes between the PBS- (1.5×105±4.8×104), PM2.5− (1.0×105±5.5×104), and PM2.5–10− (7.6×104±9.6×103) treated mice. The significant increase that we observed in the total cell count for BAL from mice instilled with PM2.5–10 is made up of predominantly neutrophils; there was a significant increase in the number of neutrophils recovered from the BAL, consistent with the increased percentage of neutrophils we observed in the BAL fluid (Fig. 2c).
Figure 2a-c.
a) Total cell count of recovered cells in lung lavage fluid 24 hours after instillation of PM2.5 or PM2.5–10 (# P<0.001). b) Percentage of macrophages (checkered bars) and neutrophils (dark bars) in lung lavage fluid 24 hours after instillation of 50ug of PM2.5 or PM2.5–10 (ΔP<0.0001). c) Number of neutrophils in lung lavage fluid 24 hours after instillation of PM2.5 or PM2.5–10 (# P<0.001). Data are shown as mean values ± SEM.
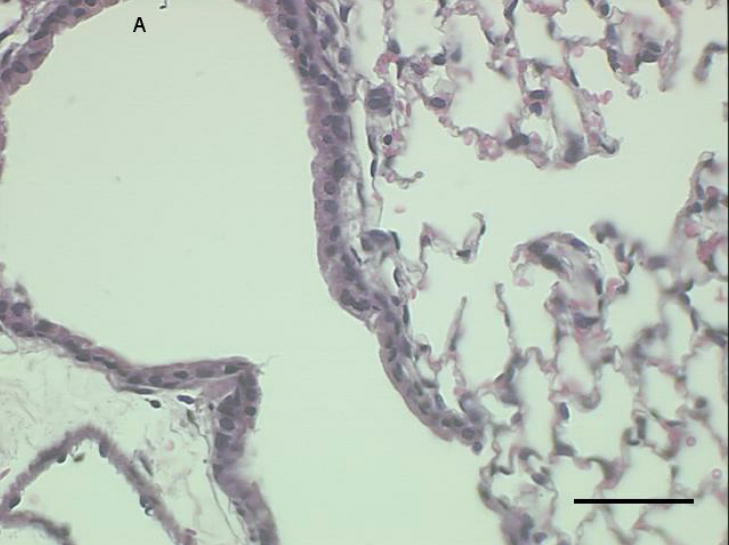
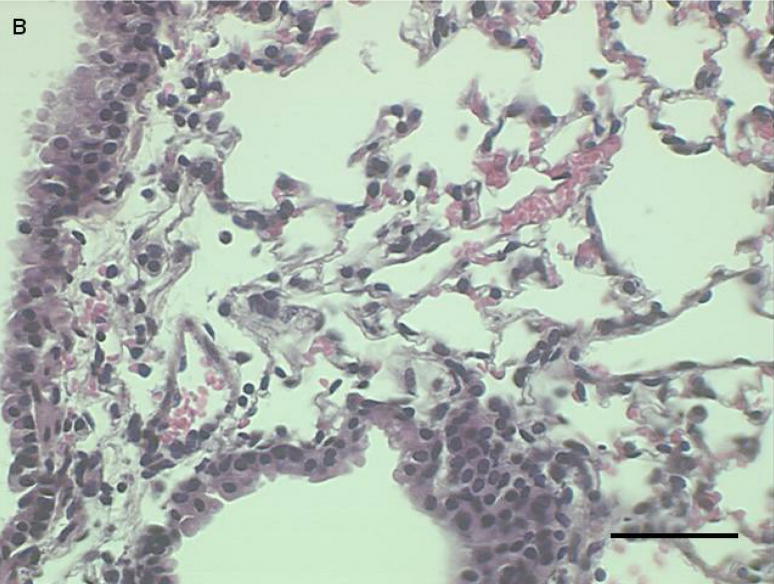
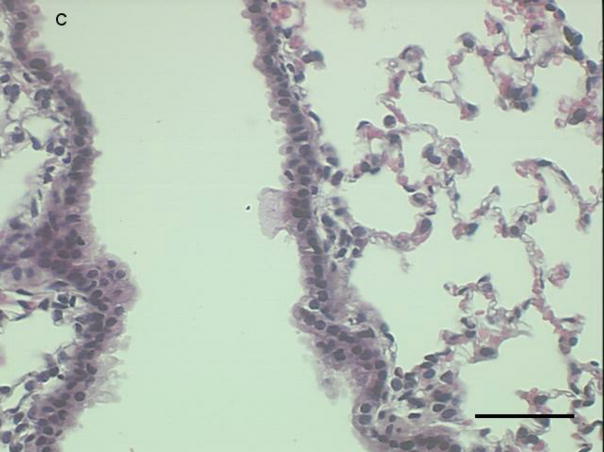
Histological sections from mice instilled with coarse or fine PM show patterns of cellular infiltration in the peribronchial regions consistent with the inflammatory response measured as total cells recovered from lung lavage fluid (Fig. 3a–c). There were no peribronchiolar inflammatory cells apparent in the PBS controls, We observed diffuse inflammatory cell infiltration in the lungs of the mice instilled with coarse PM and focal areas of inflammation in the lungs of mice instilled with fine PM,
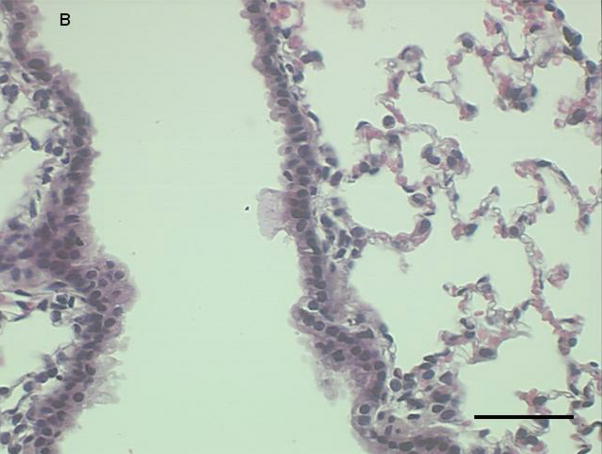
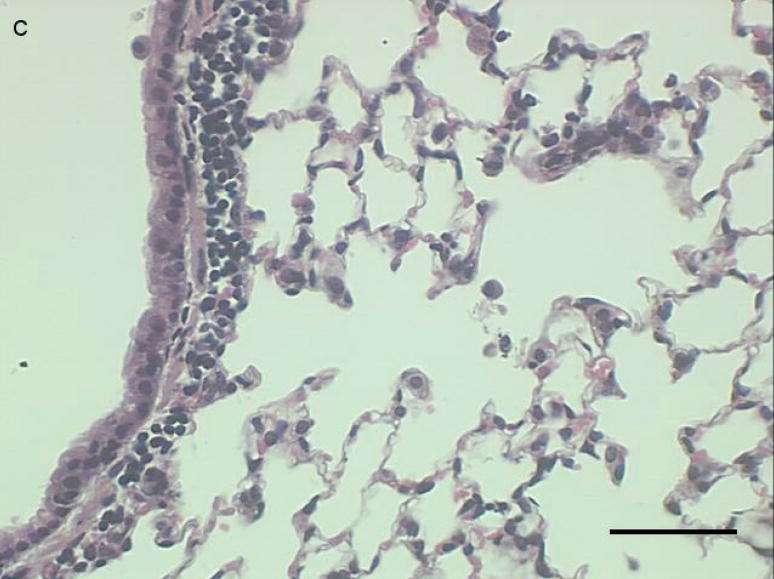
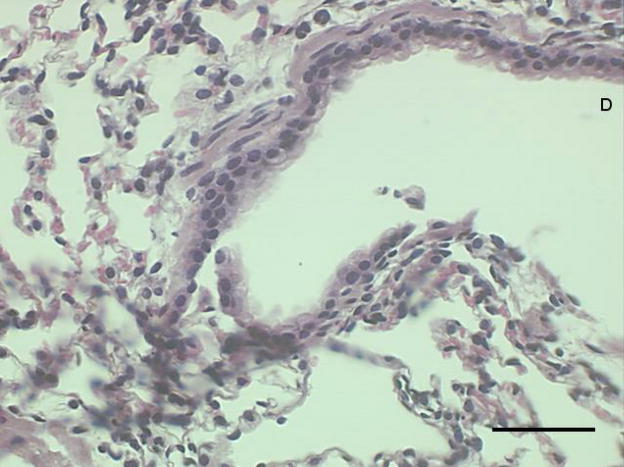
Figure 3a-c.
Representative proximal lung sections with conducting airways from mice instilled 24 hours previously with [a] PBS, [b] PM2.5, or [c]. PM2.5–10. Bar = 50um.
There were no significant differences in the amounts of protein in the BAL supernatant after removal of cells (PBS: 390±21μg/ml; PM2.5: 453±23μg/ml; PM2.5–10: 436±27μg/ml).
Time course of the response to PM2.5–10 exposure
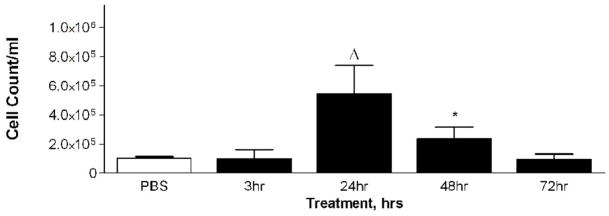
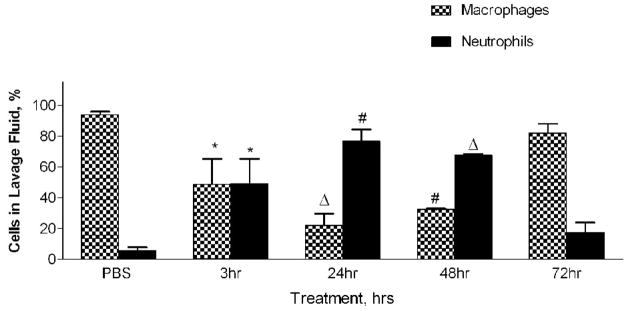
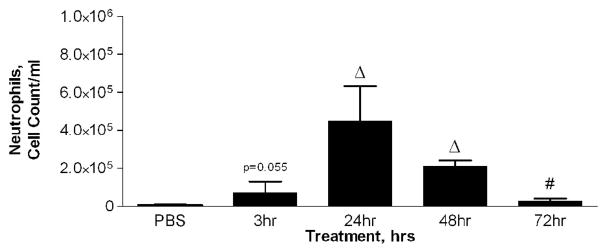
Mice were divided into four treatment groups, each receiving 50 ug of PM2.5–10, and their inflammatory response was analyzed 3, 24, 48, and 72 hours after instillation. Matched control groups that received only PBS were included at each time point after instillation. We found increases in total viable cells recovered from lung lavage fluid 24 and 48 hours after instillation (Fig. 4a), with decreased total cell numbers at 48 and at 72 hours (compared to the corresponding control values). The relative percentage of neutrophils in the lavage fluid was significantly increased at 24 hours (76%) after instillation, with gradual decreases to 67% at 48 hours and 17% at 72 hours, as compared to the corresponding values in the PBS controls (Fig. 4b). It should be noted that the absolute number of macrophages in the lavage fluid was significantly increased at 24 hours (9.3×104±1.6×104) after PM2.5–10 instillation, and remained significantly elevated at 48 (1.0×105±1.8×104) and 72 hours (1.2×105±4.0×103). Total neutrophils recovered from the lung lavage fluid are significantly increased at 3, 24, 48, and 72 hours after PM instillation compared to the PBS controls, showing a peak value at 24 hours with apparent time-related decreases at 48 and 72 hours after instillation (Fig 4c).
Figure 4a-c.
a) Total cell count of recovered cells in lung lavage fluid 3, 24, 48, or 72 hours after instillation of PM2.5–10 (* P<0.05; ΔP<0.0001). b) Percentage of macrophages (checkered bars) and neutrophils (dark bars) in lung lavage fluid 3, 24, 48, or 72 hours after instillation of 50 ug of PM2.5–10 (* P<0.05; # P<0.001; ΔP<0.0001). c) Number of neutrophils in lung lavage fluid 3, 24, 48, or 72 hours after instillation of PM2.5–10 (# P<0.001; ΔP<0.0001). PBS control groups for each time point have been pooled.
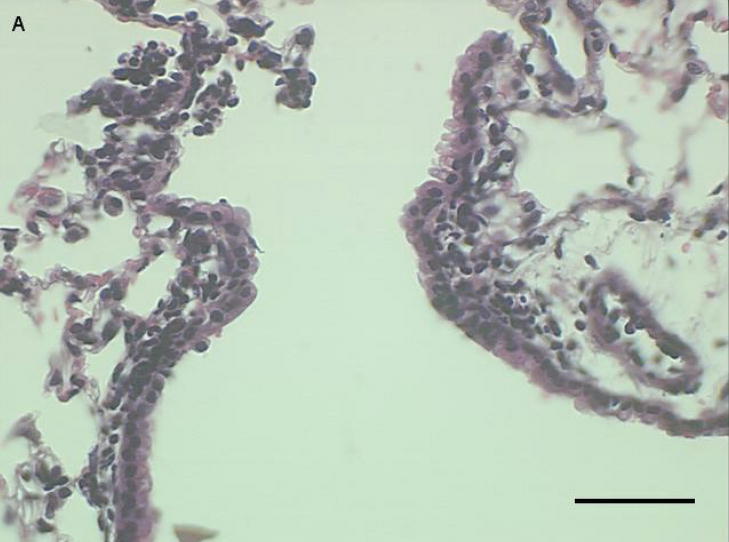
Histological examination of lung tissue from these mice was consistent with our observations in the lung lavage fluid. We observed an increase in inflammatory cells lining the peribronchial regions at 3 (Fig. 5a), 24 (Fig 5b), and 48 hours (Fig 5c), with a marked reduction in inflammatory cells at 72 hours (Fig 5d).
Figure 5a-d.
Representative proximal lung sections with conducting airways from mice instilled at [a] 3, [b] 24, [c] 48, or [d] 72 hours with PM2.5–10. Bar = 50um.
Saline extraction of PM2.5–10 and PM2.5
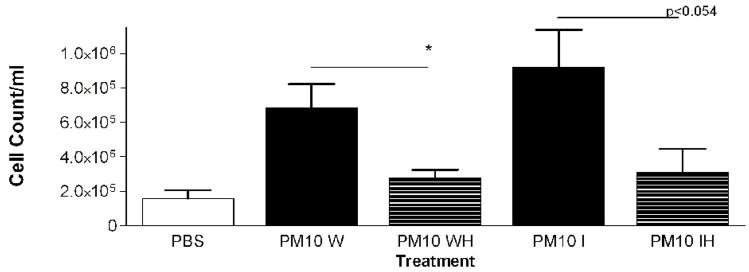
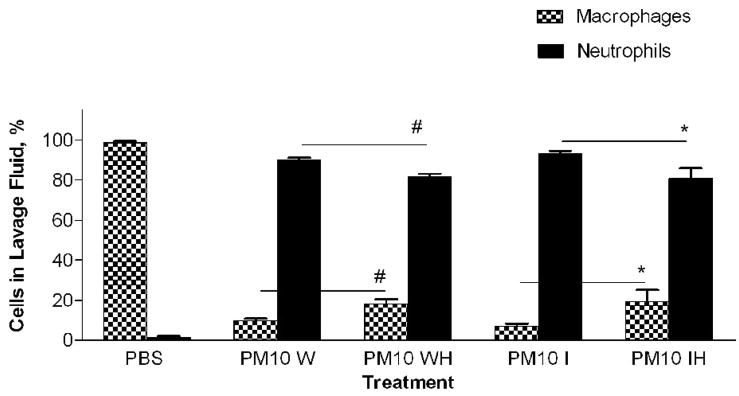
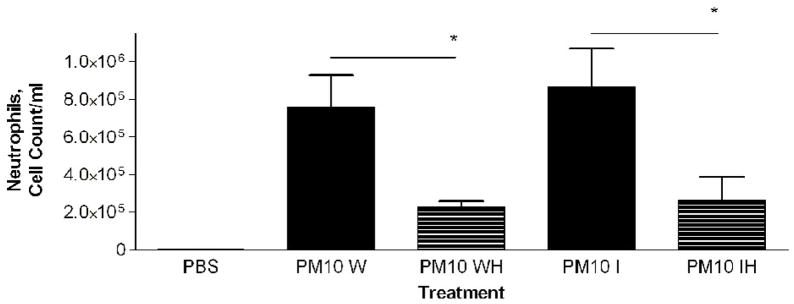
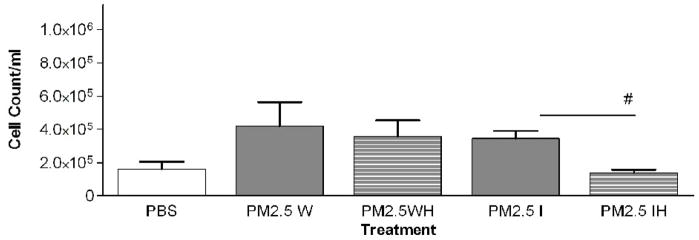
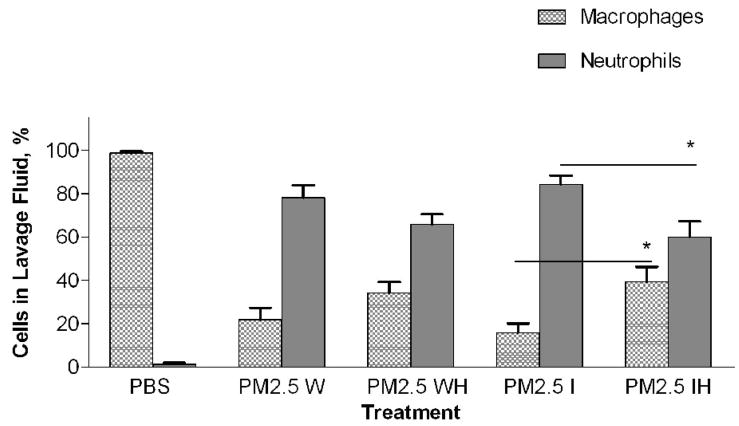
In a previous study with PM2.5–10 isolated from ambient air we found a significant difference in the response of mice treated with insoluble particulate matter versus those treated with the soluble material obtained after the whole PM sample was extracted with PBS. The pro-inflammatory material in the PM2.5–10 appeared to remain with the insoluble fraction after centrifugation (Wegesser and Last, 2008). In the current study we fractionated particulate matter suspensions (PM2.5–10 and PM2.5) obtained from the dairy barn into soluble (aqueous) and insoluble (particulate) fractions prior to instillation. Each particulate fraction was instilled at a nominal dose of 50 μg per mouse. The results of this experiment are presented in Figures 6 (coarse PM) and 7 (fine PM). Consistent with our previous study, we found that the inflammatory activity remained in the insoluble fraction from both PM2.5–10 and PM2.5 after extraction; there was no significant difference in activity between the whole and insoluble fractions. When we compare PM2.5–10 (6.8×105±1.4×105) and PM2.5 (4.1×105±1.5×105) inflammatory responses, we see that the same weight of PM2.5 gives a little more than half of the response observed with the same weight of PM.5–10. Both the PM2.5–10 and PM2.5 whole and insoluble fractions resulted in 78–93% neutrophils in the lavage fluid, thus making neutrophils the predominant cell type in the lung lavage. Even though the soluble fraction was not analyzed in this way, our results suggest that the soluble fraction does not have significant pro-inflammatory activity. It appears that the pro-inflammatory activity remains in the insoluble fraction of both PM2.5–10 and PM2.5.
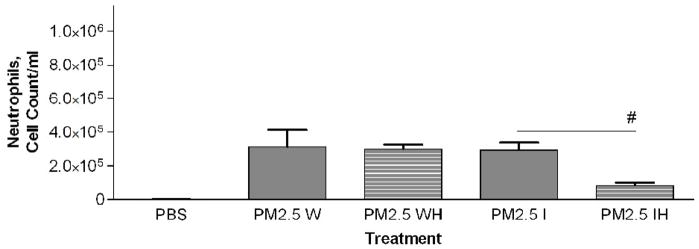
Figure 6a-c.
a) Total cell count of recovered cells in lung lavage fluid 24 hours after instillation of PM2.5–10 Whole (W), Whole Heated (WH), PM2.5–10 Insoluble (I), or Insoluble Heated (IH) Fraction (* P<0.05). b) Percentage of macrophages (checkered bars) and neutrophils (dark bars) in lung lavage fluid 24 hours after instillation of PM2.5–10 Fractions (* P<0.05; # P<0.001). c) Number of neutrophils in lung lavage fluid 24 hours after instillation of PM2.5–10 Fractions (* P<0.05).
PM2.5–10 and PM2.5: Activity of heated versus unheated particles
PM2.5–10 and PM2.5 were treated for 24 hours at 130°C to inactivate biological materials present in the samples, a protocol we have used previously for this purpose (Wegesser and Last, 2008). We created five treatment groups: 1) PM2.5–10 whole fraction (W), 2) PM2.5–10 Whole Heated Fraction (WH), 3) PM2.5 Whole Fraction (W), 4) PM2.5 Whole Heated Fraction (WH), 5) PBS control. Heated or normal PM samples were instilled in mice 24 hours before analysis. Heating of the PM2.5–10 resulted in a significant (P<0.05) loss of about 59% (6.8×105±1.4×105 versus 2.8×105±4.5×104) of the total inflammatory activity associated with the particles, while heating of the PM2.5 resulted in an apparent loss of about 16% (4.2×105±1.5×105 versus 3.5×105±9.8×104, not significant) of the corresponding activity (Fig. 6a, 7a). There was a significant increase (Fig 6b) in the relative percentage of macrophages recovered from the lung lavage fluid with the WH fraction from mice instilled with PM2.5–10 (18.3±3.8%) as compared to the W fraction (9.7±2.0%), with no significant change in the absolute number of macrophages when the equivalent comparison is made. All of the treatment groups show significant differences when they are compared with the PBS control values (Fig. 6b), suggesting that although we see significant decreases in inflammatory activity after particles are heated, some residual inflammatory activity remains in the PM after heating. On the other hand, neither the percentage nor the number of macrophages recovered from mice instilled with the PM2.5 W or WH fraction was significantly different (Fig 7b). PM2.5–10 instillation also caused significant decreases in both the percentage of neutrophils recovered from the lavage fluid and the number of total neutrophils (see figure 6b). Unlike the PM2.5–10 fraction, instillation of the PM2.5 W or WH fraction did not cause any significant decreases in the percentage or number of neutrophils recovered (Fig 6b, c; 7b, c). These results suggest that more than half of the pro-inflammatory activity present in the whole PM2.5–10 fraction results from its content of heat-labile components such as endotoxin, but that the active agent in whole PM2.5 is not heat labile.
Figure 7a-c.
a) Total cell count of recovered cells in lung lavage fluid 24 hours after instillation of PM2.5 Whole (W), Whole Heated (WH), Insoluble (I), or Insoluble Heated (IH) Fraction (# P<0.001). b) Percentage of macrophages (checkered bars) and neutrophils (dark bars) in lung lavage fluid 24 hours after instillation of PM2.5 Fractions (* P<0.05). c) Number of neutrophils in lung lavage fluid 24 hours after instillation of PM2.5 Fractions (# P<0.001).
PM2.5–10 and PM2.5: Activity of heated versus unheated fractionated particles
We compared the activity of Insoluble PM2.5–10 (I)) and the PM2.5–10 Insoluble Heated (IH) Fraction, Figure 6a–c. The mice instilled with heat-treated insoluble PM2.5–10 showed an apparent reduction of both total cell counts (9.2×105±2.1×105 vs. 3.1×105±1.4×105, p=0.054) and the number of neutrophils (p<0.05), Fig. 6c) recovered in lavage fluid as compared to mice instilled with unheated insoluble PM2.5–10. There was a significant reduction in total cells in the lavage fluid from mice treated with PM2.5 (IH) versus PM2.5 (I), Fig 7a. This response to heat treatment is also reflected in the number (and relative percentage) of neutrophils (2.9×105±4.5×104 vs. 8.4×104±1.7×104). There was no significant change in the number of macrophages (5.1×104±1.9×104 vs. 5.2×104±1.9×104) recovered from lung lavage fluid of mice treated with PM2.5 (I) or heat-treated PM2.5 (IH) (Fig 7b, c). These results suggest that biologically active material such as endotoxin is present in PM2.5–10 (I), the inflammatory effects of which are significantly reduced by heat inactivation. Comparable material may be present in the insoluble fraction prepared from PM2.5, or the active components in PM2.5 (I) may be volatile.
The percentage of neutrophils and macrophages in the BAL from the mice treated with PM2.5 followed the same trends: we did not see a difference in response between (W) and (WH), but we did see a significant reduction in neutrophils recovered from mice treated with (IH) versus (I) alone, Fig 7c. Although we see a clear response in mice treated with whole and fractionated PM2.5 above control, the heat-labile component of the response seems to be measurable only in the insoluble fraction, and is reduced after heat treatment of the insoluble PM2.5 material.
Time course of heat inactivation of PM2.5–10 versus LPS
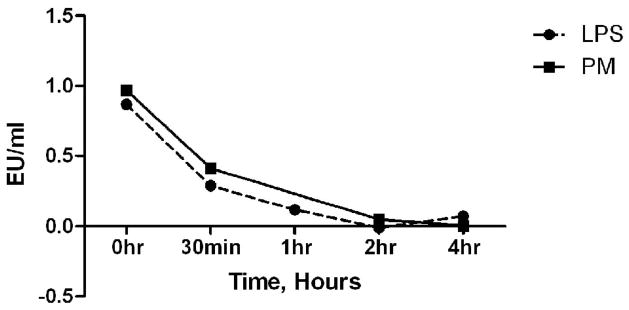
Previously (Wegesser and Last, 2008) we showed that heating pure LPS (E. Coli 055:B5) or PM2.5–10 (from ambient air) spiked with LPS for 24 hours at 130°C decreased lung inflammation after intratracheal injection as measured by total cells recovered in the lavage fluid. We did not find decreased activity after heating PM2.5–10 alone, and concluded from this negative result that endotoxin did not contribute significantly to the inflammatory response caused by instillation of PM2.5–10 isolated from ambient air into mice. In the present study we used heat treatment at 130°C for shorter periods of time than 24 hours to examine whether inflammatory activity caused by pure LPS (Escherichia. coli 0111B4) and inflammatory activity caused by the heat-labile component of the dairy barn-derived PM2.5–10 were inactivated at the same rate. We adjusted the concentration of LPS and PM2.5–10 to have the same relative activity in the Limulus bioassay at 0 time, and incubated tubes of either LPS (0.8 EU) or PM2.5–10 (1:128 dilution) for 0.5, 1, 2, or 4 hours at 130°C. The rate of reduction in the activity of LPS and PM2.5–10 was similar, as shown in Figure 8. Thus, our data are consistent with the heat-labile inflammatory activity in the PM2.5–10 being endotoxin.
Figure 8.
Reduction of Endotoxin Units (EU) per ml after heat treatment (130°C) measured by Limulus bioassay at 0 time, 30 minutes, or 1, 2, or 4 hours. 0-time data, PM2.5–10= 0.97 EU/ml and LPS=0.87 EU/ml.
Discussion
The state of California routinely experiences some of the highest levels of particulate matter air pollution reported in the United States (U.S. EPA: http://www.epa.gov/oar/oaqps/greenbk/anay.html). Eleven of the highest producing 20 agricultural counties in the U.S.A. are located in California’s Central Valley (http://www.cdfa.ca.gov/statistics.html), and include several of these high PM areas. Thus, we have become interested in developing methodology to determine which specific constituents of coarse and fine PM in the Central Valley of California are responsible for their toxicity, especially the relative contribution of bioaerosol components such as endotoxin. In earlier studies (Wegesser and Last, 2008; Wegesser et al., 2009) we examined the inflammatory activity of coarse (and, to a lesser extent, fine) PM collected from summer ambient air from rural areas in the San Joaquin Valley. Based upon the heat stability of the activity in the PM tested, we concluded that the pro-inflammatory activity of the PM from ambient air collected under hot and dry conditions was not due to any measurable extent to endotoxin. In the present study we deliberately sought to collect comparable PM fractions that were rich in endotoxin to test whether our bioassay system would allow us to determine the contribution of endotoxin to PM-induced lung inflammation when particles actually did contain this agent. Our long-term goal is to determine whether mouse bioassay can ultimately be used to identify specific toxic constituents in active PM preparations.
Our studies demonstrate that dairy barn and non-dairy particles of the same size range collected in the same season from the same region differ in size, shape, and pattern density. Under polarized light, we do not find birefringent particles in either the coarse or fine dairy barn particles. Birefringence under polarized light is a convenient and simple method for detection of crystalline material such as silica, as compared to using a scanning electron microscope (McDonald and Roggli, 1995). Birefringence, a physical property unique to crystalline materials such as silica and silicates (Kleinerman et al., 1979), has been used to detect these particles in lung tissue (McDonald and Roggli, 1995) and lung lavage fluid (Fireman et al., 1999). Pinkerton and colleagues (2000) have identified silicates in inhaled PM from the same region where our ambient air PM were collected using detection of birefringent particles in lung sections by polarized light microscopy. Thus we can conclude that silica and silicates (from rocks, soil, crustal material, and sediment) are minor components in the dairy environment we studied as compared to non-dairy PM sources from the same region. It should be noted that Kullman and colleagues (1998) found evidence of birefringent particles [feed (starch) and bedding (lime)] during an environmental survey of 85 working dairy barns; however, this study was done in central Wisconsin, where feed and bedding conditions differ from those in use in California.
Coarse and fine particulate matter cause different effects in the lung. Epidemiological studies infer that there is a stronger correlation between fine PM content in ambient air and enhanced detrimental health effects as compared to coarse PM (Dockery et al., 1993; Pope et al., 2002; Schwartz et al., 1996; Schwartz and Neas, 2000). However, it has been shown by in vivo and in vitro studies that the coarse fraction has greater potency at equivalent masses when compared to the fine fraction (Monn et al., 1999; Soukup et al., 2001; Schins et al., 2004; Hetland et al., 2005; Lipsett et al., 2006; Yeatts et al. 2007). Likewise, in the present study we show that a single dose of instilled coarse PM has more inflammatory potential after 24 hours as compared to a single instillation of fine material collected simultaneously from the same location. Both total lavagable cells and the relative number of neutrophils are increased more by coarse than by fine PM administered to mice at the same dose. If we assume that the time course of response to fine PM is the same as the time course we demonstrate for coarse PM in Figure 4, we can conclude that the coarse PM fraction collected from a working dairy barn has a higher inflammatory potential per unit of mass than the fine PM fraction. We have tested different PM samples obtained from other locations and have observed similar findings (data not shown).
There have been several attempts to elucidate which constituents from coarse PM are responsible for toxic and pro-inflammatory effects in the lung such as biological agents (Becker et al., 1996; Dong et al., 1996), metals (Pope et al., 1989; Costa et al., 1997; Ghio et al., 1999), organic compounds (Li et al., 2002), or ultrafine particles adhered to coarse particles (Oberdorster et al., 1994). Particulate matter not only varies by size fraction but also by seasonal (Heinrich et al., 2003; Seagrave et al., 2006; Sillanpää et al., 2006), location (Gilmour et al. 2007; Happo et al., 2007), and compositional patterns (Sillanpää et al., 2006).
In this study we used intratracheal instillation as opposed to inhalation or other exposure methods due to the relatively small amount of PM available per sampling period. We also wanted to continue to develop a bioassay method that would facilitate comparative activity studies between different spatial and temporal batches of PM collected using high-volume air samplers. Intratracheal instillation has been demonstrated to be an appropriate and relevant method of determining potential toxicity and lung injury caused by inhalable particulates, (Costa et al., 2006). Both in vitro and in vivo studies have been used previously to evaluate respiratory tract inflammation and toxicity from particulate matter; both approaches have their advantages and disadvantages. One clear advantage of in vivo methodology is the relevance of quantifying lung inflammation and toxicity resulting from particulate matter exposure of animals to estimating human risk. However, there are experimental limitations to the use of inhalation exposure methods (Driscoll et al., 2000; Costa et al., 2006), including estimation of the total dose delivered to the lungs and the large amount of PM material required to perform such exposures. One of the most powerful advantages of intratracheal instillation as an exposure route is the delivery of an actual known dose to the lung; this can be very beneficial in characterizing dose response to a particulate matter sample. Clearly there are limitations to using intratracheal instillation, as it does not mimic a natural route of exposure. However, this method is an effective technique for the identification of individual toxic constituents of complex mixtures such as PM, and for comparison of the toxicity of different PM samples.
Others have previously fractionated PM to attempt to identify bioactive components in either in vivo or in vitro systems, (Ghio et al., 1999; Imrich et al., 2000; Soukup et al., 2001; Becker et al., 2003; Adamson et al., 2004). Due to the heterogeneity of PM, determining which specific constituents cause their toxicity has been difficult. However, there are numerous studies that have taken an additive or subtractive approach to determine the principal toxic components of PM. In this paper, similar to our past study (Wegesser and Last, 2008), we took a simple subtractive approach to ask whether there a decrease in total activity if we fractionate the whole PM into soluble and insoluble fractions, and whether coarse and fine particulate matter behave differently after fractionation. We found a decrease in bioactivity between the coarse soluble fraction (aqueous) and the coarse insoluble fraction (particulate); within the detection limits of the bioassays used, the bioactivity observed in the whole coarse PM was completely retained in the insoluble fraction (Fig 6a). We observed the same trend with the fine PM, where bioactivity was retained in the insoluble fraction after aqueous extraction (Fig 7a). Imrich and colleagues (2000) demonstrated in an in vitro system that alveolar macrophages were responsive to the insoluble fraction of CAPs (concentrated ambient particle) suspensions, whereas macrophages incubated with the soluble fraction did not exhibit increased cytokine release upon exposure. Others have found that the soluble fraction, specifically metals, is the primary component responsible for inflammatory activity with other types of PM preparations (Kodavanti et al., 1998; Adamson et al., 1999). We should note, however, that bioactivity may also be preferentially found in the insoluble fraction because it contains (by far) the highest percentage of the overall particle mass (Linton et al., 1976).
As shown in Figure 8, LPS and coarse PM have similar heat inactivation curves. After 2 hours of heating at 130°C the activity, measured by Limulus assay, had decreased by 98% and 94%, respectively. Tsuji and Harrison (1978) showed 99.9% destruction of LPS (E. Coli, O127:B8) after heating at 170°C for 4 hours and 99.6% destruction after heating at 190°C for 40 minutes. Alexis and colleagues (2006) heat treated PM2.5–10 at 120°C for 20 hours. Their results demonstrated that heat inactivation resulted in a significant reduction of cell-surface marker expression (mCD14, CD11b/CR3, and HLA-DR/MHC class II) and phagocytic capabilities of macrophages; however, in contrast to our results, they found that heat inactivation had no effect on neutrophil influx into the airways as measured by sputum analysis. Endotoxin biological activity was not measured via Limulus assay but rather by measuring Polymixin B-inhibition of cytokine induction in the cited study (Alexis et al., 2006). Although the Limulus assay has become the gold standard for endotoxin determination in environmental samples, the assay is subject to some limitations, such as its inability to measure insoluble endotoxin and other bioaerosol components (Rylander, 1997, 2002), and that the biological activity of measured endotoxin does not correlate with in vivo activity or toxicity (Binding et al., 2004). We should note that our finding in Figure 8 that LPS and coarse PM have similar heat inactivation curves as measured by the Limulus assay supports our conclusion that heat treatment is destroying endotoxin in the PM, but we cannot view this observation as a rigorous demonstration that all of the heat-sensitive material in the coarse PM fraction is endotoxin. Endotoxin, which contains protein and lipid components, is a more complex molecule than LPS purified from E. coli, and there are many different forms of endotoxin arising from different bacterial species in nature. Thus, better methods to specifically quantify and/or neutralize the effects of endotoxin activity in PM preparations from ambient air are a desirable goal. Some of the residual activity observed in heat-treated PM may be attributed to insoluble endotoxin. However, our experiments do not rule out the possibility that the heat treatment step may also cause dissociation of pro-inflammatory constituents from the PM mixture whose activity may be inhibited or masked in the whole PM preparations as collected by high volume sampling at ambient temperatures. We are not aware of any specific endotoxin measurements that can be used to determine whether insoluble endotoxin remains in PM after heat treatment. Analytical methods exist for quantification of 3-hydroxy fatty acids from the lipid A portion of LPS; however, results obtained by this assay do not correlate to those obtained by Limulus assay (Binding et al., 2004). In addition, quantification of 3-hydroxy fatty acids of LPS via GC-MS (gas chromatography-mass spectroscopy) does not always infer the absolute amounts of LPS due to the many different species with differing 3-hydroxy fatty acid sequences (Binding et al., 2004). Kullman and colleagues (1998) sampled organic dust from 85 working dairy barns and found that organic dust was composed of: histamine, endotoxin, cow urine antigen, Tyrophagus putrescentiae mite antigen, spores, yeasts, mesophilic molds, mesophilic bacteria, and thermophilic bacteria. We believe that the existing literature does not definitively address whether measurement of LPS by Limulus assay or 3-hydroxy fatty acid content clearly quantifies the bioactivity of environmental endotoxin in PM. Additional research is needed to accurately characterize the contributions of bioaerosol components to the toxicity of coarse and fine PM in ambient air and in the emissions from agricultural sources in humid and dry climates.
In summary, this study demonstrates that dairy barn coarse PM instilled into mouse lungs results in an inflammatory response, which is predominantly caused by heat labile material, especially endotoxin. Additionally, we show that coarse PM from this source has a greater inflammatory activity on an equal mass basis than fine PM, consistent with the fact that the coarse fraction is more likely to contain any endotoxin present (Monn et al., 1999; Soukup et al., 2001; Schins et al., 2004). When the coarse (or fine) PM is fractionated into soluble (aqueous) and insoluble (particulate) fractions and the insoluble fraction is heated there is a significant reduction in activity, suggesting a major contribution by endotoxin to the total toxicity observed. We do not understand the basis for the different heat lability observed with whole PM2.5 and the insoluble fraction prepared from PM2.5, but the relevant experiments have been replicated and this is a reproducible observation. Additional experiments with different PM2.5 samples will need to be performed to explain this apparently paradoxical finding. However, it is noteworthy that addition of a simple heating step to our bioassay system allows us to determine the quantitative role of endotoxin in PM-induced lung inflammation when particles actually contain this agent. This approach should be an important addition to Limulus bioassay in evaluating the quantitative importance of endotoxin activity in total PM toxicity.
Acknowledgments
We thank Drs. Steven Cliff, Frank Mitloehner, and Kent Pinkerton for providing the high-volume PM sampler used in these experiments, and for many helpful discussions, and Doug Gisi for access to the sampling site. We also acknowledge Lisa M. Franzi for her skilled technical assistance and generous support. Teresa C. Wegesser was supported by an NIH training grant (ES-07059-30) during this study.
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson IYR, Prieditis H, Renaud V. Pulmonary Toxicity of an Atmospheric Particulate Sample Is Due to the Soluble Fraction. Toxicol Appl Pharmacol. 1999;157:45–50. doi: 10.1006/taap.1999.8658. [DOI] [PubMed] [Google Scholar]
- Adamson IYR, Prieditis H, Renaud V. Soluble and Insoluble Air Particle Fractions Induce Differential Production of Tumor Necrosis Factor-α in Rat Lung. Exp Lung Res. 2004;30(5):355–368. doi: 10.1080/01902140490438933. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Clin Immunol. 2006;117(6):1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Gilmour IM, Devlin RB. Stimulation of human and rat alveolar macrophages by urban air particulates: Effects of oxidant radical generation and cytokine production. Toxicol Appl Pharmacol. 1996;141:637–648. doi: 10.1006/taap.1996.0330. [DOI] [PubMed] [Google Scholar]
- Becker S, Soukup JM, Sioutas C, Cassee FR. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp Lung Res. 2003;29:29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- Binding N, Jaschinski S, Welich S, Bletz S, Witting U. Quantification of bacterial lipopolysaccharides (endotoxin) by GC-MS determination of 3-hydroxy fatty acids. J Environ Monit. 2004;6:65–70. doi: 10.1039/b309237b. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Jerrett M, Burnett RT, Kaufman JD, Miller KA, Sheppard L. Air Pollution and Cardiovascular Events. NEJM. 2007;356:2104–2106. [PubMed] [Google Scholar]
- Cassee FR, Fokkens PHB, Lesemann DLAC, Bloemen HJTh, Boere AJF. Respiratory allergy and inflammation due to ambient particles (RAIAP); collection and characterisation of particulate matter samples from 5 European sites. RIVM; Bilthoven: Report 863001001/2003. [Google Scholar]
- Costa DL, Dreher KL. Bioavailable transition metals in particulate matter mediate cardiopulmonary injury in healthy and compromised animal models. Environ Health Perspect. 1997;105(suppl 5):1053–1060. doi: 10.1289/ehp.97105s51053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DL, Lehmann JR, Winsett D, Richards J, Ledbetter AD, Dreher KL. Comparative pulmonary toxicological assessment of oil combustion particles following inhalation or instillation exposure. Toxicol Sci. 2006;91(1):237–246. doi: 10.1093/toxsci/kfj123. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Pope CA, III, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;29:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- Donaldson KT, Mill S, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005;207(2 suppl 1):483–488. doi: 10.1016/j.taap.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Dong W, Lewtas J, Luster MI. Role of endotoxin in tumor necrosis factor α expression from alveolar macrophages treated with urban air particles. Exp Lung Res. 1996;22:577–592. doi: 10.3109/01902149609046043. [DOI] [PubMed] [Google Scholar]
- Dreher KL. Particulate Matter Physiochemistry and Toxicology: In Search of Causality – A Critical Perspective. Inhal Toxicol. 2000;12 (Supplement):45–57. doi: 10.1080/08958378.2000.11463230. [DOI] [PubMed] [Google Scholar]
- Driscoll KE, Costa DL, Hatch G, Henderson R, Oberdoster G, Salem H, Schlesinger RB. Forum: Intratracheal Instillation as an Exposure Technique for the Evaluation of Respiratory Tract Toxicity: Uses and Limitations. Toxicol Sci. 2000;55:24–35. doi: 10.1093/toxsci/55.1.24. [DOI] [PubMed] [Google Scholar]
- Fireman E, Greif J, Schwarz Y, Man A, Ganor E, Ribak Y, Lerman Y. Assessment of Hazardous Dust Exposure by BAL and Induced Sputum. Chest. 1999;115:1720–1728. doi: 10.1378/chest.115.6.1720. [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Stonehuerner J, Dailey LA, Carter JD. Metals associated with both the water soluble and insoluble fractions of an ambient air pollution particle catalyze an oxidative stress. Inhal Toxicol. 1999;11:37–49. doi: 10.1080/089583799197258. [DOI] [PubMed] [Google Scholar]
- Gilmour PS, McGee J, Duvall RM, Dailey L, Daniels M, Boykin E, Cho SH, Doerfler D, Gordon T, Devlin RB. Comparative Toxicity of Size-Fractionated Airborne Particulate Matter Obtained from different Cities in the United States. Inhal Toxicol. 2007;19(suppl 1):7–16. doi: 10.1080/08958370701490379. [DOI] [PubMed] [Google Scholar]
- Happo MS, Salonen RO, Hälinen AI, Jalava PI, Pennanen AS, Kosma VM, Sillanpää M, Hillamo R, Brunekreef B, Katsouyanni K, Sunyer J, Hirvonen MR. Dose and Time Dependency of Inflammatory Responses in the Mouse Lung to Urban Air Coarse, Fine, and Ultrafine Particles From Six European Cities. Inhal Toxicol. 2007;19:227–246. doi: 10.1080/08958370601067897. [DOI] [PubMed] [Google Scholar]
- Heinrich J, Pitz M, Bischof W, Krug N, Borm PJA. Endotoxin in fine (PM2.5) and coarse (PM2.5–10) particle mass of ambient aerosols. A temporo-spatial analysis. Atmos Environ. 2003;37(26):3659–3667. [Google Scholar]
- Hetland RB, Cassee FR, Lag M, Refsnes M, Dybing E, Schwarze PE. Cytokine release from alveolar macrophages exposed to ambient particulate matter: heterogeneity in relation to size, city and season. Part Fibre Toxicol. 2005;2:4. doi: 10.1186/1743-8977-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SL, Cheng WL, Lee CT, Huang HC, Chan CC. Contribution of endotoxin in macrophage cytokine response to ambient particles in vitro. J Toxicol Environ Health. 2002;A 65:1261–1272. doi: 10.1080/152873902760125741. [DOI] [PubMed] [Google Scholar]
- Imrich A, Ning Y, Kobzik L. Insoluble components of concentrated air particles mediate alveolar macrophage responses in vitro. Toxicol Appl Pharmacol. 2000;167:140–150. doi: 10.1006/taap.2000.9002. [DOI] [PubMed] [Google Scholar]
- Kenyon NJ, Last MS, Eiserich JP, Morrissey BM, Temple LM, Last JA. Differentiation of the roles of NO from airway epithelium and inflammatory cells in ozone-induced lung inflammation. Toxicol Appl Pharmacol. 2006;215:250–259. doi: 10.1016/j.taap.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman J, Green F, Harley RA, et al. Pathology standards for coal workers’ pneumoconioses. Arch Pathol Lab Med. 1979;103:375–431. [PubMed] [Google Scholar]
- Kodavanti UP, Hauser R, Christiani DC, Meng ZH, McGee J, Ledbetter A, Richards J, Costa DL. Pulmonary responses to oil fly ash particles in the rat differ by virtue of their specific soluble metals. Toxicol Sci. 1998;171:20–26. doi: 10.1006/toxs.1998.2460. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, McGee JK, Walsh L, Gilmour PS, Highfill JW, Davies D, Pinkerton KE, Richards JH, Crissman K, Andrews D, Costa DL. Consistent Pulmonary and Systemic Responses from Inhalation of Fine Concentrated Ambient Particles: Roles of Rat Strains Used and Physicochemical Properties. Environ Health Perspect. 2005;113(11):1561–1568. doi: 10.1289/ehp.7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullman GJ, Thorne PS, Waldron PF, Marx JJ, Ault B, Lewis DM, Siegel PD, Olenchock SA, Merchant JA. Organic dust exposures from work in dairy barns. Am Ind Hyg Assoc J. 1998;59(6):403–413. doi: 10.1080/15428119891010668. [DOI] [PubMed] [Google Scholar]
- Li N, Wang M, Oberley TD, Sempf JM, Nel AE. Comparison of the Pro-Oxidative and Proinflammatory Effects of Organic Diesel Exhaust Particle Chemicals in Bronchial Epithelial Cells and Macrophages. J Immunol. 2002;169:4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- Linton R, Loh A, Natusch D, Evans C, Williams P. Surface predominance of trace elements in airborne particles. Science. 1976;191:852–854. doi: 10.1126/science.1251197. [DOI] [PubMed] [Google Scholar]
- Lipsett MJ, Tsai FC, Roger L, Woo M, Ostro BD. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ Health Perspect. 2006;114(8):1215–1220. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald WJ, Roggli VL. Detection of silica particles in lung tissue by polarizing light microscopy. Arch Pathol Lab Med. 1995;119:242–246. [PubMed] [Google Scholar]
- Mishell BB, Shiigi SM. Selected Methods in Cellular Immunology. Freeman and Co.; San Francisco: 1980. pp. 16–19. [Google Scholar]
- Monn C, Becker S. Cytotoxicty and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol Appl Pharmacol. 1999;155:245–252. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Ferin J, Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect. 1994;102(suppl 5):173–179. doi: 10.1289/ehp.102-1567252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161(6):585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Green FHY, Saiki C, Vallyathan V, Plopper CG, Gopal V, Hung D, Bahne EB, Lin SS, Ménache MG, Schenker MB. Distribution of Particulate Matter and Tissue Remodeling in the Human Lung. Environ Health Perspect. 2000;108:1063–1069. doi: 10.1289/ehp.001081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CAIII. Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79:623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, III, Thun MJ, Namboodiri MM, Dochery DW, Evans JS, Speizer FE, Clark CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U. S. adults. Am J Respir Cri Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston JD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylander R. Evaluation of the Risks of Endotoxin Exposures. Int J Occup Environ Health. 1997;3(1):S32–36. [Google Scholar]
- Rylander R. Endotoxin in the Environment – Exposure and Effects. J Endotoxin Res. 2002;8(4):241–252. doi: 10.1179/096805102125000452. [DOI] [PubMed] [Google Scholar]
- Schwartz J. Air pollution and daily mortality. A review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Dockery DW, Neas LM. Is daily mortality associated specifically with fine particles? J Air Waste Manage Assoc. 1996;46:927–993. [PubMed] [Google Scholar]
- Schwartz J, Neas LM. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology. 2000;11(1):6–10. doi: 10.1097/00001648-200001000-00004. [DOI] [PubMed] [Google Scholar]
- Schwarze PE, Ovrevik J, Lag M, Refsnes M, Nafstad P, Hetland RB, Dybing E. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25:559–579. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- Schins RPF, Lightbody JH, Borm PJA, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Seagrave JC, McDonald JD, Bedrick E, Edgerton ES, Gigliotti AP, Jansen JJ, Ke L, Naeher LP, Seilkop SK, Zheng M, Mauderly JL. Lung toxicity of ambient particulate matter from Southeastern U.S. sites with different contributing sources: Relationship between composition and effects. Environ Health Perspect. 2006;114:1387–1393. doi: 10.1289/ehp.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää M, Hillamo R, Saarikoski S, Frey A, Pennanen AS, Makkonen U, Spolnik Z, van Greiken R, Branis M, Brunekreef B, Chalbot MC, Kuhlbusch T, Sunyer J, Kerminen VM, Kulmala M, Salonen RO. Chemical Composition and Mass Closure of Particulate Matter at Six Urban Sites in Europe. Atmos Environ. 2006;40:212–222. [Google Scholar]
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Spaan S, Schinkel J, Wouters IM, Preller L, Tielemans E, Nij ET, Heederik D. Variability in endotoxin exposure levels and consequences for exposure assessment. Ann Occup Hyg. 2008;52(5):303–316. doi: 10.1093/annhyg/men024. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Withagen CE, van Dalen WJ, Dormans JA, Cassee FR, Heisterkamp SH, van Loveren H. Adjuvant activity of ambient particulate matter of different sites, sizes, and seasons in a respiratory allergy mouse model. Toxicol Appl Pharmacol. 2004;200:186–200. doi: 10.1016/j.taap.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Harrison SJ. Dry-Heat Destruction of Lipopolysaccharide: Dry-Heat Destruction Kinetics. Appl Environ Microbiol. 1978;36(5):710–714. doi: 10.1128/aem.36.5.710-714.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [Accessed 16 December 2008];USEPA: Nonattainment Status for Each County by Year. Available http://www.epa.gov/oar/oaqps/greenbk/anay.html.
- Wegesser TC, Last JA. Lung response to coarse PM: Bioassay in mice. Toxicol Appl Pharmacol. 2008;230(2):159–166. doi: 10.1016/j.taap.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesser TC, Pinkerton KE, Last JA. California Wildfires of 2008: Coarse and Fine Particulate Matter Toxicity. Environ Health Perspect. 2009 doi: 10.1289/ehp.0800166. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatts K, Svendsen E, Creason J, Alexis N, Herbst M, Scott J, Kupper L, Williams R, Neas L, Cascio W, Devlin RB, Peden DB. Coarse Particulate Matter (PM2.5–10) Affects Heart Rate Variability, Blood Lipids, and Circulating Eosinophils in Adults with Asthma. Environ Health Perspect. 2007;115(5):709–714. doi: 10.1289/ehp.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]