Abstract
Src-family protein tyrosine kinases (PTKs) transduce signals to regulate neuronal development and synaptic plasticity. However, the nature of their activators and molecular mechanisms underlying these neural processes are unknown. Here, we show that brain-derived neurotrophic factor (BDNF) and platelet-derived growth factor enhance expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptor 1 and 2/3 proteins in rodent neocortical neurons via the Src-family PTK(s). The increase in AMPA receptor levels was blocked in cultured neocortical neurons by addition of a Src-family-selective PTK inhibitor. Accordingly, neocortical cultures from Fyn-knockout mice failed to respond to BDNF whereas those from wild-type mice responded. Moreover, the neocortex of young Fyn mutants exhibited a significant in vivo reduction in these AMPA receptor proteins but not in their mRNA levels. In vitro kinase assay revealed that BDNF can indeed activate the Fyn kinase: It enhanced tyrosine phosphorylation of Fyn as well as that of enolase supplemented exogenously. All of these results suggest that the Src-family kinase Fyn, activated by the growth factors, plays a crucial role in modulating AMPA receptor expression during brain development.
Src-family protein tyrosine kinases (PTKs), such as Src and Fyn, are non-receptor-type PTKs that can be activated by various extracellular factors, including growth factors and neuronal factors (1). The abundance of Src-family PTKs in the brain, especially at developing growth cones and postsynaptic sites, indicates their potential participation in the intracellular signaling necessary for synaptic development and plasticity (2, 3), although the precise molecular mechanism of their signaling is still under investigation. Src-family PTKs are reported to modulate the function of N-methyl-d-aspartate (NMDA) channels (4) and potassium channels (5) and to regulate long term potentiation (LTP) (6). Analyses of Fyn-knockout mice (7, 8) have confirmed the idea that Fyn-mediated signaling is essential for normal neural development (9, 10), synaptic plasticity (10, 11), and brain functions (12, 13).
Neurotrophins share significant structural homology with nerve growth factor and initiate signals through their receptor-type protein tyrosine kinases and the 75-kDa, low affinity neurotrophin receptor (14, 15) to regulate neuronal development and synaptic plasticity (16, 17). For example, brain-derived neurotrophic factor (BDNF) can change the activities of NMDA channels and calcium channels (18, 19) and can modulate basal synaptic transmission (20–22) as well as LTP (23–26). PTKs of these neurotrophin receptors are associated with various signaling molecules, including Src-family PTKs (27, 28). Another growth factor, platelet-derived growth factor (PDGF), also is known to bind to the receptor-type PTK and activates a variety of signaling cascades, including the Src-family PTKs (29). PDGF is shown to influence the gating properties of calcium channels (30) as well as NMDA channels (31).
The above similarities in the biological activities of these growth factors and Src-family PTKs have raised the question of whether the Src-family PTKs may mediate signal transduction from the growth factors that regulate synaptic and developmental plasticity (30, 32). In the present study, we demonstrate that Fyn is indeed involved in the signaling of BDNF and PDGF, which both influence the expression of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors.
MATERIALS AND METHODS
Animals.
For culture experiments, rats (Sprague–Dawley) were obtained from Shizuoka Laboratory of Animal Center (Shizuoka Japan). Fyn knockout mice in the C57BL/6 genetic background (5 backcrosses) (8) were maintained in the animal facility of the University of Tokyo. Wild-type C57BL/6 mice were used as a control. For determination of AMPA receptor levels in vivo, three litters (23 animals total) were produced from crosses between heterozygotes in the C57BL/6 genetic background (6 backcrosses) (7) in the animal facility of the Cold Spring Harbor Laboratory (Cold Spring Harbor, NY).
Neuronal Culture.
Whole cerebral neocortices of embryonic day 18 rats or neonatal mice were mechanically dissociated and plated onto poly-d-lysine-coated dishes at a lower density (500–800 cells/mm2). Cortical neurons were grown with DMEM containing 0.5 mM pure glutamine (Ajinomoto, Tokyo), 2% fetal bovine serum, nutrient mixture N2, and 10 mM Hepes (pH 7.3) (33). The following day, the original medium was replaced by the medium lacking serum (serum-free N2 medium). This procedure reduced glial contamination to <5% of total cells. Purified human recombinant BDNF (50 ng/ml), epidermal growth factor (EGF) (20 ng/ml), basic fibroblast growth factor (bFGF) (20 ng/ml), PDGF (α/β heterodimer) (30 ng/ml), and a Src-family-selective tyrosine kinase inhibitor, 4-amino-5-(4-methylphenyl)-7-(t-butyl) pyrazolo [3,4d] pyrimidine (PP1, Calbiochem; 1 μM), were added daily (34). Cultures were used for experiments 5 days after plating. Prolonged or a higher density of culture reduced the effect of growth factors, presumably because endogenous growth factors or cell–cell contacts mimicked a similar effect (data not shown; see Discussion). BDNF was a gift from Sumitomo Chemical, Osaka. EGF, PDGF, and bFGF all were purchased from Sigma.
Immunodetections.
Immunoblot analysis was done with protein extracted from neocortical culture and tissue. Protein was separated by SDS/PAGE and was blotted onto nitrocellulose membranes. Immunoblots were treated with various rabbit affinity-purified antibodies or mouse mAbs: anti-glutamate receptor 1 (GluR1), anti-NMDAR1, anti-NMDAR2A/B (all from Chemicon), anti-GluR2/3 (a gift from K. Sakimura (Niigata University, Japan), made to 50-aa peptide corresponding to the carboxy terminus of GluR2), anti-neurofilament (NF) 200/150-kDa (a gift from Y. Takahashi, Niigata University), anti-Fyn (Santa Cruz Biotechnology), monoclonal anti-Fyn (clone fyn301; Wako Pure Chemicals, Osaka), anti-Src (clone 327; Oncogene Science), anti-phosphotyrosine (clone PY20; Transduction Laboratories, Lexington, KY), and anti-α-tubulin (Sanbio, Uden, The Netherlands) antibodies. Immunoreactivity was visualized by chemiluminescence reaction (ECL kit; Amersham).
Northern Blotting.
RNA analysis was carried out according to standard procedures as described (35). 32P-labeled cDNA probes for mouse GluR2 (a 1.5-kilobase fragment; a gift from K. Sakimura) and rat GluR3 (a 269- to 1,337-nt fragment produced by reverse transcription–PCR) were generated by using the Random Primed DNA Labeling kit (Boehringer Mannheim). These probes were sequentially hybridized to an RNA filter.
In Vitro Kinase Assay.
PTK activity was monitored by the in vitro kinase assay as follows. Hippocampi and neocortices from young rats (4–6 days old) were homogenized in lysis buffer (10 mM Tris⋅HCl, pH 8.0/140 mM NaCl/2 mM EDTA/0.5 μg/ml leupeptin/1 mM Na3VO4/1% Nonidet P-40) containing 5 μg/ml affinity-purified chicken anti-BDNF antibodies. The postnuclear supernatant was obtained by centrifugation and was pretreated with excess amounts of protein A Sepharose. The cleared lysate was treated with 200 ng/ml BDNF, then was incubated with an anti-Fyn antibody or a control Ig at 4°C for 90 min. The immunocomplex was precipitated with protein A Sepharose. Kinase reactions were carried out with the immunoprecipitates at 37°C for 10 min in 50 μl of kinase buffer (25 mM Hepes, pH 7.2/3 mM MnCl2/0.1% Nonidet P-40) containing 10 μCi 32P-γ-ATP (Amersham). In addition, the enzyme activity of Fyn that phosphorylates tyrosine residues of target proteins was monitored by supplementing an exogenous substrate, enolase (Sigma; 0.125 mg/ml), to the reaction mixture. The reaction was stopped by adding 2× Laemmli sample loading buffer and was analyzed by SDS/PAGE under reducing conditions. To visualize tyrosine phosphorylation, the gel was treated with KOH and was subjected to a film autoradiography. 32P incorporation into enolase (41 kDa) was measured by densitometory.
RESULTS
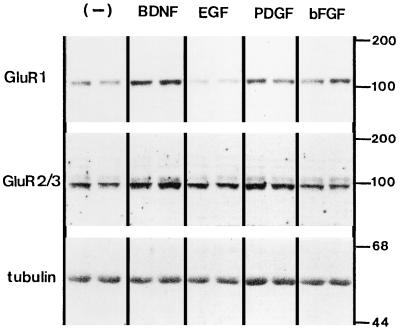
Recently, it was reported that daily application of BDNF to cultured neocortical neurons specifically up-regulated the protein levels of AMPA receptors (GluR1 and GluR2/3 receptors) but not NMDA receptors (NMDAR1 and NMDAR2A/B (33). In addition to BDNF, we studied the AMPA receptor-increasing activity of other growth factors—bFGF, EGF, and PDGF—whose receptors are expressed on neurons and elicit similar signaling in tyrosine phosphorylation (36). Among these, PDGF and BDNF markedly increased the protein expression of GluR1 and GluR2/3 receptors without an apparent change in α-tubulin levels (Fig. 1). Only EGF appeared to have an inhibitory activity on GluR1 expression with unknown mechanisms. These observations raised the possibility that a common signaling pathway for the AMPA receptor increase is shared by both BDNF receptors (TrkB) and PDGF receptors. We then added a Src-family-selective PTK inhibitor, PP1 (34), to the cultures, together with BDNF or PDGF (Fig. 2), and quantitated the changes in the receptor protein levels. Immunoblotting revealed that PP1 completely blocked the increase of the AMPA receptor subunits triggered by BDNF and PDGF whereas protein levels of NMDAR2A/B were not altered by these growth factors nor by the kinase inhibitor. The action of PP1 was ascertained by the reduction in the tyrosine phosphorylation level of the 60-kDa protein (p60), as revealed by using an antiphosphotyrosine antibody, but the PP1 treatment did not affect cell viability as monitored by protein recovery from the cultures (see Fig. 2 legend). All of these results are consistent with the previous reports indicating that Src-family PTKs are associated with TrkB and PDGF receptors and are involved in their signal transduction (28, 29).
Figure 1.
Effects of growth factors on AMPA receptor expression in cultured rat neocortical neurons. Neocortical neurons were grown at a lower density (<800 cells/mm2) for 5 days with or without (−) daily applications of BDNF (50 ng/ml), EGF (20 ng/ml), PDGF (30 ng/ml), and bFGF (20 ng/ml). Protein was extracted from two sister cultures (for each factor), was separated by SDS/PAGE, was transferred to a membrane, and probed with antibodies directed against GluR1, GluR2/3, and α-tubulin. Note that the treatment of EGF suppressed GluR1 levels (P < 0.001) but not GluR2/3 levels. In contrast, bFGF effects on the AMPA receptors displayed an increasing tendency (57), but its effect failed to reach statistical significance. Control protein levels were 100 ± 4.9% for GluR1 and 100 ± 12.7% for GluR2/3, EGF-treated levels were 63 ± 3.7% for GluR1 and 111 ± 4.5% for GluR2/3, and bFGF-treated levels were 130 ± 13% for GluR1 and 140 ± 10% for GluR2/3 as determined by four independent blots (n = 4). Numbers on the right indicate the positions of protein size markers (kDa). See Fig. 2 for the effects of the other factors.
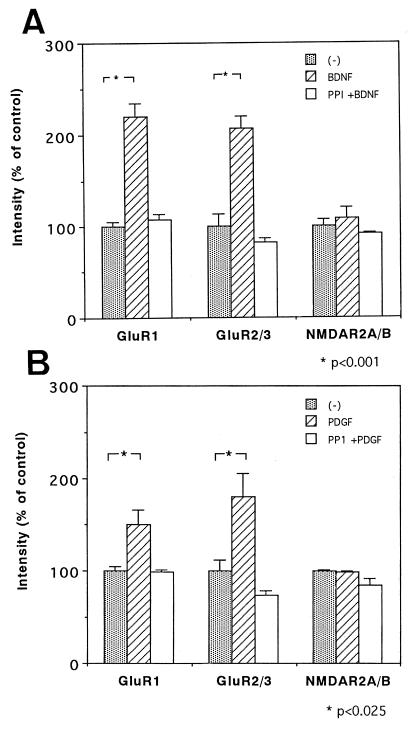
Figure 2.
Effects of a Src-family-specific inhibitor on the growth factor-mediated increases in AMPA receptors. Rat neocortical cultures were prepared as shown in Fig. 1 and were treated with BDNF (A) or PDGF (B) in the presence or absence of a Src-family PTK inhibitor, PP1 (n = 3–4 for each condition). The effects of these factors and PP1 on the level of GluR1, GluR2/3, and NMDAR2A/B proteins were determined by immunoblotting. For statistical analyses, independent blots were made, and each immunoreactivity on the blots was measured by densitometry. The average control receptor level was set as 100% for each immunoblot. Statistical analysis was performed by using one-way ANOVA followed by the Bonferroni test. Protein levels of NMDAR2A/B and α-tubulin (data not shown) were not significantly altered by the treatments (F [2,6] = 1.1 in A and F [2,9] = 3.7 in B for NMDAR2A/B, and F [2,6] = 1.2 in A and F [2,9] = 0.7 in B for α-tubulin). The action of the inhibitor was controlled by monitoring the basal tyrosine phosphorylation level with an antiphosphotyrosine antibody (PY20); tyrosine phosphorylation of p60 was reduced to 58.1 ± 0.4% (P <0.001; n = 3). Protein recovery was not affected significantly by the growth factors or by PP1; control for BDNF; 0.550 ± 0.017 mg/dish, BDNF; 0.578 ± 0.022 mg/dish and PP1 plus BDNF; 0.480 ± 0.031 mg/dish (F [2,6] = 4.43), and control for PDGF; 0.616 ± 0.022 mg/dish, PDGF; 0.600 ± 0.025 mg/dish; PP1 plus PDGF; 0.578 ± 0.006 mg/dish (F [2,6] = 0.43).
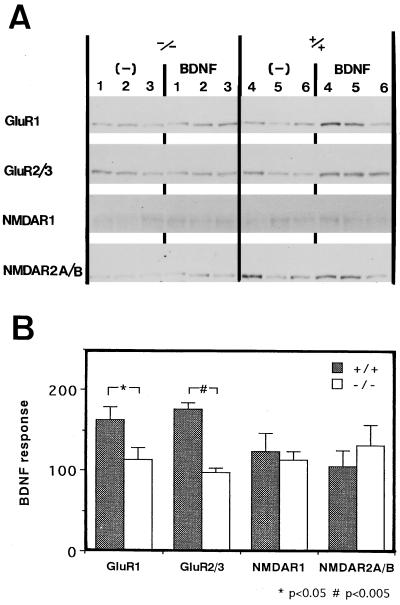
We speculated that one of the Src-family PTKs, Fyn, is involved in the growth factor-mediated increase in AMPA receptor proteins because Fyn is enriched in the forebrain regions and increases during brain development (37–39). To examine the Fyn participation, primary cultures of neocortical neurons were prepared from neonatal Fyn knockout and wild-type mice (8) and were treated with BDNF, the most effective factor for the AMPA receptor increase. Expression of AMPA receptors, NMDA receptors, and α-tubulin (an internal standard) was examined by immunoblotting (Fig. 3). Cultures from wild-type mice showed increases in the protein levels of GluR1 and GluR2/3 in response to BDNF applications whereas cultures from homozygous Fyn knockout mice did not. NMDA receptors did not reveal a significant increase in their protein levels even with BDNF treatment. This result demonstrated that the Src-family kinase Fyn is involved in the growth factor-mediated increase of AMPA receptors in culture.
Figure 3.
Altered BDNF responses in neocortical culture prepared from Fyn knockout mice. (A) Neocortical cultures were prepared separately from neonates of three homozygous Fyn-knockout mice (lanes 1–3) and three wild-type mice (lanes 4–6). Each number represents a pair of cultures prepared from an individual animal. One set of sister cultures from each animal was treated for 4 days with 50 ng/ml BDNF administered daily, and the other set was used as a control. Cultures were processed individually for immunoblotting and were probed with antibodies against GluR1, GluR2/3, NMDAR1, NMDAR2A/B, and α-tubulin (an internal standard). (B) Protein levels of the glutamate receptors were measured and normalized with those of α-tubulin. Statistical analysis was performed on averaged values obtained from two independent blots using one-way ANOVA followed by the Bonferroni test. Filled bars represent BDNF-dependent protein levels (%) in neocortical cultures prepared from wild-type (+/+) mice, and clear bars represent those from homozygous Fyn-knockout (−/−) mice. Results are expressed as means ± SEM (n = 3).
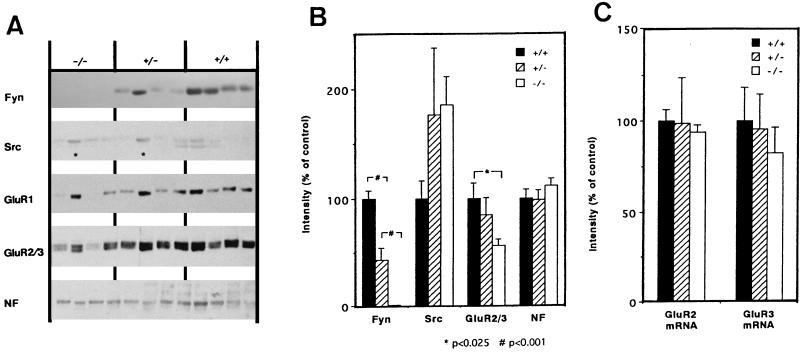
To confirm the effect of the Fyn deficiency in vivo, immunoblot analysis was used to determine the protein levels of AMPA receptors, Fyn, Src, and NF in the neocortex of young wild-type (5 animals) and Fyn knockout mice (7) (8 heterozygotes and 10 homozygotes) on postnatal days 13–14. Immunoblots of representative samples are shown in Fig. 4A. NF was used as an internal control to normalize the intensity of the immunoreactivity quantified from the blots (Fig. 4B). The level of Fyn in heterozygotes was half of that in wild-type mice, and Fyn was not detected in homozygotes. In contrast, Src levels in both Fyn homozygotes and heterozygotes tended to be up-regulated but variable among animals. Compared with wild-type mice, the protein levels of GluR2/3 and GluR1 in the Fyn homozygotes were reduced significantly whereas GluR2/3 mRNA levels for these receptors were not (Fig. 4C). Strikingly, some of the Fyn knockout mice that displayed higher levels of the AMPA receptor proteins exhibited increased expression of Src (marked with asterisks in Fig. 4A), as if the higher levels of Src expression compensated for the loss of Fyn and thus resulted in the higher expression of the AMPA receptors.
Figure 4.
Impaired expression of the AMPA receptors in the neocortex of young Fyn knockout mice. (A) Protein from the neocortex of Fyn knockout mice (postnatal 13–14 days old) was processed for immunoblotting with anti-Fyn, anti-Src, anti-GluR1, anti-GluR2/3, and anti-NF antibodies. Five wild-type mice (+/+), eight heterozygotes (+/−), and 10 homozygotes (−/−) were examined. Four examples representative for each genotype are shown. Note that some Fyn mutants exhibited rather normal GluR2/3 levels but expressed higher levels of Src (indicated with asterisks). (B) Fyn, Src, GluR1, GluR2/3, and NF immunoreactivity was quantified by densitometry. Protein levels for Fyn, Src, GluR1, and GluR2/3 were normalized by those of NF in each animal, and the average values for wild-type mice were set as 100%. Statistical analysis was performed with one-way ANOVA followed by Bonferroni test. Results are expressed as means ± SEM. GluR1 levels also were altered by Fyn mutation (F [2,20] = 3.52): 100 ± 16.9% (+/+), 59.5 ± 15.4% (+/−), and 50.3 ± 7.5% (−/−). In contrast, NF levels were not changed significantly among genotypes (F [2,20] = 0.80). (C) mRNA levels for GluR2, GluR3, and cyclophilin (an internal control) were measured by RNA blotting in four wild-type mice as well as four heterozygous and five homozygous Fyn mutants. Signal intensities for mRNA in each animal were measured and normalized with those for cyclophilin mRNA levels. Control values of wild-type mice were set as 100%. Statistical analysis was performed with one-way ANOVA (F [2,13] = 0.064 for GluR2 mRNA and F [2,13] = 0.31 for GluR3 mRNA).
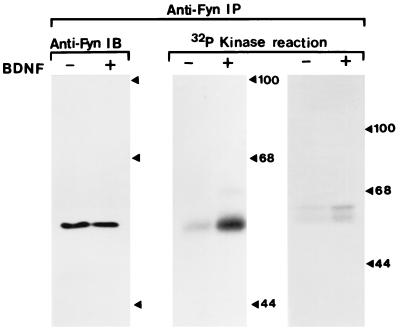
We performed kinase assays with Fyn immune complexes isolated from rat hippocampal and neocortical tissues to confirm that BDNF stimulation indeed resulted in Fyn activation. In the hippocampal sample, BDNF increased the phosphorylation level of Fyn whereas the amount of Fyn protein was not altered (Fig. 5). This increase of phosphorylation led to an increase in the PTK activity of Fyn, as monitored with an exogenous Fyn substrate, enolase (321 ± 92%). BDNF also enhanced the phosphorylation of Fyn in the immune complexes prepared from the neocortex. These results suggest that the Src-family PTK, Fyn, indeed is activated by BDNF in these neurons.
Figure 5.
Effects of BDNF on tyrosine phosphorylation of Fyn. Fyn activation by BDNF was examined by an in vitro kinase assay of the immunoprecipitates obtained with an anti-Fyn antibody. Tissue homogenate was prepared from the hippocampus of young rats (postnatal 4–6 days), was treated with (+) or without (−) BDNF, and was subjected to immunoprecipitation with an anti-Fyn polyclonal antibody. Half of the anti-Fyn immunoprecipitate from the hippocampus was used for immunoblotting to control for the quality of the immunoprecipitation (Left). Half of the anti-Fyn immunoprecipitate was incubated with [32P]-γ-ATP, and SDS/PAGE then was performed. Phosphorylation of Fyn was visualized by film autoradiography (Center). Fyn immunoprecipitates also were taken from the neocortex, and the kinase assay was performed similarly (Right). The tyrosine kinase activity of Fyn was monitored by measuring phosphorylation levels of enolase that was supplemented exogenously into other sets of the reaction mixture (data not shown).
DISCUSSION
Neurotrophic factors, growth factors, and cytokines regulate a large variety of molecular processes in both neuronal differentiation and maturation (39). Our previous study has shown that BDNF specifically enhances the expression of AMPA receptor proteins without any significant change in their mRNA levels (33), suggesting that the neurotrophin might modulate the translational or post-translational processes of AMPA receptor expression. Here, we demonstrate that Fyn is involved in this growth factor signaling to enhance the expression of AMPA receptor in neocortical neurons, using Fyn-knockout mice and a specific inhibitor of Src-family PTKs. The present results also suggest that (i) BDNF is not the only factor that influences the receptor expression, (ii) the activity to increase the AMPA receptors may involve not only Fyn but also other PTKs in the Src-family, and (iii) the increases in GluR1 protein parallel those in GluR2/3 protein for all manipulations of BDNF, PDGF, and the mouse gene targeting used.
Among several Src-family PTKs found in the brain, Fyn and Src are the most enriched in the forebrain regions (37, 41), and their expression is regulated during development (38, 41). Similarly, levels of BDNF and AMPA receptors are changed most remarkably at early postnatal stages of rodents (42, 43). In agreement, the cortical impairment of AMPA receptor expression was most apparent in Fyn-knockout mice, at the juvenile stage when the BDNF/Fyn signal is supposed to become more active, but was less clear at the embryonic stage as well as at the adult stage (data not shown). The redundant expressions of Src-family PTKs in the brain suggest that Fyn signaling from BDNF or PDGF is not the only pathway regulating the expression of AMPA receptors and that any Src-family PTKs, activated by other endogenous activators, are also able to trigger this signal transduction. Recently, a family of cell adhesion molecules that interact with Fyn has been characterized (44). The Fyn activation by such cell adhesion molecules may explain the reason why the neuronal density in culture also influenced the AMPA receptor expression (45). Future studies will characterize downstream signaling from Fyn kinase to AMPA receptors as well as other activators of Fyn that regulate these receptors.
The BDNF receptor TrkB, Src-family PTKs, and AMPA receptors are all intrinsic components of the post-synaptic density (18, 39, 46, 47) whereas some of BDNF is thought to be stored in presynaptic terminals and released in an activity-dependent manner (48–50). The studies reported here indicate that Fyn mediates the increase in levels of AMPA receptors in response to the activation of TrkB or PDGF receptors, presumably at postsynaptic sites. At the neuromuscular junction, as well as in the Torpedo electric organ, Src-family PTKs are associated with nicotinic acetylcholine receptors, and they have been implicated in receptor clustering and stabilization at post-synaptic sites (51–53). Among the PTKs, Src appears to be the one that regulates the phosphorylation of nicotinic acetylcholine receptors in the muscle. All of the existing evidence supports the idea that Src-family PTKs interact with various channel molecules and modulate their synaptic stability and function. In the present study, the changes of the individual AMPA receptor subunits always paralleled after the treatment with BDNF and PDGF in all of the types of cultures prepared from wild animals. This phenomenon would be more reasonable if Fyn signaling might act on the AMPA receptor complex rather than the individual receptor subunits independently. These observations indicate that the PTK Fyn might act on the AMPA receptor complex, possibly through a molecular mechanism similar to that shown at the neuromuscular junction.
Of interest, there are significant similarities between GluR2 (54, 55) and Fyn knockout mice in phenotypes (3, 12): Both mutants have significant hippocampal LTP impairment as well as higher susceptibility to seizure induction. BDNF knockout mice also exhibit an impairment in synaptic plasticity (25, 26). Our findings of lower AMPA receptor expression in Fyn knockout mice provide a potential explanation for the previous finding that LTP induction in the Fyn knockout mice requires higher levels of synaptic stimulation than in wild-type controls (3). It is likely that the lower expression of AMPA receptors in these mutants results in lower levels of membrane depolarization and in decreases in NMDA receptor currents during LTP induction. Thus, a decline in AMPA receptor expression may be responsible for the abnormal synaptic plasticity and behavior observed in the Fyn knockout mice.
The activities of neurotrophins in synaptic plasticity have been examined by using various electrophysiological techniques (20, 22–24). Changes in hippocampal basal neurotransmission, which presumably reflects AMPA receptor activity, occur within hours after BDNF application and depend on translation but not on transcription (56). The increase in AMPA receptor protein observed in our study was similar at the translational or post-translational level but required chronic applications of BDNF over several days. This provides circumstantial evidence to suggest that the mechanisms underlying these two phenomena may differ. Further physiological study on the chronic effects of BDNF as well as those of PDGF should help to characterize the signaling of Src-family PTKs in the acute responses to BDNF reported previously. Here, we demonstrated that the molecular signaling of the Src-family PTKs triggered by the growth factors plays a crucial role in regulation of AMPA receptor levels in the brain. These results provide insights into the mechanisms regulating AMPA receptor expression, a key determinant of synaptic plasticity.
Acknowledgments
We thank Dr. P. L. Stein, Dr. P. Soriano, and Dr. T. Yagi for providing parental Fyn mutant mice, Miss J. H. Kogan and Mrs. C. Fujikawa for technical assistance, Dr. K. Sakimura for mouse GluRB cDNA and GluRB/C antibodies, Dr. S. Koizumi, Mr. H. Jourdi, and Dr. H. Umemori for advice, and Dr. K. Horikawa for maintaining the mutant mice. This work was supported by the Japan Society for the Promotion of Science (Grant RFTF-96L00203), the Uehara Memorial Foundation, and a grant from Kyowa Hakko Kogyo Co.
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- BDNF
brain-derived neurotrophic factor
- LTP
long term potentiation
- NMDA
N-methyl-d-aspartate
- PDGF
platelet-derived growth factor
- PTK
protein tyrosine kinase
- EGF
epidermal growth factor
- bFGF
basic fibroblast growth factor
- PP1
4-amino-5-(4-methylphenyl)-7-(t-butyl) pyrazolo [3,4d] pyrimidine
- GluR1
glutamate receptor 1
- NF
neurofilament
Footnotes
To whom reprint requests should be addressed.
References
- 1.Thomas S M, Brugge J S. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 2.Hanissian S H, Chatila T, Sahyoun N E. J Neurobiol. 1992;23:803–813. doi: 10.1002/neu.480230703. [DOI] [PubMed] [Google Scholar]
- 3.Grant S G, O’Dell T J, Karl K A, Stein P L, Soriano P, Kandel E R. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 4.Yu X M, Askalan R, Keil G J, II, Salter M W. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 5.Holmes T C, Fadool D A, Ren R, Levitan I B. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 6.Lu Y M, Roder J C, Davidow J, Salter M W. Science. 1998;279:1363–1367. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- 7.Stein P L, Lee H M, Rich S, Soriano P. Cell. 1992;70:741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 8.Yagi T, Aizawa S, Tokunaga T, Shigetani Y, Takeda N, Ikawa Y. Nature (London) 1993;366:742–745. doi: 10.1038/366742a0. [DOI] [PubMed] [Google Scholar]
- 9.Beggs H E, Soriano P, Maness P F. J Cell Biol. 1994;127:825–833. doi: 10.1083/jcb.127.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Umemori H, Sato S, Yagi T, Aizawa S, Yamamoto T. Nature (London) 1994;367:572–576. doi: 10.1038/367572a0. [DOI] [PubMed] [Google Scholar]
- 11.Kojima N, Wang J, Mansuy I M, Grant S G, Mayford M, Kandel E R. Proc Natl Acad Sci USA. 1997;94:4761–4765. doi: 10.1073/pnas.94.9.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyakawa T, Yagi T, Taniguchi M, Matsuura H, Tateishi K, Niki H. Mol Brain Res. 1995;28:349–352. doi: 10.1016/0169-328x(94)00251-9. [DOI] [PubMed] [Google Scholar]
- 13.Miyakawa T, Yagi T, Kitazawa H, Yasuda M, Kawai N, Tsuboi K, Niki H. Science. 1997;278:698–701. doi: 10.1126/science.278.5338.698. [DOI] [PubMed] [Google Scholar]
- 14.Barbacid M. Ann NY Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan D R, Miller F D. Curr Opin Cell Biol. 1997;9:213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- 16.Thoenen H. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- 17.Lewin G R, Barde Y A. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 18.Suen P C, Wu K, Levine E S, Mount H T, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lesser S S, Sherwood N T, Lo D C. Mol Cell Neurosci. 1997;10:173–183. doi: 10.1006/mcne.1997.0656. [DOI] [PubMed] [Google Scholar]
- 20.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 21.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka T, Saito H, Matsuki N. J Neurosci. 1997;17:2959–2966. doi: 10.1523/JNEUROSCI.17-09-02959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 24.Akaneya Y, Tsumoto T, Hatanaka H. J Neurophysiol. 1996;76:4198–4201. doi: 10.1152/jn.1996.76.6.4198. [DOI] [PubMed] [Google Scholar]
- 25.Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, Bonhoeffer T. Proc Natl Acad Sci USA. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patterson S L, Abel T, Deuel T A, Martin K C, Rose J C, Kandel E R. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- 27.Kremer N E, D’Arcangelo G, Thomas S M, DeMarco M, Brugge J S, Halegoua S. J Cell Biol. 1991;115:809–819. doi: 10.1083/jcb.115.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Gay B, Wada K, Koizumi S. J Neurochem. 1998;71:106–111. doi: 10.1046/j.1471-4159.1998.71010106.x. [DOI] [PubMed] [Google Scholar]
- 29.Kypta R M, Goldberg Y, Ulug E T, Courtneidge S A. Cell. 1990;62:481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- 30.Hu X Q, Singh N, Mukhopadhyay D, Akbarali H I. J Biol Chem. 1998;273:5337–5342. doi: 10.1074/jbc.273.9.5337. [DOI] [PubMed] [Google Scholar]
- 31.Valenzuela C F, Xiong Z, MacDonald J F, Weiner J L, Frazier C J, Dunwiddie T V, Kazlauskas A, Whiting P J, Harris R A. J Biol Chem. 1996;271:16151–16159. doi: 10.1074/jbc.271.27.16151. [DOI] [PubMed] [Google Scholar]
- 32.Hilborn M D, Vaillancourt R R, Rane S G. J Neurosci. 1998;18:590–600. doi: 10.1523/JNEUROSCI.18-02-00590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Neuroscience. 1998;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- 34.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 36.Loughlin S E, Fallon J H. Neurotrophic Factors. San Diego: Academic; 1993. [Google Scholar]
- 37.Umemori H, Wanaka A, Kato H, Takeuchi M, Tohyama M, Yamamoto T. Mol Brain Res. 1992;16:303–310. doi: 10.1016/0169-328x(92)90239-8. [DOI] [PubMed] [Google Scholar]
- 38.Inomata M, Takayama Y, Kiyama H, Nada S, Okada M, Nakagawa H. J Biochem (Tokyo) 1994;16:386–392. doi: 10.1093/oxfordjournals.jbchem.a124536. [DOI] [PubMed] [Google Scholar]
- 39.Grant S G, Karl K A, Kiebler M A, Kandel E R. Genes Dev. 1995;9:1909–1921. doi: 10.1101/gad.9.15.1909. [DOI] [PubMed] [Google Scholar]
- 40.Patterson P H, Nawa H. Cell/Neuron. 1992;10:123–137. [Google Scholar]
- 41.Cartwright C A, Simantov R, Cowan W M, Hunter T, Eckhart W. Proc Natl Acad Sci USA. 1988;85:3348–3352. doi: 10.1073/pnas.85.10.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katoh-Semba R, Takeuchi I K, Semba R, Kato K. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 43.Standley S, Wagle N, Baudry M. Dev Brain Res. 1998;107:277–283. doi: 10.1016/s0165-3806(98)00036-4. [DOI] [PubMed] [Google Scholar]
- 44.Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien R J, Mammen A L, Blackshaw S, Ehlers M D, Rothstein J D, Huganir R L. J Neurosci. 1997;17:7339–7350. doi: 10.1523/JNEUROSCI.17-19-07339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petralia R S, Wenthold R J. J Comp Neurol. 1992;318:329–354. doi: 10.1002/cne.903180309. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T, Okumura-Noji K. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 48.Conner J M, Lauterborn J C, Yan Q, Gall C M, Varon S. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altar C A, Cai N, Bliven T, Juhasz M, Conner J M, Acheson A L, Lindsay R M, Wiegand S J. Nature (London) 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- 50.Canossa M, Griesbeck O, Berninger B, Campana G, Kolbeck R, Thoenen H. Proc Natl Acad Sci USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swope S L, Huganir R L. J Biol Chem. 1993;268:25152–25161. [PubMed] [Google Scholar]
- 52.Fuhrer C, Hall Z W. J Biol Chem. 1996;271:32474–32481. doi: 10.1074/jbc.271.50.32474. [DOI] [PubMed] [Google Scholar]
- 53.Colledge M, Froehner S C. J Neurosci. 1997;17:5038–5045. doi: 10.1523/JNEUROSCI.17-13-05038.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brusa R, Zimmermann F, Koh D S, Feldmeyer D, Gass P, Seeburg P H, Sprengel R. Science. 1995;270:1677–1680. doi: 10.1126/science.270.5242.1677. [DOI] [PubMed] [Google Scholar]
- 55.Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna F A, Velumian A, MacDonald J, Carlen P, et al. Neuron. 1996;17:945–956. doi: 10.1016/s0896-6273(00)80225-1. [DOI] [PubMed] [Google Scholar]
- 56.Kang H, Schuman E M. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 57.Cheng B, Furukawa K, O’Keefe J A, Goodman Y, Kihiko M, Fabian T, Mattson M P. J Neurochem. 1995;65:2525–2536. doi: 10.1046/j.1471-4159.1995.65062525.x. [DOI] [PubMed] [Google Scholar]