Abstract
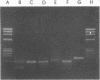
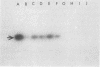
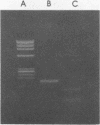
The polymerase chain reaction (PCR) was used to identify mycobacterial DNA sequences in uncultured clinical specimens. Two oligonucleotide primers derived from the sequence of a gene that codes for the 65-kilodalton antigen of Mycobacterium tuberculosis amplified DNA from all 11 species of mycobacteria tested. Amplified DNAs of nontuberculosis mycobacteria were found to be approximately 20 to 40 bases shorter than those from M. tuberculosis and Mycobacterium bovis BCG. DNA equivalent to that present in as few as 40 M. tuberculosis cells either alone or in the presence of DNA equivalent to that in 10(6) human cells could be detected. Results from analysis of cultured bacteria and clinical specimens showed PCR was sensitive and specific both in detecting mycobacteria and in differentiating M. tuberculosis and BCG from other species of mycobacteria. The PCR method with the primers reported here may become a useful tool in the early and rapid detection of mycobacterial infections in uncultured clinical specimens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates J. H. Diagnosis of tuberculosis. Chest. 1979 Dec;76(6 Suppl):757–763. doi: 10.1378/chest.76.6_supplement.757. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Drake T. A., Hindler J. A., Berlin O. G., Bruckner D. A. Rapid identification of Mycobacterium avium complex in culture using DNA probes. J Clin Microbiol. 1987 Aug;25(8):1442–1445. doi: 10.1128/jcm.25.8.1442-1445.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Lin C. Y., Maa J. S., Lai C. H., Wu S. Y., Soong Y. K. Detection of human papillomaviruses in cervicovaginal cells using polymerase chain reaction. J Infect Dis. 1990 Jan;161(1):113–115. doi: 10.1093/infdis/161.1.113. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Lin S. S., Wu S. Y., Juang W. M., Chang C. H., Lin J. Y. The detection of mycobacterial DNA sequences in uncultured clinical specimens with cloned Mycobacterium tuberculosis DNA as probes. Tubercle. 1988 Mar;69(1):27–36. doi: 10.1016/0041-3879(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., McMillan C., Coyle M. B. Whole chromosomal DNA probes for rapid identification of Mycobacterium tuberculosis and Mycobacterium avium complex. J Clin Microbiol. 1987 Jul;25(7):1239–1243. doi: 10.1128/jcm.25.7.1239-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. A., Fisher J. H., Scoggin C. H. Techniques of DNA hybridization detect small numbers of mycobacteria with no cross-hybridization with non-mycobacterial respiratory organisms. Am Rev Respir Dis. 1985 May;131(5):760–763. doi: 10.1164/arrd.1985.131.5.760. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Keulen W. J., De Bruyn J., Kolk A. H., Groothuis D. G., Berwald L. G., Tiesjema R. H., van Embden J. D. Characterization, sequence determination, and immunogenicity of a 64-kilodalton protein of Mycobacterium bovis BCG expressed in escherichia coli K-12. Infect Immun. 1987 Jun;55(6):1466–1475. doi: 10.1128/iai.55.6.1466-1475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D., Lathigra R., Hendrix R., Sweetser D., Young R. A. Stress proteins are immune targets in leprosy and tuberculosis. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4267–4270. doi: 10.1073/pnas.85.12.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]